Abstract
BACKGROUND
Transfusion-related acute lung injury (TRALI) is a significant cause of mortality, especially after transfusions containing antibodies to major histocompatibility complex (MHC) class II antigens. We hypothesize that a first event induces both 1) polymorphonuclear neutrophils (PMNs) to express MHC class II antigens, and 2) activation of the pulmonary endothelium, leading to PMN sequestration, so that the infusion of specific MHC class II antibodies to these antigens causes PMN-mediated acute lung injury (ALI).
STUDY DESIGN AND METHODS
Rats were treated with saline (NS), endotoxin (lipopolysaccharide [LPS]), or cytokines (interferon-γ [IFNγ], macrophage colony-stimulating factor [MCSF], tumor necrosis factor-α [TNFα]); the PMNs were isolated; and the surface expression of the MHC class II antigen OX6 and priming by OX6 antibodies were measured by flow cytometry or priming assays.
RESULTS
A two-event model of ALI was completed with NS, LPS, or IFNγ/MCSF/TNFα (first events) and the infusion of OX6 (second event). Compared with NS incubation, rats treated with either LPS or IFNγ/MCSF/TNFα exhibited OX6 PMN surface expression, OX6 antibodies primed the formyl-methionyl-leucyl phenylalanine (fMLF)-activated respiratory burst, and PMN sequestration was increased. OX6 antibody infusion into LPS-incubated or IFNγ/MCSF/TNFα-incubated rats elicited ALI, the OX6 antibody was present on the PMNs, and PMN depletion abrogated ALI.
CONCLUSION
Proinflammatory first events induce PMN MHC class II surface expression, activation of the pulmonary endothelium, and PMN sequestration such that the infusion of cognate antibodies precipitates TRALI.
Despite successful mitigation strategies, transfusion-related acute lung injury (TRALI) remains the leading cause of transfusion-associated death.1–6 Donor antibodies to class II human leukocyte antigens (HLA)/major histocompatibility complex (MHC) antigens have been implicated in the pathogenesis of TRALI, and these antibodies appear to be responsible for the majority of antibody-mediated TRALI in reported hemovigilance series.1,7,8 The current models of the pathogenesis of TRALI secondary to the infusion of donor antibodies directed against recipient HLA class II antigens are based on associative data and require proinflammatory activation of the pulmonary vascular endothelium and circulating monocytes, resulting in polymorphonuclear neutrophil (PMN)-mediated endothelial damage, capillary leak, and acute lung injury (ALI).9–12
Neutrophils (PMNs) have been implicated as the effector cells in TRALI, although they do not normally express HLA class II antigens on their cell surface.13–15 Both in vitro and in vivo cytokine stimulation of PMNs may induce HLA class II antigen surface expression on PMNs.16–23 Chronic clinical conditions also induce the surface expression of MHC class II antigens on PMNs.16,24–27 Therefore, we hypothesize that antibody-mediated TRALI occurs through a direct mechanism in which the first event induces the surface expression of MHC class II antigens on the PMN membrane and proinflammatory activation of the pulmonary vascular endothelium and the second event, the transfusion of donor antibodies to this MHC class II antigen, elicits PMN activation, resulting in ALI.
MATERIALS AND METHODS
Materials
Unless otherwise stated, all reagents were purchased from Sigma Chemical Company. The antibodies to OX6, both unlabeled and phycoerythrin (PE)-labeled, and the control isotype were purchased from AbD Serotec. The rat PMN antibody was purchased from Accurate Chemical & Scientific Corporation. Rat granulocyte-macrophage–colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (MCSF), interferon-γ (IFNγ), and tumor necrosis factor-α (TNFα) were purchased from R&D. PE50 tubing was purchased from Fisher Scientific.
Detection of MHC class II antigen surface expression
Rats received an intraperitoneal injection of either 1) equal volume 0.9% saline (NS), 2) 2 mg/kg lipopolysaccharide (LPS) (Salmonella enteritides) 2 hours before anesthesia, or 3) 3 μg/kg IFNγ +20 μg/kg MCSF in NS 24 hours before anesthesia followed by 65 ng/kg TNFα intravenously 22 hours after IFNγ/MCSF injection (IFNγ/MCSF/TNFα). Whole blood was collected in heparinized tubes, and PMNs were isolated as previously described.28 Rat PMNs (106) were incubated for 30 minutes with PE-labeled antibody to OX6 or isotype control (for each treatment group) at 4°C, fixed, and the immunoreactivity was measured by flow cytometry.29 The mean fluorescence intensity (MFI) was calculated by subtracting the isotype measurement (nonspecific binding).
PMN priming
OX6 priming of the respiratory burst was measured as previously described.28 Briefly, isolated rat PMNs from the groups described above were incubated for 5 minutes with buffer, the antibodies to OX6, mouse immunoglobulin G (IgG) (an isotypic control), and platelet-activating factor (PAF) (a positive control), and the formyl-methionyl-leucyl phenylalanine fMLF-activated respiratory burst was measured.28 For Fcγ receptor (FcγR) blocking, PMNs were incubated with Fc block (Accurate) for 10 minutes at 37°C before adding mouse IgG or the antibodies to OX6.
