Abstract
Background
Cardiac hypertrophy is highly prevalent in patients with type 2 diabetes mellitus (T2DM). Experimental evidence has implied that pregnant women with T2DM and their children are at an increased risk of cardiovascular diseases. Our previous mouse model study has revealed that maternal T2DM induces structural heart defects in their offspring.
Objective
The present study aims to determine whether maternal T2DM induces embryonic heart hypertrophy in a murine model of diabetic embryopathy.
Study design
The T2DM embryopathy model was established by feeding 4-week-old female C57BL/6J mice with a high-fat diet (HFD) for 15 weeks. Cardiac hypertrophy in embryos at embryonic day 17.5 was characterized by measuring heart size and thickness of the right and left ventricle walls and the interventricular septum, as well as the expression of β-myosin heavy chain (β-MHC), atrial natriuretic peptide (ANP), insulin-like growth factor 1 (IGF1), desmin (DES), and adrenomedullin (ADM). Cardiac remodeling was determined by collagen synthesis and fibronectin synthesis. Fibrosis was evaluated by Masson staining and determining the expression of connective tissue growth factor (CTGF), osteopontin (OPN), and Galectin 3 (GAL3) genes. Cell apoptosis also was measured in the developing heart.
Results
The thicknesses of the left ventricle walls and the interventricular septum of embryonic hearts exposed to maternal diabetes were significantly thicker than those in the nondiabetic (ND) group. Maternal diabetes significantly increased β-MHC, ANP, IGF1 and DES expression, but decreased expression of ADM. Moreover, collagen synthesis was significantly elevated, whereas fibronectin synthesis was suppressed, in embryonic hearts from diabetic dams, suggesting that cardiac remodeling is a contributing factor to cardiac hypertrophy. The cardiac fibrosis marker, GAL3, was induced by maternal diabetes. Furthermore, maternal T2DM activated the pro-apoptotic c-Jun-N-terminal kinase (JNK1/2) stress signaling and triggered cell apoptosis by increasing the number of TUNEL positive cells (10.4 ± 2.2% of the T2DM group vs. 3.8 ± 0.7% of the ND group, P < 0.05).
Conclusions
Maternal T2DM induces cardiac hypertrophy in embryonic hearts. Adverse cardiac remodeling, including elevated collagen synthesis, suppressed fibronectin synthesis, profibrosis and apoptosis, is implicated as the etiology of cardiac hypertrophy.
Keywords: pregestational type 2 diabetes, hypertrophy, cardiac remodeling, fibrosis, diabetic embryopathy
Introduction
The mechanism of “early life programming” has been well accepted, linking early growth and subsequent risk of diseases including obesity, type 2 diabetes mellitus (T2DM), and ischemic heart disease1–6. Pregestational diabetes is the leading cause for fetal intrauterine growth retardation7. It is well known that a pregnant woman with T2DM and her unborn child are both at increased risk of pregnancy complications such as pre-eclampsia, preterm births, stillbirths, macrosomia, miscarriage, intrauterine growth retardation, and congenital anomalies8–12. Specifically, heart hypertrophy was highly prevalent in asymptomatic patients with T2DM13. However, it is unknown whether pregestational type 2 maternal diabetes influences the heart of the fetus.
Neonatal echocardiographic data indicate that cardiac septal overgrowth affects 10% to 40% of neonates born to mothers with pregestational diabetes14–16. The functional impact of neonatal cardiac septal hypertrophy can range from clinically asymptomatic to potentially fatal congestive heart failure stemming from left ventricular outflow tract obstruction. The underlying mechanism has been widely investigated by using a streptozocin-induced diabetic rat or mouse model of type 1 diabetes17–19. However, there are few reports about cardiac hypertrophy in embryos from a T2DM model.
Cardiac remodeling resulting in cardiac rupture occurs in acute streptozocin-induced diabetes mouse models20. Further evidence indicated that cardiac remodeling in patients with T2DM is associated with endothelial dysfunction, collagen turnover, and a late onset of heart failure21, 22. Diabetes affects cardiac remodeling through a variety of mechanisms, including fibrosis23. However, it is unknown whether cardiac remodeling occurs in the embryo of a mother with pregestational T2DM. Therefore, it is important to investigate the effect of cardiac remodeling and fibrosis on heart of offspring whose mother suffered from T2DM.
In this study, hearts from fetuses of high-fat diet (HFD)-induced T2DM dams were investigated24. Heart hypertrophy was significantly increased in the fetus from a T2DM mother, and cardiac remodeling occurred in the embryo from a diabetic mother. Regulation of collagen and fibronectin synthesis was disturbed in these fetal hearts by the imbalance of matrix metalloproteinases (MMPs) and the tissue inhibitors of matrix metalloproteinases (TIMPs). The subsequent increased fibrosis and apoptosis caused by maternal T2DM during pregnancy may provide a direct rationale for the emergence of heart failure in a later life stage of a neonate.
Methods and Materials
Study design
In this study, two experimental groups, the T2DM dams and the nondiabetic dams, were used to determine the effect of T2DM on cardiac hypertrophy in E17.5 embryos. Cardiac hypertrophy was morphologically evaluated and further confirmed by the expression of hypertrophy markers using quantitative PCR. To elucidate the underlying mechanism of cardiac hypertrophy, cardiac remodeling and fibrosis were examined via measurement of the collagen/fibronectin synthesis markers in embryonic hearts from T2DM and nondiabetic dams. H9C2 cell culture model was used to confirm these changes in high glucose in vitro-induced cardiac hypertrophy.
Animals and the T2DM model
To investigate the effect of T2DM on embryonic hearts, T2DM mice model was established as previously described24. Briefly, 4-week-old female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were fed an HFD (Research Diets, New Brunswick, NJ, USA) for 15 weeks. The HFD contained 20% protein, 20% carbohydrates, and 60% fat. HFD-fed mice were mated with lean male mice. Embryonic day 0.5 (E0.5) of pregnancy was established at noon on the day when a vaginal plug was observed. Blood glucose measurement, a glucose tolerance test, and an insulin tolerance test proved that the HFD mice have the T2DM phenotype. Fetus’ hearts at 17.5-day pregnancy were collected and kept in −80°C for further use.
Evaluation of hypertrophy with hematoxylin and eosin staining and calculation of hearts dimensions
To determine hypertrophy in embryonic heart from T2DM dams, hematoxylin and eosin (H&E) staining was performed using heart sections. The procedures of heart hematoxylin and eosin (H&E) staining have been described25. Briefly, the pregnant dams were euthanized on E17.5, and fetuses were excised from the uteri and decapitated. Fetus’ hearts were then fixed in methacarn (60% methanol, 30% chloroform, and 10% glacial acetic acid), embedded in paraffin, and cut into 10-µm sections. After deparaffinization and rehydration, all specimens then underwent H & E staining through a standard procedure. All heart sections were photographed and examined for heart dimensions using Photoshop CS6 (Adobe, San Jose, California, USA). We measured the thickest dimension of the left ventricle, interventricular septum, and right ventricle.
mRNA changes in cardiac hypertrophy and remodeling markers
To demonstrate the expression of hypertrophy and cardiac remodeling markers in messenger RNA level, mRNA was isolated from E17.5-day hearts and H9C2 cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and reversed transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Grand Island, NY, USA). Real-time qPCR for β-myosin heavy chain (β-MHC), atrial natriuretic peptide (ANP), insulin-like growth factor 1 (IGF1), desmin (DES), adrenomedullin (ADM), connective tissue growth factor (CTGF), osteopontin (OPN), galectin-3 (GAL)3, MMP1, MMP13, TIMP1, TIMP2, TIMP3, TIMP4 COL1a, COL4a, FN1 and β-Actin were performed using the Maxima SYBR Green/ROX qPCR Master Mix Assay (Thermo Fisher Scientific, Waltham, MA, USA) in the StepOnePlus system (Applied Biosystems, Foster City, CA, USA). The primer sequences used are listed in Supplementary Table 1.
