Abstract
BACKGROUND
Allogeneic blood transfusion mediates immunosuppression in transfused recipients by an unknown mechanism. Regulatory T cells (Tregs) are suppressive CD4+CD25+Foxp3+ cells with a central role in immunosuppression in trauma victims, cancer patients, and transplant recipients. We hypothesized that transfusion-related immunosuppression is, in part, mediated by induction of Tregs, and this induction is attenuated with prestorage leukoreduction and accentuated with prolonged storage.
STUDY DESIGN
Packed red blood cell (PRBC) units were obtained and 50% of PRBCs were leukoreduced (LR) before routine storage for 1 day or 42 days and the supernatant was collected. Normal human peripheral blood mononuclear cells (PBMCs) were exposed to 1-day NLR, 42-day NLR, 1-day LR, or 42-day LR PRBC supernatants or to PRBC storage solution or washed PRBC supernatant ± anti-CD3 stimulation, and analyzed by flow cytometry for Foxp3+ Tregs or CD25+-activated T cells. PRBC supernatants and cell culture supernatants were analyzed by immunoassay for interleukin (IL)-1β, IL-2, IL-4, IL-10, interferon-γ, tumor necrosis factor–α, and transforming growth factor–β. Treg activity was evaluated by suppression assay.
RESULTS
All PRBC groups induced Tregs compared with control media in anti–CD3-stimulated PBMCs, without alteration by LR or prolonged storage. PRBC supernatant did not alter nonspecific T-cell activation from control media. PRBC-induced Tregs were suppressive, inhibiting proliferation of T-responder cells. All cytokines measured decreased with storage in LR PRBC units and no cytokines were substantially elevated in cell supernatants exposed to PRBC supernatant. PRBC storage solution did not reproduce the effects of PRBC supernatant, and washed PRBC supernatant attenuated Treg induction.
CONCLUSIONS
PRBC supernatant induces Tregs, but this induction is not altered by LR or prolonged storage. This induction appears to be independent of cytokines and is attenuated with washed PRBCs, implicating the plasma fraction.
Allogeneic blood transfusion (ABT) is recognized to have diverse immunomodulatory effects. ABT is associated with a proinflammatory response, as suggested by its mechanistic role in multisystem organ failure and transfusion-related acute lung injury.1,2 ABT also has immunosuppressive effects, which have been appreciated since it was first reported by Opelz and colleagues3 in 1973 that pretransplantation ABT could improve renal-allograft survival. In addition, ABT has other immune regulatory effects because it is associated with increased cancer recurrence, decreased severity of autoimmune disease, and increased infection risk.4–6
Storage of packed red blood cells (PRBCs) exaggerates the immune modulation of ABT because it results in progressive accumulation of immunologic mediators until the last day at which the PRBCs can be transfused (day 42).7–10 Prestorage leukoreduction (LR) has been proposed to ameliorate immunosuppression from donor leukocytes or donor leukocyte-derived mediators. LR diminishes the amount of transfused cytokines and other immunologic mediators, and this can attenuate the immunosuppressive effects of ABT by reduction of functional defects in the immune response.11,12
A variety of mediators for transfusion-related immunosuppression have been proposed, including allogeneic leukocytes, leukocyte-derived soluble mediators, and soluble HLA molecules, although the precise mechanisms of transfusion-related immunosuppression remain uncertain.13 Given their broad repertoire of targets and their critical importance in immune regulation, regulatory T cells (Tregs) represent a potential mechanism of transfusion-related immunosuppression. This population of CD4+ CD25+ T cells promotes immune tolerance, prevents autoimmunity, and is characterized by unique expression of Foxp3, a transcription factor of critical importance for Treg development and function in the forkhead/winged-helix family of transcriptional regulators.14,15 Tregs suppress CD4+ and CD8+ effector T-cell–mediated immunity, and inhibit the function of dendritic cells to substantially blunt the immune response.16,17 Treg-mediated immunosuppression has been implicated in the immunopathology of trauma and sepsis, as injured patients contain elevated levels of hypersuppressive circulating Tregs.18,19 These immune-suppressive cells also infiltrate tumors and can cause subversion of the antitumor immune response, and their presence is a poor prognostic factor in many cancers.20–24 Tregs can also play a beneficial role as they are vital in the induction of tolerance to transplant allografts.25 The importance of Treg-mediated immunosuppression is appreciated, as such, their role in other states of immune regulation is expanding. We hypothesize that exposure to ABT results in induction of Tregs, and this induction is attenuated with LR and accentuated with prolonged storage.