A two-event in vivo model of TRALI
Male Sprague-Dawley rats (Harlan) underwent ALI using a protocol approved by the Animal Care and Use Committee at the University of Colorado Denver, as previously reported.28 Rats were injected with: 1) NS, 2) LPS, or 3) IFNγ/MCSF/TNFα (first event); then, they were infused (4 mL/hour) with antibodies to OX6 (150 μg/kg), an MHC class II antigen, or with an isotypic mouse IgG antibody, mouse IgG (second event).30 For PMN depletion, rats were injected with a rat PMN antibody (Accurate) 24 hours before starting the experiments.28 Six hours later, blood was drawn, the rats were killed, a bronchoalveolar lavage was performed, and the percentage of Evans Blue dye in the bronchoalveolar lavage versus plasma (lung leak) was determined, as described.28 The right lung was removed and immediately frozen for quantification of myeloperoxidase (MPO), such that 1 gram of lung tissue was homogenized in 20 mM potassium phosphate, pH 7.4, and MPO was measured.28
Digital microscopy
For microscopic examination, the left lung was inflated with 1.5 mL optimal cutting temperature compound in 4% sucrose, embedded in paraffin, and sectioned.28 Sections (5 μm) were placed on glass slides; washed in phosphate-buffered saline (PBS), pH 7.4; and permeabilized with a 70% acetone/30% methanol solution.28 The lungs were incubated with the rat PMN-specific antibody (1:500) overnight at 4°C, secondary antibodies, which included an Alexa Fluor 488-labeled donkey anti-rabbit IgG (1:100) and an Alexa Fluor 588 donkey anti-mouse (1:100, for the OX6 antibody) overnight at 4°C, 4′,6-diami-dino-2-phenylindole (DAPI [a nuclear stain]), and Alexa Fluor 633-labeled wheat germ agglutinin to label the cell membranes overnight at 4°C. Positive colocalization of the PMN-specific antibody, the membrane marker wheat germ agglutinin, and an antibody to OX6 antibody are visualized as a white color at ×40 magnification.
Statistics
The data are reported as the means ±standard error of the means and were analyzed with repeated or independent analyses of variance and post-hoc Newman-Keuls tests for multiple comparisons based on the equality of variance. Significance was determined at p <0.05.
RESULTS
Anti-MHC class II (OX6) binding to rat PMNs
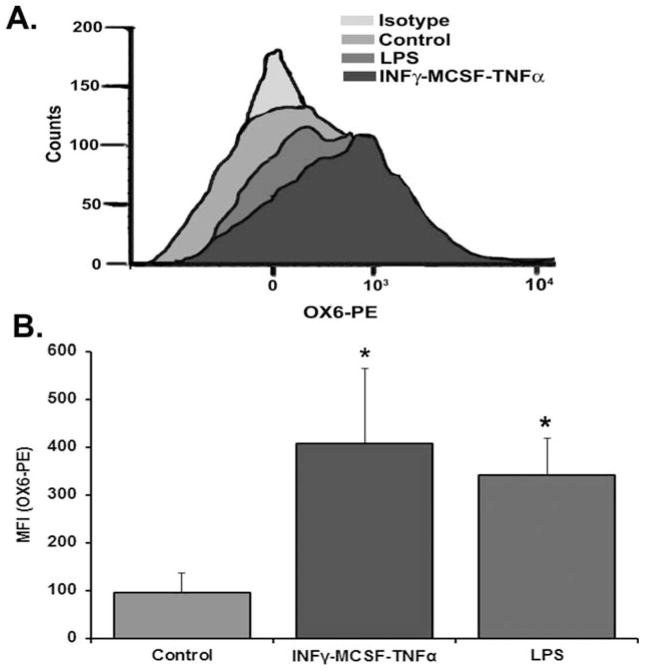
After injection of NS, LPS, or IFNγ/MCSF/TNFα into the rats, the PMNs were isolated, incubated with PE-labeled antibody to OX6 or isotype control mouse IgG for each treatment group, and examined by flow cytometry. The nonspecific binding, as determined by the isotype controls, was subtracted from the raw MFI for each treatment group. Compared with the PMNs from NS-treated rats, the PMNs from IFNγ/MCSF/TNFα-treated animals demonstrated a 3.8-fold ± 1.3-fold increase, whereas LPS induced a 3.5-fold ± 1.2-fold increase in OX6/MHC class II surface expression (IFNγ/MCSF/TNFα: MFI, 408.3 ± 156.0; LPS: MFI, 341.8 ± 76.3; NS: MFI, 96.7 ± 40.7; p <0.05 vs. NS; n = 4) (Fig. 1). Pretreatment of PMNs with Fc block in all groups did not affect antibody binding to OX6 on the PMN surface (results not shown).
Fig. 1.