Measurement of cardiac remodeling markers
To determine the molecular changes of cardiac remodeling markers, Western blotting was performed as previously described26. Briefly, hearts from the different experimental groups were sonicated in lysis buffer containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Equal amounts of protein and the Precision Plus Protein standards (Bio-Rad Laboratories, Hercules, CA, USA) were resolved by SDS-PAGE and transferred onto Immobilon-P membranes (Millipore, Billerica, MA, USA). Membranes were incubated in 5% nonfat milk for 45 minutes and then incubated for 18 hours at 4°C with the primary antibodies. To determine whether equivalent amounts of protein were loaded among all samples, membranes were stripped and incubated with a mouse antibody against β-actin (Abcam, Cambridge, MA, USA) to generate a signal used as a loading control. Signals were detected using the SuperSignal West Femto Maximum Sensitivity Substrate kit (Thermo Scientific, Waltham, MA, USA). The sources and dilutions of antibodies used in each experiment are listed in Supplementary Table 2.
Fibrosis in embryonic heart from T2DM dams
To evaluate fibrosis extent, collagen content was evaluated by the masson’s trichrome staining with a heart section of nondiabetes (ND) and T2DM offspring by Masson’s trichrome stain kit (Sigma-Aldrich, St. Louis, MO, USA). One hundred photographs were taken for each group. We randomly took 500×400 pixel area on each photography, and measured the collagen area using ImageJ 1.49v (NIH, Bethesda, MD, USA) to 100 photography, and then the average collagen area was calculated. To measure the nuclear density of cells on each paraffin section, we randomly counted nuclear on 500×400 pixel area on 50 pictures, and then calculated the average nuclear density. The cardiomyocyte area were measured by adjusting the color threshold on 500×400 pixels, which covered only the cell area, and then each cell area was divided by the amount of nuclear in this area.
Cell apoptosis in embryonic heart from T2DM dams
To investigate the effect of T2DM on cardiac cell apoptosis, the TUNEL assay was performed using the ApopTag Fluorescein Direct In Situ Apoptosis Detection Kit (Millipore, Billericam, MA, USA) as previously described26. Briefly, 10-µm paraffin heart sections were incubated with TUNEL reaction agents. Three hearts from three different dams per group were used. TUNEL-positive cells in a randomly selected area (~200 cells) of hearts were counted. The percentage of TUNEL-positive cells was calculated as a fraction of the total cell number multiplied by 100.
Cell culture
H9C2 rat cardiomyoblasts (Sigma-Aldrich, St. Louis, MO, USA) were maintained in DMEM supplemented with 10% fetal bovine serum, 90 mg/dl glucose (Invitrogen, Carlsbad, CA, USA), 100U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Sub-confluent cells were treated with high glucose (HG, 200 mg/dl) DMEM for 3 days. The cell surface area was determined with image analysis software ImageJ 1.49v (NIH, Bethesda, MD, USA).
Statistical Analyses
Data are presented as mean ± SE. Each set of experiments were repeated independently at least three times with comparable results, and embryonic samples from each replicate were taken from different dams. Statistical differences were evaluated using one-way analysis of variance (ANOVA) with Sigmaplot 12.5 software. For one-way ANOVA analysis, a Tukey test was used to estimate the significance of the results, with P < 0.05 indicating statistical significance.
Results
Maternal T2DM induces hypertrophy in the fetal heart
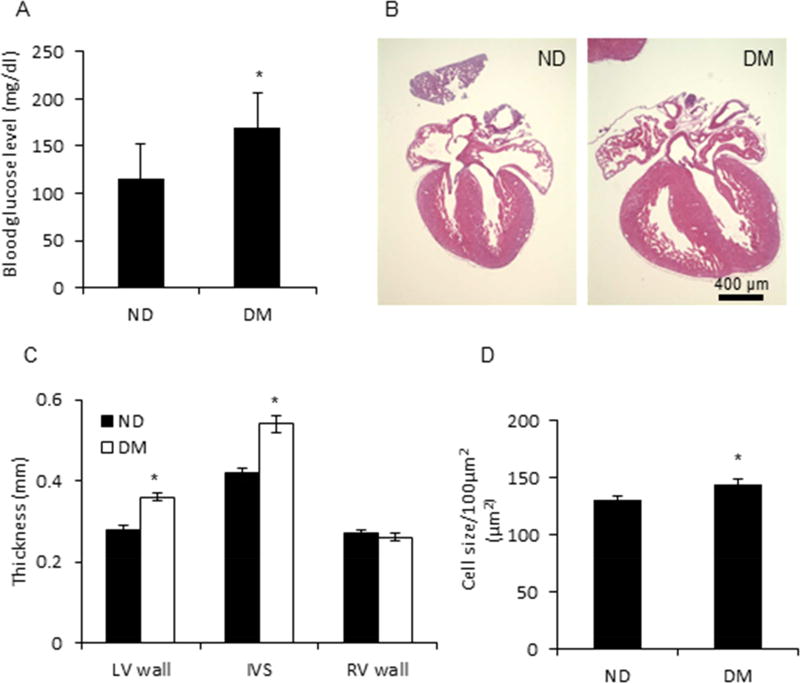
After feeding with a HFD for 15 weeks, dams had a blood glucose level that was significantly higher than that of ND dams (Fig. 1A), suggesting a successful establishment of the T2DM mouse model with modest hyperglycemia, which mimics T2DM in humans and is consistent with our previous findings24. A total of 76 fetal hearts from 16 ND dams and 40 fetal hearts from 9 T2DM dams were analyzed for heart dimension by measuring thickness of the left and right ventricle, and interventricular septum in a four-chamber view. As shown in Fig. 1B, the fetal heart size from T2DM dams was significantly larger than the size of fetal hearts from ND dams. Further investigation revealed that the thickness of the interventricular septum was higher in the T2DM group than that in the ND group (0.54 ± 0.02 mm vs. 0.42 ± 0.01 mm, P < 0.001), and the left ventricular walls were thicker (0.36 ± 0.01 mm vs. 0.28 ± 0.01 mm, P < 0.05) in the T2DM group than in the ND group (Fig. 1C). However, there was no difference in the right ventricular wall thickness between the two groups (0.26 ± 0.01 mm vs. 0.27 ± 0.01 mm) (Fig. 1C). The cardiomyocyte size in the T2DM group was significantly larger than that of the ND group (p < 0.05) (Fig. 1D).
Figure 1. Cardiac hypertrophy in embryos from a T2DM dam.
A, Blood glucose level of T2DM mouse model. ND, Nondiabetes, DM, type 2 diabetes mellitus. B, Enlarged heart in embryos from ND and DM dam. C, Thickness of left ventricle (LV) wall, interventricular septum (IVS), and right ventricle (RV) wall of embryo from ND and DM dam. D, Cardiomyocyte size in heart of embryo from ND and DM dam. 76 and 40 hearts of embryos from 16 ND dams and 40 DM dams, respectively, were removed at E17.5. * indicates significant difference (P < 0.05) when compared to ND group.