METHODS
Preparation of blood components
After informed consent according to guidelines set forth by the Colorado Multiple Institutional Review Board, healthy adult volunteers donated 1 U whole blood, which was separated into components and stored according to the American Association of Blood Banks criteria. Fifty percent, by weight, of the PRBCs were LR on day 0 using Pall BPF4 (Pall Corporate) and Fenwall-Sepacell R500-ii (Baxter-Fenwall) third-generation filters before routine storage. Samples from the LR and nonleukoreduced (NLR) PRBCs were drawn from sterile couples on days 1 and 42 routine storage from the same donor. The plasma fraction (supernatant) was isolated from each of these PRBC samples by centrifugation at 5,000g for 7 minutes followed by an additional spin of 12,500g for 5 minutes to remove acellular debris. Each supernatant was aliquoted and stored at −70°C until later use. PRBC units were washed poststorage using a Cobe 2991 Cell Processor (Gambro BCT) per published methodology, and the plasma fraction (supernatant) was isolated, aliquoted, and frozen using centrifugation at g-forces recommended for plasma isolation per AABB criteria, with a final spin at 12,500g to remove acellular debris.26–29
Peripheral blood mononuclear cell isolation and blood component exposure
Peripheral blood was collected from healthy human volunteers, and peripheral blood mononuclear cell (PBMC) fraction was isolated using a Ficoll-Paque density gradient (Amersham Biosciences AB). Cells were washed with Dulbecco’s PBS three times before their use or stored in 10% dimethyl sulfoxide solution in control media (RPMI-1640 media supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mmol/L l-glutamine, and 10% pooled human serum; Gemini Bio-Products) at −70°C until use.
For PRBC supernatant exposure experiments, 2 × 106 isolated normal human PBMCs were cultured in 1.5 mL control media or 1.5 mL PRBC supernatant at 20% concentration by volume in control media per well on a 24-well plate (Corning) with or without 1 µg/mL soluble anti-CD3 antibody (BD Biosciences) at 37°C in 5% CO2 for 5 days. In other experiments, PBMCs were cultured in various concentrations of PRBC storage solution (AS-5, which contains dextrose, adenine, mannitol, and sodium chloride; and the anticoagulant-preservative solution CPD, which contains citrate, dextrose and monobasic sodium phosphate) or washed PRBC supernatant also at 20% concentration by volume in control media. PRBC supernatant from a single donor was collected and processed for LR, NLR, storage duration, plasma fractionation, and washing, as described for use in each single experiment. To control for variability among PRBC and PBMC donors, different PRBC and PBMC donors were used for every repetition of each experiment up to 10 repetitions.
PBMCs were then harvested, stained for 15 minutes at room temperature with murine antihuman CD4 antibody (TC-conjugated; Invitrogen), murine antihuman CD25 antibody (fluorescein isothiocyanate–conjugated; BD Biosciences), and fixed with commercial fixation/permeabilization solution according to manufacturer’s instructions (eBioscience). PBMCs were then incubated for 30 minutes at room temperature with normal rat serum in permeabilization buffer (eBioscience) to prevent nonspecific binding and with rat antihuman Foxp3 antibody (phycoerythrin-conjugated; eBioscience). PBMCs were then washed in permeabilization buffer and resuspended in 1% paraformaldehyde in Dulbecco’s PBS and stored at 4°C in the dark until flow cytometric analysis was performed. Cells were analyzed by flow cytometry using a FACSCaliber (Becton Dickinson) with gating for lymphocytes, then CD4+ T cells, then on Foxp3+ T cells as Tregs, or alternatively on CD25+Foxp3− T cells as activated (non-Treg) T cells. Flow data were analyzed using the FlowJo software program (Tree Star). The Foxp3 gate was established using CD4− cells as Foxp3− controls and was identical for each sample per group. Appropriate isotype control antibodies were used to control for nonspecific staining (Invitrogen and eBioscience).
Treg suppression assay
Normal human PBMCs were isolated and cultured in control media or 20% PRBC supernatant with our without 1 µg/mL soluble anti-CD3 antibody (BD Biosciences) for 5 days as described. The PBMCs were then harvested, washed with Dulbecco’s PBS, and sorted by the magnetic bead immunosorting technique (MACS cell separation; Miltenyi Biotec) into CD4+CD25+ Tregs and CD4+ CD25+ T-responder cells. Cells were first sorted into CD4+ isolates by negative selection, then into CD25+ or CD25− isolates by positive selection according to the manufacturer’s instructions.
Sorted Tregs and T-responder cells were stained with 2 µM carboxyfluorescein diacetate, succinimidyl ester (CFSE) for 15 minutes at 37°C (Invitrogen). The 5 × 104 sorted CD4+ T-responder cells were incubated with either 5 × 104 additional CD4+ T responder cells with or without 3 µg/mL soluble anti-CD3 and 3 µg/mL soluble anti-CD28 activating antibodies (BD Biosciences), or with 5 × 104 sorted Tregs from control media, PRBC supernatant exposure, control media with anti-CD3 stimulation or PRBC supernatant with anti-CD3 stimulation, with 3 µg/mL soluble anti-CD3 and 3 µg/mL soluble anti-CD28 antibodies in 300 µL control media in a 96-well round bottom plate (Corning) at 37°C in 5% CO2.