OX6 binds to and primes rat polymorphonuclear neutrophils (PMNs). The rats received: 1) an intraperitoneal injection of interferon-γ (IFNγ) and macrophage-colony–stimulating factor (MCSF) 24 hours before exsanguination, followed by intravenous TNFα 2 hours before exsanguination; 2) 2 mg/kg lipopolysaccharide (LPS) 2 hours before exsanguination; or 3) normal saline (control) 2 hours before exsanguination. PMNs were isolated from whole blood. The isolated PMNs were then incubated with either isotypic immunoglobulin G-phycoerythrin (PE)-labeled controls or OX6-PE labeled antibodies and analyzed by flow cytometry. (A) PMNs that were pretreated with IFNγ/MCSF/tumor necrosis factor-α (TNFα), along with PMNs that were pretreated with LPS, had increased surface expression of OX6 based on the histogram shift from control PMNs, which did not demonstrate increased surface expression versus the isotype-treated controls (no difference was observed between the isotypes of each treatment group; the histograms overlapped each other), so a representative isotype is shown). (B) The increased expression of OX6 on the PMNs that were treated with LPS (medium gray) or IFNγ/MCSF/TNFα (dark gray) was significantly greater than normal saline-treated rat PMNs (light gray), as determined by the mean fluorescence intensity (the “isotypic” background from each treatment group was subtracted from the appropriate group to calculate the mean fluorescence intensity; p <0.05; n = 4).
Antibodies to OX6 primed PMNs that expressed OX6 on their surface
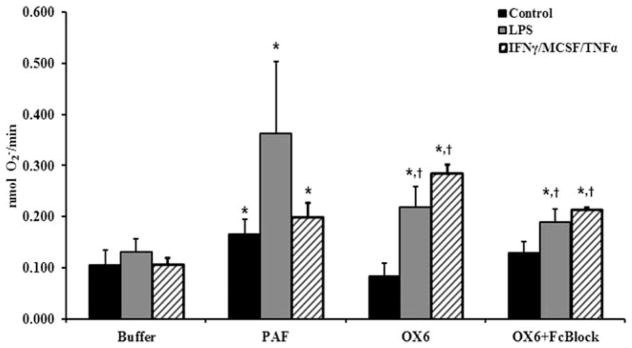
PMNs isolated from rats treated with NS, LPS, or IFNγ/MCSF/TNFα were primed with NS, PAF (a positive control), or antibodies to OX6; and the fMLF-activated oxidative burst was measured. Compared with the PMNs from groups incubated with NS and activated with fMLF alone, PAF (the positive control), caused significant priming of the respiratory burst (p <0.05; n = 4) (Fig. 2). Incubation of PMNs from saline-treated animals (controls) did not induce priming of the respiratory burst, including PMNs from saline-treated rats that were primed with isotypic mouse IgG with or without Fc block (buffer-primed controls/fMLF, 0.09 ± 0.02; mouse IgG/fMLF, 0.14 ± 0.02; mouse IgG primed plus Fc block/fMLF, 0.15 ± 0.6; n = 4; p >0.1). In addition, PMNs from LPS-treated or IFNγ/MCSF/TNFα-treated animals that were primed with antibodies to OX6 produced a significantly greater fMLF-activated respiratory burst versus PMNs from NS-treated rats that were primed with NS or OX6 and activated with fMLF (p <0.05; n = 4) (Fig. 2). Furthermore, pretreatment with Fc block had no significant effect on the ability of OX6 to prime PMNs from IFNγ/MCSF/TNFα-treated or LPS-treated rats, which remained significantly greater compared with that of PMNs from NS-treated rats, buffer, or OX6 primed and activated with fMLF (p <0.05; n = 4,).
Fig. 2.
OX6 causes polymorphonuclear neutrophil (PMN) priming of rat PMNs. Rats received: 1) an intraperitoneal injection of interferon-γ (IFNγ) and macrophage-colony–stimulating factor (MCSF) 24 hours before exsanguination, followed by intravenous tumor necrosis factor-α (TNFα) 2 hours before exsanguination, 2) 2 mg/kg lipopolysaccharide (LPS) 2 hours before exsanguination, or 3) normal saline (control) 2 hours before exsanguination. PMNs were isolated from whole blood, and isolated PMNs were pretreated with Fc block for 10 minutes; stimulated with a buffer control, platelet-activating factor (PAF) (a positive control), or OX6 antibody (10 μg/mL) for 5 minutes; activated with formyl-methionyl-leucyl phenylalanine (fMLF); and the superoxide release was measured. PAF caused a significant increase in priming (superoxide release) in all groups compared with fMLF (buffer) alone (*). PMNs from rats treated with IFNγ/MCSF/TNFα and LPS had significant priming (p <0.05; n = 4) with OX6 from fMLF (buffer) alone (*) and compared with PMNs from control rat PMNs primed with OX6 (†). PMNs that were pretreated with Fc block had no effect on the priming of OX6 in the rat PMNs treated with IFNγ/MCSF/TNFα or LPS, because OX6 caused significant priming in both groups from both fMLF (buffer) alone and control PMNs.