Maternal T2DM increases the expression of hypertrophic markers in the developing heart
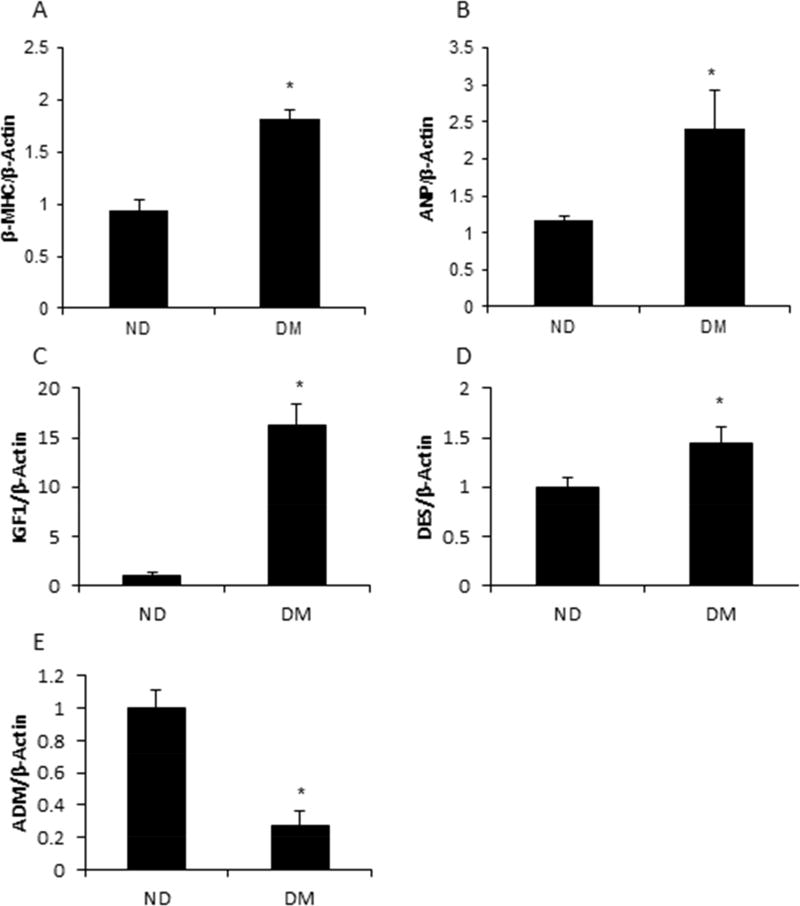
Re-expression of the early developmental gene, β-MHC, is well documented in contributing to the pathogenesis of cardiac hypertrophy in adults with T2DM27. Therefore, it is of interest to determine the effect of maternal diabetes on the expression of β-MHC and ANP, another cardiac hypertrophic marker, in the developing heart. The expression levels of β-MHC and ANP were significantly higher in the fetal hearts of the T2DM group compared with the ND group (p < 0.01 and p < 0.05 respectively) (Fig. 2A and 2B). It has been reported that IGF128, 29, DES30, 31, and ADM32–34 are dysregulated along with progression of cardiac hypertrophy. Consistent with findings of others, the expression of IGF1 and DES were significantly increased in the fetal hearts of T2DM dams compared with those from ND dams (p < 0.001 and p < 0.05 respectively) (Fig. 2C and 2D). ADM attenuates hypertrophic remodeling33, and maternal T2DM suppressed the expression of ADM (Fig. 2E).
Figure 2. Alteration of hypertrophy-associated genes in embryonic heart in T2DM pregnancy.
Hyperglycemia increased the expression of fetal genes, β-MHC (A), and ANP (B). Hypertrophy-associated genes, IGF1 (C), DES (D), and ADM (E), were influenced by T2DM in the embryonic heart. Each experiment was repeated three times. * indicates significant difference (P < 0.05) when compared to ND group.
Collagen synthesis genes were partially induced by T2DM
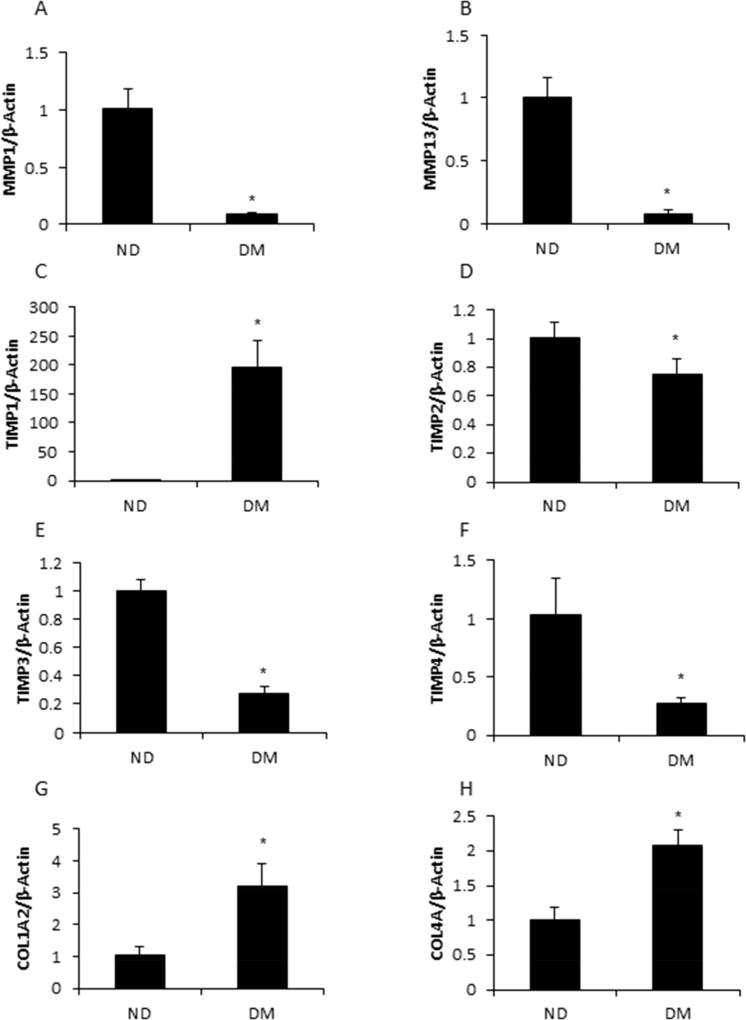
Cardiac hypertrophy and remodeling are pathological features of many cardiac diseases, including diabetic cardiomyopathy35. Cardiac remodeling and stiffness are the consequence of alteration in key components of the extracellular matrix, including collagen and fibronectin36. Numerous reports have demonstrated the effect of collagen concentrations and collagenolysis on hypertrophic cardiomyopathies37. However, the mechanism is still unclear. Because collagen dynamics are maintained by MMPs and TIMPs, we determined the expression of MMP1, MMP2, MMP8, MMP9, MMP13, and four genes of the TIMPs family. Two out of the five MMPs, MMP1 and MMP13, were significantly decreased in the fetal hearts from the DM group compared with those of the ND group (p < 0.01) (Fig. 3A and 3B). The expression of TIMP1 was significantly increased by maternal diabetes (p < 0.001) (Fig. 3C), whereas the expression of TIMP2, TIMP3, and TIMP4 was decreased by maternal diabetes (p < 0.05, p < 0.01 and p < 0.05 respectively) (Fig. 3D, 3E, and 3F). Furthermore, the collagen synthesis genes, COL1 and COL4, were up-regulated in the fetal hearts by maternal diabetes (Fig. 3G and 3H). All of the data demonstrated that the collagen synthesis genes, Col1 and Col4, were induced by maternal T2DM.
Figure 3. Collagen synthesis was increased by hyperglycemia.
Hyperglycemia suppressed the expression of matrix metalloproteinases, MMP1 (A) and MMP13 (B). T2DM influenced the expression of TIMP1 (C), TIMP2 (D), TIMP3 (E), and TIMP4 (F). Hyperglycemia activated collagen genes, COL1A2 (G) and COL4A (H). Each experiment was repeated three times. * indicates significant difference (P < 0.05) when compared with the ND group.