Antibodies to OX6 elicit ALI in rats treated with IFNγ/MCSF/TNFα or LPS and require PMNs
The two-event model of ALI consisted of 1) intraperitoneal NS, LPS, or IFNγ/MCSF/TNFα as the first event; and 2) the infusion of NS or 150 μg/mL of a murine monoclonal antibody to OX6 as the second event. To ensure that the first event induced sequestration of PMNs in the lung, total lung MPO was measured. Compared with lungs from rats treated with intraperitoneal NS, IFNγ/MCSF/TNFα-treated animals demonstrated significant increases in total lung MPO, suggesting that IFNγ/MCSF/TNFα as the first event elicited PMN sequestration (NS [control], 122 ± 7 MPO activity/gram of lung tissue vs. IFNγ/MCSF/TNFα, 176 ± 11 MPO activity/gram of lung tissue; n = 5; p <0.05 vs. NS), similar to previous LPS data.28
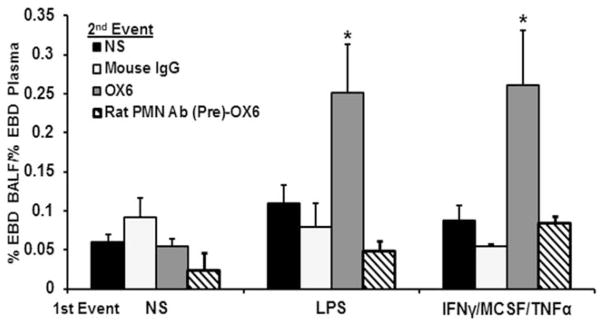
In the two-event model, none of the first events induced ALI with NS as the second event (Fig. 3). However, compared with NS/OX6-treated, LPS/NS-treated, and IFNγ/MCSF/TNFα/NS-treated, NS/mouse IgG (isotypic antibody control)-treated, or IFNγ/MCSF/TNFα/mouse IgG-treated rats, those that were pretreated with IFNγ/MCSF/TNFα or LPS and infused with antibodies to OX6 demonstrated a significant amount of Evans Blue dye leak (p <0.05; n = 6) (Fig. 3). Furthermore, to determine whether PMNs were required for the observed ALI secondary to the MHC class II antibody OX6, rats were granulocyte depleted with an antibody specific for rat PMNs that was administered 24 hours before the first event.28 Leukodepletion of these treated animals was confirmed by a blood smear drawn before the first event that was stained with a modified Wright’s stain, as previously described.28 Granulocyte depletion abrogated OX6-mediated injury in LPS-treated and IFNγ/MCSF/TNFα-treated rats (Fig. 3).
Fig. 3.
Major histocompatibility complex class II antibody, OX6, causes lung injury. Rats received an intraperitoneal injection of either 1) interferon-γ (IFNγ) and macrophage-colony–stimulating factor (MCSF) 24 hours before the infusion followed by intravenous tumor necrosis factor-α (TNFα) 2 hours before the infusion, or 2) normal saline (NS) or 2 mg/kg lipopolysaccharide (LPS) 2 hours before the infusion of 150 μg/kg of OX6 or mouse immunoglobulin G (IgG) (antibody isotype control). After transfusion, the rats were injected with Evans Blue Dye (EBD); and, 6 hours later, blood was removed, and a bronchoalveolar lavage (BAL) was performed. The plasma and BAL fluid were analyzed for acute lung injury. Rats that were pretreated with either LPS or IFNγ/MCSF/TNFα followed by OX6 transfusion had significant lung injury based on the percentage of EBD leakage into the BAL fluid, compared with the rats that received NS as the second event regardless of treatment group and the rats that were pretreated with NS and infused with OX6 (p <0.05; n = 6). Rats were also granulocyte-depleted by an intraperitoneal injection 24 hours before the first event with an antibody to rat PMNs, followed by either IFNγ/MCSF/TNFα, LPS, or NS as a first event, then followed by an infusion of antibodies to OX6. No lung injury was observed in the granulocyte-depleted rats based on EBD leakage (n = 3).