Maternal T2DM suppresses fibronectin synthesis
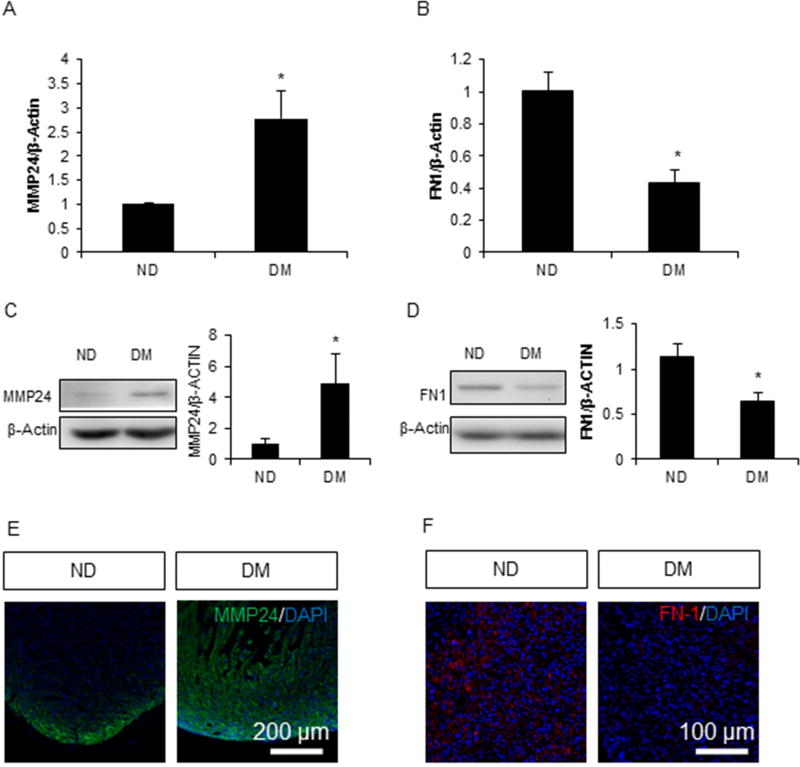
Fibronectin is another important component of extracellular matrix and contributes to the pathogenesis of cardiac hypertrophy38. MMP24, which degrades fibronectin but not collagen, was significantly increased at both the messenger RNA level and the protein level in fetal hearts by maternal diabetes (p < 0.05) (Fig. 4A and 4C). MMP24 was mainly expressed in the peripheral areas of the heart in the ND group. However, in the DM group, MMP24 was overexpressed not only in the peripheral tissue, but also inside of the myocardium of the fetal heart (Fig. 4E). The overall expression of MMP24 in the DM group was higher than that of the ND group. Correlated with MM24 up-regulation, messenger RNA and protein levels of fibronectin were significantly down-regulated by maternal diabetes (p < 0.05) (Fig. 4B and 4D).
Figure 4. Fibronectin synthesis was suppressed by hyperglycemia.
mRNA expression of MMP24 (A) and FN1 (B) in the embryonic heart. Western blot of MMP24 (C) and FN1 (D) in embryonic heart. Immunofluorescence of MMP24 (E) and FN1 (F) in embryonic heart. Each group contained three samples, * indicates significant difference (P < 0.05) when compared with the ND group.
Maternal T2DM induces pro-fibrosis in cardiomyocytes of the developing heart
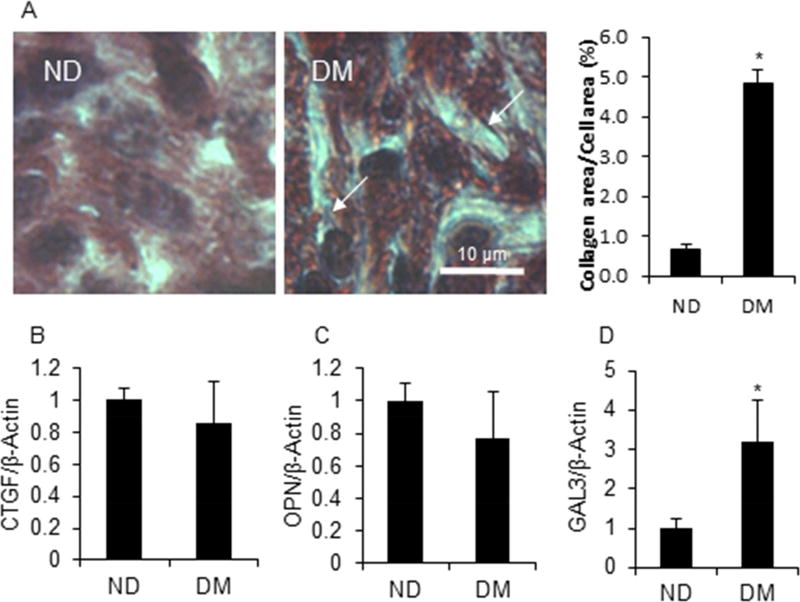
Hyperglycemia induces progressive cardiomyocyte fibrosis in diabetic patients23; however, little is known regarding the effect of maternal diabetes on fetal cardiomyocyte fibrosis. Fibrosis was evaluated by Masson’s trichrome staining in fetal heart sections. No fibrosis was observed in the fetal hearts of either group (Fig. 5A); however, collagen staining was much higher in the fetal hearts from DM dams compared with those from ND dams (p < 0.01) (Fig. 5A), confirming our previous findings that T2DM increased collagen synthesis, but did not induce fibrosis. Furthermore, the expression of pro-fibrosis markers, CTGF, OPN, and GAL3 was detected. Among them, only GAL3 expression in the DM group was significantly higher than that in the ND group (p < 0.05) (Fig. 5B, 5C, and 5D).
Figure 5. T2DM induced pro-fibrosis in the embryo heart.
A. Masson staining of the embryo heart section. Arrow represents collagen staining (blue line). mRNA expression of pro-fibrosis markers, CTGF (B), OPN (C), and GAL3 (D). Each group contained three samples. * indicates significant difference (P < 0.05) when compared with the ND group.
Maternal T2DM induces cell apoptosis in the developing heart
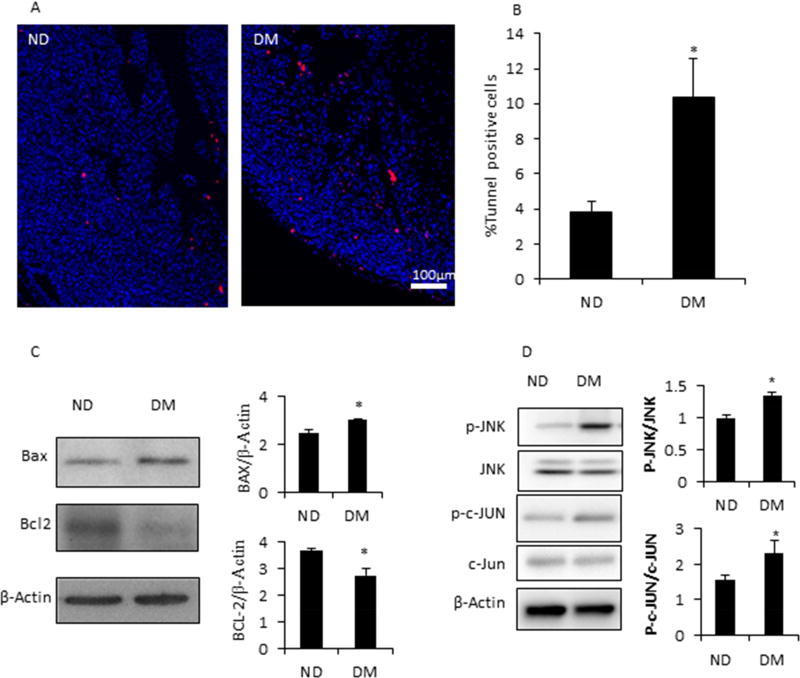
Cell apoptosis is involved in the induction of diabetic embryopathy24, 39. We hypothesized that maternal diabetes-induced cardiac hypertrophy was associated with enhanced cell apoptosis. Indeed, the percentage of TUNEL positive cells was significantly higher in the DM group comparing with that of the ND group (10% vs. 4%) (Fig. 6A and 6B). Increased levels of BAX, a pro-apoptotic factor, and decreased levels of Bcl-2, a pro-survival factor, were observed in the fetal hearts from the DM group (Fig. 4C). The activation of the pro-apoptotic Jun-N terminal kinase (JNK) signaling pathway is a central mechanism in diabetic embryopathy40. The levels of phosphorylated JNK and c-Jun were significantly increased in the DM group compared with the ND group (Fig. 4D).
Figure 6. T2DM induced apoptosis of cardiomyocytes in embryo heart.
A, TUNEL assay of embryo heart from ND and DM dams. B, Quantification of TUNEL positive cells based on panel A. C, Western blot of pro-apoptotic factor, BAX, and anti-apoptotic factor, BCL-2, in embryo hearts from ND and DM dams. D, Activation of JNK and c-JUN in heart of embryos from ND and DM dams. * indicates significant difference (P < 0.05) when compared with the ND group.
Cardiac hypertrophy was induced in H9C2 cell line by high glucose in vitro
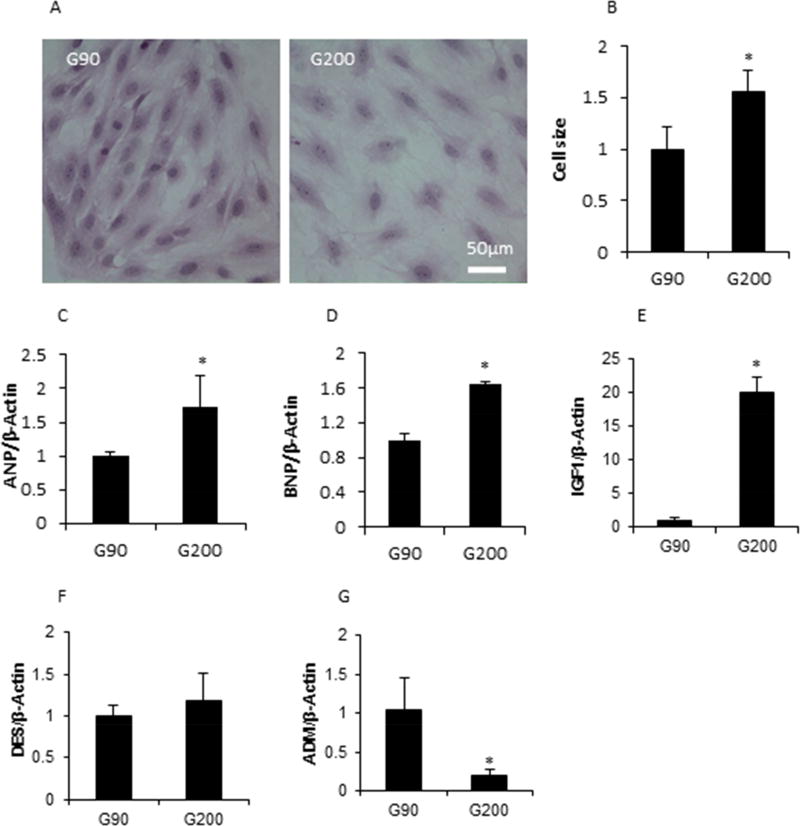
The H9C2 cell line, a cardiomyoblast cell line, shows similar hypertrophic responses to those shown by primary neonatal cardiomyocyte cells41. To confirm our in vivo findings, H9C2 cells were treated with modestly high glucose stimulation (200 mg/dl) for 3 days in vitro. Consistent with our in vivo findings, H9C2 cells showed a hypertrophic phenotype (i.e., manifesting larger cell size) after treatment with 200 mg/dl glucose compared with cells cultured in normal glucose (90 mg/dl) (Fig. 7A and 7B). Furthermore, the expression of hypertrophy genes, ANP, BNP, and IGF1, were all significantly increased by modestly high glucose stimulation (p < 0.05, p < 0.01 and p < 0.001 respectively) (Fig. 7C, 7D, and 7E). ADM expression, a cardiac hypertrophy attenuator, was suppressed by modest high glucose (Fig. 7G). However, no difference of DES expression was observed between the normal and the modest high glucose groups (Fig. 7F).
Figure 7. Cardiac hypertrophy was induced by high glucose in vitro.
A, The cell size was increased by high glucose (200 mg/dl) in H9C2 cell line. B, Quantification of cell hypertrophy based on panel A. High glucose influenced hypertrophy associated markers (C–G). * indicates significant difference (P < 0.05) when compared with the low glucose group.
Comment
Previous studies have shown that mild elevation of glucose seen in pregestational diabetes has a profound influence on the development of the offspring heart16, 42, 43. Our hypothesis is that chronic mild hyperglycemia during pregnancy is associated with cardiomyocyte hypertrophy of the offspring. Here, we used a T2DM mouse model with mild hyperglycemia induced by a HFD to investigate the effect of modest maternal glucose elevation during pregnancy on the developing embryonic heart. The average fasting glucose level in this model was 169 ± 38 mg/dl, which is comparable to the blood glucose level of a patient with T2DM44. Our results indicate that the fetuses of T2DM dams have hypertrophic hearts, supporting the clinical observations of babies of women with pregestational T2DM15, 16, 45, 46. It has been shown that transient hyperglycemic exposure is sufficient to induce septal overgrowth47. Thus, chronic mild hyperglycemia in T2DM is the major cause for fetal cardiac hypertrophy.
It is worth noting that our results suggest that maternal pregestational T2DM induces a significant hypertrophy in the fetus’s interventricular septum and left ventricle, but not in the right ventricle wall. The underlying mechanism for this observation needs further investigation. In adults, more blood volume/pressure loads into the left heart than the right heart48, 49. However, in fetuses, the blood volume in the right ventricle is higher than that in the left ventricle50. Therefore, the differences in responses to maternal diabetes between the right and left ventricles were probably not caused by blood volume/overload, but rather the different organogenesis of the left and right ventricles50–52. The two heart ventricles derive from different progenitors. The left ventricle is from the first heart field cells (FHF), whereas the right ventricle derives from the second heart field cells (SHF)53. Thus, it is possible that the effects of maternal diabetes on FHF and SHF are different. The impact of maternal diabetes on SHF cell differentiation is less than that of FHF cells, and, thus, there is no difference in the right ventricular wall thickness between the nondiabetic and the diabetic groups.
The pathological remodeling response of the adult heart to T2DM is characterized by hypertrophy, ventricular dilatation, and fibrosis54. Moreover, the imbalance between MMPs and TIMPs is usually associated with adverse LV remodeling55. However, there is little evidence of the effect of the imbalance of MMPs/TIMPs on cardiac remodeling in the embryonic heart from a mother with pregestation T2DM. In our animal model system, embryonic hearts from the diabetic group expressed more collagen, but downregulated fibronectin synthesis, which may contribute to developing heart hypertrophy. In addition, maternal T2DM induced an imbalance of MMP1/13 to TIMP1 and activated collagen genes in the embryonic heart, which may have contributed to the observed increase in collagen synthesis. Meanwhile, our findings that maternal T2DM elevated MMP24 and suppressed fibronectin might be further evidence that modest hyperglycemia during pregnancy induces cardiac remodeling. In this study, we found that imbalance between MMPs and TIMPs, alterations of collagen/fibronectin synthesis, pro-fibrosis and elevated cardiac cell apoptosis are involved in the development of fetal heart hypertrophy under maternal type 2 diabetes. It is of interest to prevent cardiac hypertrophy by blocking these changes. For example, TIMP1 is a potential target in restoring the balance of MMPs and TIMPs. If diabetes-increased TIMP1 expression is decreased by a specific pharmaceutical agent, it may result in rebalance of MMPs and TIMPs, and, thus, prevent abnormal cardiac remodeling. Similarly, cell apoptosis inhibitors are possible candidates in improving fetal heart function and suppression of heart hypertrophy.