Localization of antibodies to OX6 to the PMN membrane
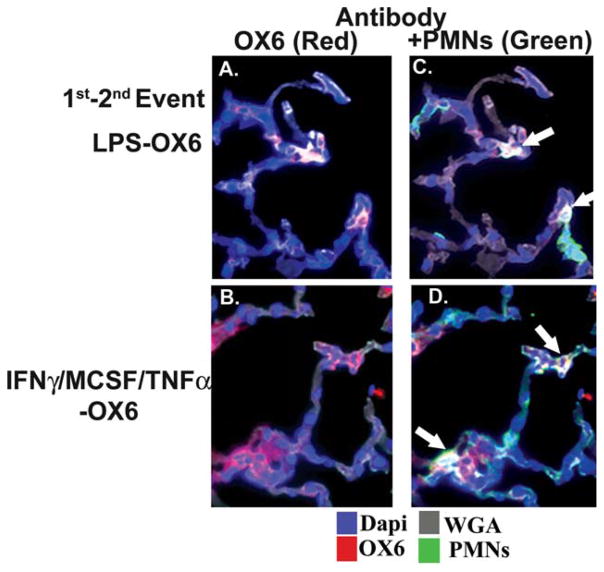
To determine the cellular location of the OX6 antibody, which elicited ALI, lung sections were incubated with a specific rat granulocyte antibody (green); a specific labeled donkey anti-mouse antibody to localize the antibody to OX6 (red); wheat germ agglutinin, which binds to the membranes (gray); and DAPI (blue), a nuclear stain. In NS-treated animals, the infusion of antibodies to OX6 did not demonstrate immunoreactivity on the PMN membranes or on the pulmonary endothelium or epithelium (data not shown). Conversely, in both LPS-treated and IFNγ/MCSF/TNFα-treated rats, the OX6 immunoreactivity (red) was present on the PMN (green) and it colocalized to the cell membrane as demonstrated by the white color, demarcated by the arrows in Fig. 4C,D.31–33 In addition, there is some increased immunoreactivity (red) of OX6 in the lung tissue from rats treated with IFNγ/MCSF/TNFα (Fig. 4B,D), although most of the red immunoreactivity appears in areas of PMN infiltration (Fig. 4C,D). The increased amount of OX6 immunoreactivity may be expected, because this cytokine “cocktail” reportedly increases MHC class II antigen expression on pulmonary endothelium and other cells and increases LPS in the specific case of OX6 immunoreactivity.31–36
Fig. 4.
OX6 colocalizes with the polymorphonuclear neutrophil (PMN) membranes. Rats received an intraperitoneal injection of either: 1) interferon-γ (IFNγ) and macrophage-colony–stimulating factor (MCSF) 24 hours before the infusion and intravenous TNFα 2 hours before the infusion, or 2) 2 mg/kg lipopolysaccharide (LPS) 2 hours before the infusion of OX6. After 6 hours, the rats were killed; and the left lung was inflated with optimal cutting temperature compound, embedded in paraffin, frozen, and sectioned. The sections were incubated with wheat germ agglutinin (WGA) (gray), which localizes to the cellular membrane (gray); 4′,6-diami-dino-2-phenylindole (DAPI) (blue), a nuclear stain; an antibody to OX6 (red); and an antibody specific for rat PMNs (green). All sections were visualized at ×40 magnification in duplicate. (A) OX6 (red) colocalized to cells, which were PMNs (green), as demonstrated in C, and this “triple colocalization” was present on the PMN membrane (white), as demarcated by white arrows. (B) Antibodies to OX6 (red) were most widespread, which were localized to both the PMNs (green) with triple colocalization to the PMN membrane (white, white arrows) in D. (C,D) There is some OX6 on the lung tissues (red) that is not associated with PMNs, although most of it is associated with infiltrating PMNs. Such immunoreactivity may be expected in rats treated with IFNγ/MCSF/TNFα, and especially IFNγ, which induces OX6 surface expression on both lung endothelium and epithelium.31–33
DISCUSSION
The reported data demonstrate that PMNs can be altered to express MHC class II antigens in vivo by the infusion of a mixture of cytokines, IFNγ/MCSF/TNFα, or LPS as confirmed by flow cytometry. Antibodies specific for the OX6 MHC class II antigen can prime the respiratory burst of these PMNs, which express OX6 on their cell surface, but not OX6-PMNs from NS-treated control animals. In the two-event animal model of ALI, the first event, LPS or IFNγ/MCSF/TNFα, induced PMN sequestration, as defined by the total lung MPO level, and infusion of the OX6 antibody induced ALI and localized to the PMN membrane, as determined by digital microscopy. There was increased immunoreactivity in the lung tissue, as expected in rats treated with the cytokine mix IFNγ/MCSF/TNFα, that was not present in the LPS-treated rats.31–36 Importantly, the infusion of antibodies to OX6 did not cause ALI if the PMNs did not express the OX6 antigen, and the observed ALI secondary to OX6 was abrogated by PMN depletion before the first event irrespective of the mediators given and identical to previous data.28
Sachs and colleagues used an in situ, isolated, perfused rat lung model infused with HLA class II antibodies to demonstrate the pathophysiology of TRALI.12 TRALI was dependent on several factors, including: 1) endothelial activation using LPS in vivo before isolating the lungs, 2) infusion of the supernatant from a monocyte in vitro culture consisting of monocytesDR7+, DR52+ incubated with cognate antibodies or monocytesDR7+, DR52+ plus antibodies to Dr7 plus DR52 antibodies, and 3) PMNs.12 If any of these components were deleted, then TRALI did not occur.12 In addition, immunohistochemistry of these lungs did not demonstrate HLA class II antigens on the lung tissue nor on pulmonary leukocytes; rather, HLA class II immunoreactivity was present only on pulmonary macrophages.9,12 Importantly, models that use isolated perfused lungs obviate the removal or modification of mediators through the kidney or liver.12,37 Thus, several questions become apparent with this model. First, how many monocytes are required? Second, because normal monocyte counts in peripheral blood are approximately 300/mL, is this number sufficient? Third, do human monocytes release the described chemokines and lipids in vivo over the prescribed time limits (6 hours), and do these events occur clinically?