Cardiac hypertrophy from pathological stimuli often results in heart failure58. Previous work indicates that hypertrophic cardiomyocytes are more sensitive to cell stress, which will increase cell apoptosis59–63. In this study we observed an increased level of apoptosis in the fetal heart cells of the T2DM group compared with the nondiabetic group. Moreover, the proapoptotic marker, BAX, was upregulated and the antiapoptotic marker, Bcl2, was downregulated in the embryonic hearts from diabetic dams. This increased apoptosis, along with proapoptotic changes, may contribute to the development of heart failure for the offspring later in life. The most profound finding in this study is the interventricular septum hypertrophy in embryos of diabetic dams. Therefore, it is pivotal to determine which signaling pathway is involved in cardiac cell apoptosis. Because the JNK signaling pathway implicates in the dysmorphogenesis of the outflow tract of the developing heart64–66, we hypothesized that, in the fetal heart from a T2DM mother, JNK and its downstream signaling are activated. Indeed, phosphorylation of JNK and c-JUN are increased in the T2DM group. JNKs belong to the superfamily of MAP kinases, which regulate cell proliferation, differentiation, and apoptosis67. Moreover, the JNK pathway is involved in the pathological process of fibrosis68. Therefore, the JNK pathway may attribute to apoptosis and increased collagen synthesis in the heart of embryos from T2DM dams.
According to the World Health Organization, cardiovascular disease is a major contributor to the growing public health epidemic in chronic diseases, such as chronic lung disease, chronic kidney disease and diabetes69. These chronic diseases are responsible for nearly two-thirds of all global deaths70. Therefore, to prevent the epidemic of chronic diseases, it is important to recognize who may be at high-risk for developing cardiovascular disease. Maternal diabetes during pregnancy is clinically associated with a higher risk of cardiovascular disease in the offspring71–77, which suggests that prenatal exposure to hyperglycemia may cause cardiac changes which might increase a person’s risk of developing cardiovascular disease in adulthood. Therefore, it is important to determine whether maternal diabetes in pregnancy has any long-term influence on the postnatal heart. Generally, hypertrophic cardiomyopathy observed in the infant of a mother with pregestational diabetes is regarded as a relatively benign phenomenon43, 78–80. Most infants are clinically asymptomatic and have resolution of the hypertrophy within months after birth. However, the infant heart is relatively stiff and sensitive to excessive stress from hypertension and diabetes at adult stage, which ultimately leads to cardiovascular diseases46, 71, 81. Therefore, periodically evaluating the heart of a person whose mother had T2DM during pregnancy should be performed, just as it should be performed for the children suffering from intrauterine growth restriction due to maternal diabetes in pregnancy82, 83, which would help to recognize the high-risk people and has prevention significance.
The clinical implications of this study particularly relate to adverse pregnancy outcomes under various maternal conditions. In addition to maternal diabetes and obesity84, 85, alcohol intake86, tobacco exposure87, and maternal medication use88 can also lead to fetal cardiac dysfunction. Current preconception care for women with diabetes can reduce cardiac defects in the offspring10; however, the birth defect rates associated with maternal diabetes are still several times higher than that in the general population46, 89. Based on the findings from animal studies75, antioxidants and multi-vitamins have been used in the prevention of adverse pregnancy outcomes90, 91. Mechanistic studies including the current study inform the molecular changes in the pathogenesis of cardiac hypertrophy in diabetic pregnancy, and such studies may not be feasible in humans because of the inaccessibility of human fetal cardiac tissues. Once animal studies reveal the detailed morphological changes, molecular signaling pathways and epigenetic alterations, clinical applications using advanced imaging technologies, peripheral miRNA determinations, metabolomic and proteomic approaches92–95 can be designed for early diagnosis, monitoring prognosis after interventions, and the development of effective treatments. High glucose is the major teratogen in diabetic pregnancy75, 96. Future studies may focus on the downstream molecular targets of glucose and recapitulate the key findings of animal studies in possible vascular tissues such as blood vessels in the placenta. The animal model described in this study allows deep mechanistic studies; however, it may not faithfully reflect the human conditions. This concern is significantly alleviated because both diabetic animal models and human pregestational diabetes induce a same set of structural birth defects62, 63, 72, 73, 75–77, 97–99.
Conclusions
Pregestational type 2 maternal diabetes appears to be a major cause of cardiac hypertrophy in the embryonic heart. We observed that cardiac remodeling and profibrosis are involved in the underlying mechanism. Our findings indicate that an imbalance of MMPs/TIMPs contributes to the reorganization of collagen, and, when combined with the alteration of fibronectin, results in cardiac remodeling. Furthermore, we showed that pregestational type 2 maternal diabetes induces profibrosis and apoptosis, which may be responsible for the dysfunction of the embryonic heart under modest hyperglycemic conditions.
Supplementary Material
Acknowledgments
We thank Ms. Hua Li for her technical support, and Dr. Julie A. Rosen for critical reading and editing. This work was supported by the NIH NIDDK grants R01DK083243, R01DK101972, R01HL131737 (P. Y) and R01DK103024 (to P. Y and E. A. R), and an American Diabetes Association Basic Science Award (1-13-BS-220) (P. Y). We thank the support of the Office of Dietary Supplements, National Institute of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have a conflict of interest.
Authors’ contributions
XL, Peng Y, and Pei Y designed the study. XL and Peng Y performed the experiment and analyzed the data. XL, Peng Y, and Pei Y wrote the manuscript. All authors revised the manuscript critically and approved the final version.