The relevance of MHC class II surface expression on PMNs may appear to be elusive; however, such PMN MHC class II antigen surface expression has been documented, especially with the expanded roles of PMNs in antigen presentation and dendritic cell function.15,19,21,27,38–44 Furthermore, PMNs may express MHC class II antigens as the result of chronic diseases, and such expression may be induced or modified by infectious pathogens, including both Gram-positive and Gram-negative bacteria.16,24,25,45–49 PMNs may express MHC class II antigens simply as the result of β2-integrin engagement, in response to infection or inflammation, or as a function of in vivo cytokine administration, which suggests that such changes in antigen surface expression are not an uncommon, selective response.17,18,20,22,23,42,46,47,49,50 The increases in MHC class II antigen surface expression on PMNs also are not restricted to selected sites and have been identified in PMNs isolated from patients with interstitial pneumonitis.45
The amount of lung leak was less than that from the identical model using the supernatant from stored red blood cells or monoclonal antibodies to MHC class I antigens, which may be due to the expression of MHC class II (OX6) on the PMN surface, with its inherent signaling leading to PMN activation and ALI, or to the relative strength of the antibody to OX6.28 The reported model also required IFNγ/MCSF/TNFα or LPS to induce MHC class II antigen expression on the rat PMNs and to activate the pulmonary endothelium, similar to both in vivo and in vitro reports that used these identical cytokines or pathogens to increase MHC class antigen surface expression on the PMN membrane.20–23,26,42,46,50 Priming agents may change quiescent PMNs to adherent and hyper-reactive, i.e., proinflammatory, such that stimuli that do not normally activate the microbicidal arsenal of PMNs elicit the release of cytotoxic oxidative and nonoxidative components, resulting in PMN-mediated cytotoxicity and ALI.28,37,51–55
These data do not rule out a possible role for circulating monocytes; however, ALI could be elicited by LPS-mediated or IFNγ/MCSF/TNFα-mediated activation of the pulmonary endothelium followed by activation of these sequestered PMNs with antibodies to OX6, without monocyte activation. In this model, the activation of monocytes results in PMN activation; however, in the reported data, there does not appear to be a role for monocytes; rather, PMNs, which had OX6 surface expression, were required for the observed ALI. To date, although much has been written about TRALI caused by HLA class II antibodies, little work, save that of Sachs and colleagues, has delved into the mechanism of the observed ALI.7,9–11,56–62 Thus, the reported data provide a simpler two-event model that depends on: 1) the clinical condition of the individual, including both proinflammatory activation of the pulmonary endothelium and the surface expression of MHC class II antigens on the host PMNs; and 2) the infusion of a specific antibody to the cognate MHC class II antigen on the PMN surface, which results in PMN activation, endothelial damage, capillary leak, and ALI. Although simpler than previous modeling, further work is needed to ensure that this direct pathogenesis has clinical utility, especially sampling of the patient’s PMNs to ascertain whether they express the cognate HLA class II antigens and that ligation of these antigens primes PMNs. Because pulmonary endothelium can synthesize and release GM-CSF, this cytokine may induce the MHC class II antigen surface expression on PMNs, and ligation of these antigens may induce the synthesis and release of interleukin-8.63,64 Finally, TRALI may be obviated by prestorage filtration, because an experimental filter has been developed that removes antibodies to MHC class I, class II, and granulocyte antigens and also inhibits the accumulation of lipids during red blood cell storage.54
Acknowledgments
These studies were supported by Bonfils Blood Center and by grant HL59355 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and grant P50 GM049222 from the National Institute of General Medical Sciences, National Institutes of Health.