References
- 1.Blackmore HL, Ozanne SE. Programming of cardiovascular disease across the life-course. J Mol Cell Cardiol. 2015;83:122–30. doi: 10.1016/j.yjmcc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Dearden L, Ozanne SE. Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol. 2015;39:3–16. doi: 10.1016/j.yfrne.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016;40:229–38. doi: 10.1038/ijo.2015.178. [DOI] [PubMed] [Google Scholar]
- 4.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–12. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Vaiserman A. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clin Epigenetics. 2015;7:96. doi: 10.1186/s13148-015-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis. 2014;237:391–9. doi: 10.1016/j.atherosclerosis.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Kanguru L, Bezawada N, Hussein J, Bell J. The burden of diabetes mellitus during pregnancy in low- and middle-income countries: a systematic review. Glob Health Action. 2014;7:23987. doi: 10.3402/gha.v7.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat M, Ramesha KN, Sarma SP, Menon S, Ganesh Kumar S. Outcome of gestational diabetes mellitus from a tertiary referral center in South India: a case-control study. J Obstet Gynaecol India. 2012;62:644–9. doi: 10.1007/s13224-012-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544 e1–544 e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Peterson C, Grosse SD, Li R, et al. Preventable health and cost burden of adverse birth outcomes associated with pregestational diabetes in the United States. Am J Obstet Gynecol. 2015;212:74, e1–9. doi: 10.1016/j.ajog.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Doss AE, Caughey AB. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. 2012;206:309, e1–7. doi: 10.1016/j.ajog.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yogev Y, Chen R, Ben-Haroush A, Hod M, Bar J. Maternal overweight and pregnancy outcome in women with Type-1 diabetes mellitus and different degrees of nephropathy. J Matern Fetal Neonatal Med. 2010;23:999–1003. doi: 10.3109/14767050903544744. [DOI] [PubMed] [Google Scholar]
- 13.Somaratne JB, Whalley GA, Poppe KK, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovasc Diabetol. 2011;10:29. doi: 10.1186/1475-2840-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aman J, Hansson U, Ostlund I, Wall K, Persson B. Increased fat mass and cardiac septal hypertrophy in newborn infants of mothers with well-controlled diabetes during pregnancy. Neonatology. 2011;100:147–54. doi: 10.1159/000323741. [DOI] [PubMed] [Google Scholar]
- 15.Cooper MJ, Enderlein MA, Tarnoff H, Roge CL. Asymmetric septal hypertrophy in infants of diabetic mothers. Fetal echocardiography and the impact of maternal diabetic control. Am J Dis Child. 1992;146:226–9. doi: 10.1001/archpedi.1992.02160140092027. [DOI] [PubMed] [Google Scholar]
- 16.Gutgesell HP, Speer ME, Rosenberg HS. Characterization of the cardiomyopathy in infants of diabetic mothers. Circulation. 1980;61:441–50. doi: 10.1161/01.cir.61.2.441. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan N, Treacy A, Brazil DP, McAuliffe FM. Cardiomyopathy and diastolic dysfunction in the embryo and neonate of a type 1 diabetic mouse model. Reprod Sci. 2013;20:781–90. doi: 10.1177/1933719112466298. [DOI] [PubMed] [Google Scholar]
- 18.Dowling D, Corrigan N, Horgan S, et al. Cardiomyopathy in offspring of pregestational diabetic mouse pregnancy. J Diabetes Res. 2014;2014:624939. doi: 10.1155/2014/624939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehtoranta L, Vuolteenaho O, Laine VJ, et al. Maternal hyperglycemia leads to fetal cardiac hyperplasia and dysfunction in a rat model. Am J Physiol Endocrinol Metab. 2013;305:E611–9. doi: 10.1152/ajpendo.00043.2013. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi M, Xu G, Li RK, Sweeney G. Diabetes influences cardiac extracellular matrix remodelling after myocardial infarction and subsequent development of cardiac dysfunction. J Cell Mol Med. 2012;16:2925–34. doi: 10.1111/j.1582-4934.2012.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez WE, Joshua IG, Falcone JC, Tyagi SC. Pioglitazone prevents cardiac remodeling in high-fat, high-calorie-induced Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2006;291:H81–7. doi: 10.1152/ajpheart.01331.2005. [DOI] [PubMed] [Google Scholar]
- 22.Scognamiglio R, Negut C, Palisi M, Dioguardi FS, Coccato M, Iliceto S. Effects of oral amino acid supplements on cardiac function and remodeling in patients with type 2 diabetes with mild-to-moderate left ventricular dysfunction. Am J Cardiol. 2008;101:111E–115E. doi: 10.1016/j.amjcard.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2012;92:601–8. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular Stress, Excessive Apoptosis, and the Effect of Metformin in a Mouse Model of Type 2 Diabetic Embryopathy. Diabetes. 2015;64:2526–36. doi: 10.2337/db14-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu FH, Fu SB, Leng X, et al. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell Physiol Biochem. 2013;31:728–43. doi: 10.1159/000350091. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide Dismutase 1 in vivo Ameliorates Maternal Diabetes-Induced Apoptosis and Heart Defects through Restoration of Impaired Wnt Signaling. Circ Cardiovasc Genet. 2015;8:665–76. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci U S A. 2006;103:16864–9. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Repudi SR, Patra M, Sen M. WISP3-IGF1 interaction regulates chondrocyte hypertrophy. J Cell Sci. 2013;126:1650–8. doi: 10.1242/jcs.119859. [DOI] [PubMed] [Google Scholar]
- 29.Bisping E, Ikeda S, Sedej M, et al. Transcription factor GATA4 is activated but not required for insulin-like growth factor 1 (IGF1)-induced cardiac hypertrophy. J Biol Chem. 2012;287:9827–34. doi: 10.1074/jbc.M111.338749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed JS, Hajira A, Li Z, Paulin D, Boriek AM. Desmin regulates airway smooth muscle hypertrophy through early growth-responsive protein-1 and microRNA-26a. J Biol Chem. 2011;286:43394–404. doi: 10.1074/jbc.M111.235127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monreal G, Nicholson LM, Han B, et al. Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life Sci. 2008;83:786–94. doi: 10.1016/j.lfs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Zhang BH, Yu YR, Tang CS, Qi YF. Adrenomedullin protects against fructose-induced insulin resistance and myocardial hypertrophy in rats. Peptides. 2011;32:1415–21. doi: 10.1016/j.peptides.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Bell D, Campbell M, Wang X, Earle JA, Cosby SL, McDermott BJ. Adrenomedullin gene delivery is cardio-protective in a model of chronic nitric oxide deficiency combining pressure overload, oxidative stress and cardiomyocyte hypertrophy. Cell Physiol Biochem. 2010;26:383–94. doi: 10.1159/000320562. [DOI] [PubMed] [Google Scholar]
- 34.Bell D, Zhao YY, Kelso EJ, et al. Upregulation of adrenomedullin and its receptor components during cardiomyocyte hypertrophy induced by chronic inhibition of nitric oxide synthesis in rats. Am J Physiol Heart Circ Physiol. 2006;290:H904–14. doi: 10.1152/ajpheart.00152.2005. [DOI] [PubMed] [Google Scholar]
- 35.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–30. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koshy SK, Reddy HK, Shukla HH. Collagen cross-linking: new dimension to cardiac remodeling. Cardiovasc Res. 2003;57:594–8. doi: 10.1016/s0008-6363(02)00877-5. [DOI] [PubMed] [Google Scholar]
- 37.Badenhorst D, Maseko M, Tsotetsi OJ, et al. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res. 2003;57:632–41. doi: 10.1016/s0008-6363(02)00733-2. [DOI] [PubMed] [Google Scholar]
- 38.Konstandin MH, Volkers M, Collins B, et al. Fibronectin contributes to pathological cardiac hypertrophy but not physiological growth. Basic Res Cardiol. 2013;108:375. doi: 10.1007/s00395-013-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wentzel P, Gareskog M, Eriksson UJ. Decreased cardiac glutathione peroxidase levels and enhanced mandibular apoptosis in malformed embryos of diabetic rats. Diabetes. 2008;57:3344–52. doi: 10.2337/db08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357:749–54. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2010;47:125–31. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 42.Hatem MA, Zielinsky P, Hatem DM, et al. Assessment of diastolic ventricular function in fetuses of diabetic mothers using tissue Doppler. Cardiol Young. 2008;18:297–302. doi: 10.1017/S1047951108002138. [DOI] [PubMed] [Google Scholar]
- 43.Hornberger LK. Maternal diabetes and the fetal heart. Heart. 2006;92:1019–21. doi: 10.1136/hrt.2005.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Diabetes Federation Guideline Development G. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Sheehan PQ, Rowland TW, Shah BL, McGravey VJ, Reiter EO. Maternal diabetic control and hypertrophic cardiomyopathy in infants of diabetic mothers. Clin Pediatr (Phila) 1986;25:266–71. doi: 10.1177/000992288602500507. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez AB, Young L, Doll JA, Morgan GM, Crawford SE, Plunkett BA. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am J Obstet Gynecol. 2014;211:290, e1–7. doi: 10.1016/j.ajog.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Gordon EE, Reinking BE, Hu S, et al. Maternal Hyperglycemia Directly and Rapidly Induces Cardiac Septal Overgrowth in Fetal Rats. J Diabetes Res. 2015;2015:479565. doi: 10.1155/2015/479565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin BR, Schinstock CA. Thinking beyond new clinical guidelines: update in hypertension. Mayo Clin Proc. 2015;90:273–9. doi: 10.1016/j.mayocp.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol. 1989;257:H1–9. doi: 10.1152/ajpheart.1989.257.1.H1. [DOI] [PubMed] [Google Scholar]