ABBREVIATIONS
- ALI
acute lung injury
- IFNγ
interferon-γ
- MCSF
macrophage colony-stimulating factor
- MHC
major histocompatibility complex
- PMN(s)
polymorphonuclear neutrophil(s)
- TNFα
tumor necrosis factor-α
- TRALI
transfusion-related acute lung injury
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
References
- 1.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemo-vigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 2.Eder AF, Benjamin RJ. TRALI risk reduction: donor and component management strategies. J Clin Apher. 2009;24:122–9. doi: 10.1002/jca.20198. [DOI] [PubMed] [Google Scholar]
- 3.Eder AF, Herron RM, Jr, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–42. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 4.Eder AF, Dy BA, Perez JM, et al. The residual risk of transfusion-related acute lung injury at the American Red Cross (2008–2011): limitations of a predominantly male-donor plasma mitigation strategy. Transfusion. 2013;53:1442–9. doi: 10.1111/j.1537-2995.2012.03935.x. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 6.Holness L, Knippen MA, Simmons L, et al. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–8. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang. 2011;100:122–8. doi: 10.1111/j.1423-0410.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 8.Keller-Stanislawski B, Reil A, Gunay S, et al. Frequency and severity of transfusion-related acute lung injury—German haemovigilance data (2006–2007) Vox Sang. 2010;98:70–7. doi: 10.1111/j.1423-0410.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 9.Kao GS, Wood IG, Dorfman DM, et al. Investigations into the role of anti-HLA class II antibodies in TRALI. Transfusion. 2003;43:185–91. doi: 10.1046/j.1537-2995.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Kopko PM, Popovsky MA, MacKenzie MR, et al. HLA class II antibodies in transfusion-related acute lung injury. Transfusion. 2001;41:1244–8. doi: 10.1046/j.1537-2995.2001.41101244.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura M, Mitsunaga S, Ishikawa Y, et al. Possible mechanisms underlying development of transfusion-related acute lung injury: roles of anti-major histocompatibility complex class II DR antibody. Transfus Med. 2003;13:141–7. doi: 10.1046/j.1365-3148.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Sachs UJ, Wasel W, Bayat B, et al. Mechanism of transfusion-related acute lung injury induced by HLA class II antibodies. Blood. 2011;117:669–77. doi: 10.1182/blood-2010-05-286146. [DOI] [PubMed] [Google Scholar]
- 13.Fung YL, Silliman CC. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev. 2009;23:266–83. doi: 10.1016/j.tmrv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–7. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 15.Vella A, Sartoris S, Bambara L, et al. Cell contact-dependent PMN HLA-DR and CD69 membrane expression induced by autologous mono-lymphocytes and cell lines. Inflammation. 2002;26:143–52. doi: 10.1023/a:1016514927365. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Schmitz JL, Yang J, et al. DRB1*15 allele is a risk factor for PR3-ANCA disease in African Americans. J Am Soc Nephrol. 2011;22:1161–7. doi: 10.1681/ASN.2010101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosselin EJ, Wardwell K, Rigby WF, et al. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 18.Mudzinski SP, Christian TP, Guo TL, et al. Expression of HLA-DR (major histocompatibility complex class II) on neutrophils from patients treated with granulocyte-macrophage colony-stimulating factor for mobilization of stem cells. Blood. 1995;86:2452–3. [PubMed] [Google Scholar]
- 19.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–28. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porretti L, Coluccio E, Prati D, et al. Flow-cytometric approach to the prompt laboratory diagnosis of TRALI: a case report. Eur J Haematol. 2004;73:295–9. doi: 10.1111/j.1600-0609.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 21.Radsak M, Iking-Konert C, Stegmaier S, et al. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinisch W, Lichtenberger C, Steger G, et al. Donor dependent, interferon-gamma induced HLA-DR expression on human neutrophils in vivo. Clin Exp Immunol. 2003;133:476–84. doi: 10.1046/j.1365-2249.2003.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spagnoli GC, Juretic A, Rosso R, et al. Expression of HLA-DR in granulocytes of polytraumatized patients treated with recombinant human granulocyte macrophage-colony-stimulating factor. Hum Immunol. 1995;43:45–50. doi: 10.1016/0198-8859(94)00131-9. [DOI] [PubMed] [Google Scholar]
- 24.Hansch GM, Radsak M, Wagner C, et al. Expression of major histocompatibility class II antigens on polymorphonuclear neutrophils in patients with Wegener’s granulomatosis. Kidney Int. 1999;55:1811–8. doi: 10.1046/j.1523-1755.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 25.Iking-Konert C, Vogt S, Radsak M, et al. Polymorphonuclear neutrophils in Wegener’s granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–62. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 26.Salazar JC, Pope CD, Sellati TJ, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–70. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 27.Sandilands GP, McCrae J, Hill K, et al. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology. 2006;119:562–71. doi: 10.1111/j.1365-2567.2006.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelher MR, Masuno T, Moore EE, et al. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–87. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silliman CC, Elzi DJ, Ambruso DR, et al. Lysophosphatidyl-cholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003;73:511–24. doi: 10.1189/jlb.0402179. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama H, Itagaki S, McGeer PL. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J Neurosci Res. 1988;20:147–57. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- 31.Chang SC, Hsu HK, Perng RP, et al. Increased expression of MHC class II antigens in rejecting canine lung allografts. Transplantation. 1990;49:1158–63. doi: 10.1097/00007890-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DJ, McMenamin C, McWilliam AS, et al. Development of the airway intraepithelial dendritic cell network in the rat from class II major histocompatibility (Ia)-negative precursors: differential regulation of Ia expression at different levels of the respiratory tract. J Exp Med. 1994;179:203–12. doi: 10.1084/jem.179.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suda T, Sato A, Sugiura W, et al. Induction of MHC class II antigens on rat bronchial epithelial cells by interferon-gamma and its effect on antigen presentation. Lung. 1995;173:127–37. doi: 10.1007/BF02981472. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim L, Dominguez M, Yacoub M. Primary human adult lung epithelial cells in vitro: response to interferon-gamma and cytomegalovirus. Immunology. 1993;79:119–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Kittur DS, Wilasrusmee C, Han WF, et al. Locally derived cytokines and upregulation of MHC class II genes in allografts. J Heart Lung Transplant. 2002;21:882–9. doi: 10.1016/s1053-2498(02)00407-2. [DOI] [PubMed] [Google Scholar]
- 36.McDouall RM, Batten P, McCormack A, et al. MHC class II expression on human heart microvascular endothelial cells: exquisite sensitivity to interferon-gamma and natural killer cells. Transplantation. 1997;64:1175–80. doi: 10.1097/00007890-199710270-00016. [DOI] [PubMed] [Google Scholar]
- 37.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Salam BK. T-cell proliferation by surface molecules expression on polymorphonuclear neutrophils stimulated with IL-4 in superantigen presence. Allergol Immunopathol (Madr) 2012;40:81–7. doi: 10.1016/j.aller.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Beauvillain C, Delneste Y, Scotet M, et al. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–73. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 40.Culshaw S, Millington OR, Brewer JM, et al. Murine neutrophils present class II restricted antigen. Immunol Lett. 2008;118:49–54. doi: 10.1016/j.imlet.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–35. [PubMed] [Google Scholar]
- 42.Geng S, Matsushima H, Okamoto T, et al. Emergence, origin, and function of neutrophildendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandilands GP, Ahmed Z, Perry N, et al. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology. 2005;114:354–68. doi: 10.1111/j.1365-2567.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashiro S, Wang JM, Yang D, et al. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 45.Abe S, Seo Y, Hayashi H, et al. Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia: a pilot study. Blood Purif. 2010;29:321–6. doi: 10.1159/000287232. [DOI] [PubMed] [Google Scholar]
- 46.Davey MS, Morgan MP, Liuzzi AR, et al. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J Immunol. 2014;193:3704–16. doi: 10.4049/jimmunol.1401018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drifte G, Dunn-Siegrist I, Tissieres P, et al. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41:820–32. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira R, Napoleao P, Banha J, et al. Crosstalk between inflammation, iron metabolism and endothelial function in Behcet’s disease. Clin Hemorheol Microcirc. 2014;56:175–85. doi: 10.3233/CH-131725. [DOI] [PubMed] [Google Scholar]
- 49.Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- 50.Zarco MA, Ribera JM, Villamor N, et al. Phenotypic changes in neutrophil granulocytes after G-CSF administration in patients with acute lymphoblastic leukemia under chemotherapy. Haematologica. 1998;83:573–5. [PubMed] [Google Scholar]
- 51.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silliman CC, Bjornsen AJ, Wyman TH, et al. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 53.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silliman CC, Kelher MR, Khan SY, et al. Experimental pre-storage filtration removes antibodies and decreases lipids in RBC supernatants mitigating TRALI in vivo. Blood. 2014;123:3488–95. doi: 10.1182/blood-2013-10-532424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wyman TH, Bjornsen AJ, Elzi DJ, et al. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am J Physiol Cell Physiol. 2002;283:C1592–603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 56.Aysola AE, Deisting B, Mair DC. Review of 16 blood components from a donor with HLA class II antibodies [abstract] Transfusion. 2005;45(Suppl):83A. [Google Scholar]
- 57.Curtis BR. Is TRALI caused by HLA class II too? Blood. 2011;117:378–9. doi: 10.1182/blood-2010-11-317180. [DOI] [PubMed] [Google Scholar]
- 58.Nicolle AL, Chapman CE, Carter V, et al. Transfusion-related acute lung injury caused by two donors with anti-human leucocyte antigen class II antibodies: a look-back investigation. Transfus Med. 2004;14:225–30. doi: 10.1111/j.0958-7578.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 59.Nishimura M, Hashimoto S, Satake M, et al. Interference with TRALI-causing anti-HLA DR alloantibody induction of human pulmonary microvascular endothelial cell injury by purified soluble HLA DR. Vox Sang. 2007;93:78–82. doi: 10.1111/j.1423-0410.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura M, Hashimoto S, Takanashi M, et al. Role of anti-human leucocyte antigen class II alloantibody and monocytes in development of transfusion-related acute lung injury. Transfus Med. 2007;17:129–34. doi: 10.1111/j.1365-3148.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 61.Varela M, Mas A, Nogues N, et al. TRALI associated with HLA class II antibodies. Transfusion. 2002;42:1102. doi: 10.1046/j.1537-2995.2002.00225.x. [DOI] [PubMed] [Google Scholar]
- 62.Win N, Brown C, Navarrete C. TRALI associated with HLA class II antibodies. Transfusion. 2003;43:545–6. doi: 10.1046/j.1537-2995.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 63.Burg J, Krump-Konvalinkova V, Bittinger F, et al. GM-CSF expression by human lung microvascular endothelial cells: in vitro and in vivo findings. Am J Physiol Lung Cell Mol Physiol. 2002;283:L460–7. doi: 10.1152/ajplung.00249.2001. [DOI] [PubMed] [Google Scholar]
- 64.Lei L, Altstaedt J, von der Ohe M, et al. Induction of interleukin-8 in human neutrophils after MHC class II cross-linking with superantigens. J Leukoc Biol. 2001;70:80–6. [PubMed] [Google Scholar]