- 51.Holland MR, Gibson AA, Kirschner CA, Hicks D, Ludomirsky A, Singh GK. Intrinsic myoarchitectural differences between the left and right ventricles of fetal human hearts: an ultrasonic backscatter feasibility study. J Am Soc Echocardiogr. 2009;22:170–6. doi: 10.1016/j.echo.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinson CW, Morton MJ, Thornburg KL. An anatomic basis for fetal right ventricular dominance and arterial pressure sensitivity. J Dev Physiol. 1987;9:253–69. [PubMed] [Google Scholar]
- 53.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107:1428–44. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–76. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavadzkas JA, Stroud RE, Bouges S, et al. Targeted overexpression of tissue inhibitor of matrix metalloproteinase-4 modifies post-myocardial infarction remodeling in mice. Circ Res. 2014;114:1435–45. doi: 10.1161/CIRCRESAHA.114.303634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balli S, Pac FA, Ece I, Oflaz MB, Kibar AE, Kandemir O. Assessment of cardiac functions in fetuses of gestational diabetic mothers. Pediatr Cardiol. 2014;35:30–7. doi: 10.1007/s00246-013-0734-0. [DOI] [PubMed] [Google Scholar]
- 57.Turan S, Turan OM, Miller J, Harman C, Reece EA, Baschat AA. Decreased fetal cardiac performance in the first trimester correlates with hyperglycemia in pregestational maternal diabetes. Ultrasound Obstet Gynecol. 2011;38:325–31. doi: 10.1002/uog.9035. [DOI] [PubMed] [Google Scholar]
- 58.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 59.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H72–80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 60.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am J Obstet Gynecol. 2010;203:185, e5–11. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205:84, e1–6. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am J Obstet Gynecol. 2012;206:448, e1–7. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Am J Obstet Gynecol. 2015;212:802, e1–8. doi: 10.1016/j.ajog.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–46. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. Am J Obstet Gynecol. 2008;198:321, e1–7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130, e1–7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 67.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X, Fu J, Xu A, et al. Gankyrin drives malignant transformation of chronic liver damage-mediated fibrosis via the Rac1/JNK pathway. Cell Death Dis. 2015;6:e1751. doi: 10.1038/cddis.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeates K, Lohfeld L, Sleeth J, Morales F, Rajkotia Y, Ogedegbe O. A Global Perspective on Cardiovascular Disease in Vulnerable Populations. Can J Cardiol. 2015;31:1081–93. doi: 10.1016/j.cjca.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization; 2014. [DOI] [PubMed] [Google Scholar]
- 71.Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:402–4. doi: 10.2337/dc09-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Y, Reece EA, Zhong J, et al. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am J Obstet Gynecol. 2016;215:366 e1–366 e10. doi: 10.1016/j.ajog.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong D, Reece EA, Lin X, Wu Y, AriasVillela N, Yang P. New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. Am J Obstet Gynecol. 2016;214:192–202. doi: 10.1016/j.ajog.2015.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. 2016;215:484 e1–484 e14. doi: 10.1016/j.ajog.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 75.Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstet Gynecol. 2015;212:569–79. doi: 10.1016/j.ajog.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang F, Reece EA, Yang P. Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. Am J Obstet Gynecol. 2015;212:650, e1–11. doi: 10.1016/j.ajog.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. Am J Obstet Gynecol. 2015;213:125–34. doi: 10.1016/j.ajog.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart RD, Nelson DB, Matulevicius SA, et al. Cardiac magnetic resonance imaging to assess the impact of maternal habitus on cardiac remodeling during pregnancy. Am J Obstet Gynecol. 2016;214:640, e1–6. doi: 10.1016/j.ajog.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Cipolla M, Osol G. Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am J Obstet Gynecol. 1994;171:805–11. doi: 10.1016/0002-9378(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 80.Martin C, Yu AY, Jiang BH, et al. Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 1998;178:527–34. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 81.Dincer UD. Fetal exposure to a diabetic intrauterine environment resulted in a failure of cord blood endothelial progenitor cell adaptation against chronic hypoxia. Stem Cells Cloning. 2015;8:1–14. doi: 10.2147/SCCAA.S73658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Comas M, Crispi F, Cruz-Martinez R, Martinez JM, Figueras F, Gratacos E. Usefulness of myocardial tissue Doppler vs conventional echocardiography in the evaluation of cardiac dysfunction in early-onset intrauterine growth restriction. Am J Obstet Gynecol. 2010;203:45, e1–7. doi: 10.1016/j.ajog.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 83.Iruretagoyena JI, Gonzalez-Tendero A, Garcia-Canadilla P, et al. Cardiac dysfunction is associated with altered sarcomere ultrastructure in intrauterine growth restriction. Am J Obstet Gynecol. 2014;210:550, e1–7. doi: 10.1016/j.ajog.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 84.Peng TY, Ehrlich SF, Crites Y, et al. Trends and racial and ethnic disparities in the prevalence of pregestational type 1 and type 2 diabetes in Northern California: 1996–2014. Am J Obstet Gynecol. 2016;216:177 e1–177 e8. doi: 10.1016/j.ajog.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel AM, Tita A, Biggio JR, Harper LM. Evaluating gestational weight gain recommendations in pregestational diabetes. Am J Obstet Gynecol. 2015;213:563, e1–5. doi: 10.1016/j.ajog.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lo JO, Schabel MC, Roberts VH, et al. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol. 2017;216:302 e1–302 e8. doi: 10.1016/j.ajog.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoyt AT, Canfield MA, Romitti PA, et al. Associations between maternal periconceptional exposure to secondhand tobacco smoke and major birth defects. Am J Obstet Gynecol. 2016;215:613 e1–613 e11. doi: 10.1016/j.ajog.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 88.Howley MM, Carter TC, Browne ML, Romitti PA, Cunniff CM, Druschel CM. Fluconazole use and birth defects in the National Birth Defects Prevention Study. Am J Obstet Gynecol. 2015;214:657, e1–9. doi: 10.1016/j.ajog.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parker SE, Yazdy MM, Tinker SC, Mitchell AA, Werler MM. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. Am J Obstet Gynecol. 2013;209:239, e1–8. doi: 10.1016/j.ajog.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hooijschuur MC, Ghossein-Doha C, Al-Nasiry S, Spaanderman ME. Maternal metabolic syndrome, preeclampsia, and small for gestational age infancy. Am J Obstet Gynecol. 2015;213:370, e1–7. doi: 10.1016/j.ajog.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 91.Ferrari F, Facchinetti F, Ontiveros AE, et al. The effect of combined inositol supplementation on maternal metabolic profile in pregnancies complicated by metabolic syndrome and obesity. Am J Obstet Gynecol. 2016;215:503, e1–8. doi: 10.1016/j.ajog.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 92.Schellen C, Ernst S, Gruber GM, et al. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am J Obstet Gynecol. 2015;213:392, e1–7. doi: 10.1016/j.ajog.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 93.Mouillet JF, Ouyang Y, Coyne CB, Sadovsky Y. MicroRNAs in placental health and disease. Am J Obstet Gynecol. 2015;213:S163–72. doi: 10.1016/j.ajog.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy MS, Bytautiene E, Saade G, Smith GN. Alterations to the maternal circulating proteome after preeclampsia. Am J Obstet Gynecol. 2015;213:853, e1–9. doi: 10.1016/j.ajog.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Bahado-Singh RO, Ertl R, Mandal R, et al. Metabolomic prediction of fetal congenital heart defect in the first trimester. Am J Obstet Gynecol. 2014;211:240 e1–240 e14. doi: 10.1016/j.ajog.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 96.Saad AF, Dickerson J, Kechichian TB, et al. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am J Obstet Gynecol. 2016;215:378, e1–6. doi: 10.1016/j.ajog.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 97.Zhong J, Xu C, Reece EA, Yang P. The green tea polyphenol EGCG alleviates maternal diabetes-induced neural tube defects by inhibiting DNA hypermethylation. Am J Obstet Gynecol. 2016;215:368 e1–368 e10. doi: 10.1016/j.ajog.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209:345, e1–7. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237, e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.