All cells were harvested after 5 days, washed in Dulbecco’s PBS, and stained with murine antihuman CD4 antibody (allophycocyanin-conjugated; Invitrogen) as described. Cells were then washed and fixed using commercial fixation/permeabilization solution as described (eBioscience). Cells were then washed and resuspended in 1% paraformaldehyde in Dulbecco’s PBS and stored at 4°C in the dark until flow cytometric analysis was performed. Cells were acquired on a FACSCaliber flow cytometer (Becton Dickinson). Flow data were analyzed using the FlowJo software program (Tree Star) with gating based on forward versus side scatter for live lymphocytes, then on CD4+ T cells, then on CFSE with the proportion of divided cells estimated as the population of cells with diluted CFSE signal compared with the undivided CFSEhigh population. Negatively or singly stained cells were used as compensation controls and the appropriate isotype controls for nonspecific staining (Invitrogen).
Cytokines
Supernatants from the PRBC supernatants and from PRBC supernatant exposure experiments were collected, centrifuged at 1,000g to remove acellular debris, and analyzed for interleukin (IL)-1β, IL-2, IL-4, IL-10, interferon-γ, and tumor necrosis factor–α by multiplex bead array according to manufacturer’s instructions (Bio-Rad). Total transforming growth factor–β1 levels were detected by standard ELISA (ELISATech).
Statistics
Statistical analyses were performed using JMP software package version 5.0.1 for Windows (SAS Institute). Results are expressed as mean values ± SEM unless otherwise indicated. For comparisons of three or more groups, ANOVA was used and a p value < 0.05 was considered statistically significant.
RESULTS
PRBC supernatant induces Tregs
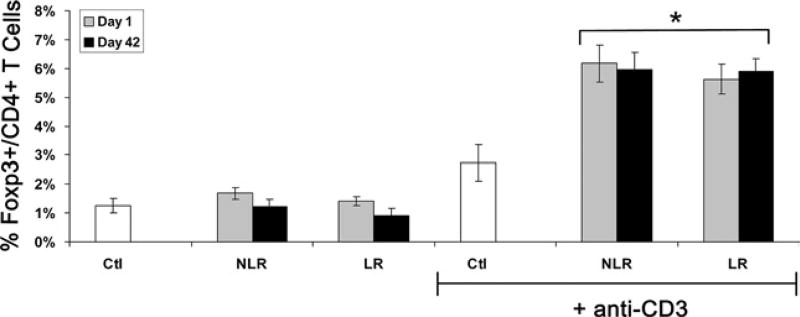
To determine if PRBC supernatant induces Tregs, and if storage or LR might alter this effect, normal human PBMCs from healthy volunteers were exposed to control media alone (Ctl) or the acellular plasma fraction from PRBC (PRBC supernatant) units from a single donor stored for 1-day or 42-day LR or NLR at 20% concentration by volume in Ctl for 5 days. One-day and 42-day supernatants and LR and NLR supernatants were collected from the same PRBC donor per group. Twenty-percent concentration was selected as a clinically relevant amount to approximate a patient transfused approximately 10 U PRBCs. PBMCs were analyzed by flow cytometry after exposure for the percentage of Foxp3+ Tregs in all CD4+ T cells. Without any stimulus, the PRBC supernatant did not alter Treg induction (1.7% ± 0.2%, 1.2% ± 0.3%, 1.4% ± 0.2%, and 0.9% ± 0.3% Foxp3+ Tregs per CD4+ T cells for 1-day NLR, 42-day NLR, 1-day LR, and 42-day LR, respectively) compared with Ctl (1.3% ± 0.3%; Fig. 1). With anti-CD3 stimulation, exposure to PRBC supernatant more than doubled the percentage of Tregs compared with Ctl plus anti-CD3, and prolonged storage or LR did not alter this induction (6.2% ± 0.6%, 6.0% ± 0.6%, 5.6% ± 0.5%, and 5.9% ± 0.4% for 1-day NLR, 42-day NLR, 1-day LR, and 42-day LR, respectively, versus 2.7% ± 0.6% for Ctl; p < 0.05 for all groups versus Ctl, n = 10 independent experiments with 10 different PRBC donors; Fig. 1). In sum, PRBC supernatant, regardless of duration of storage or LR, induces Foxp3+Tregs from PBMCs stimulated with anti-CD3.
Figure 1.
Regulatory T cells (Tregs) are induced from stimulated peripheral blood mononuclear cells by packed red blood cell supernatant. Packed red blood cells were exposed to control media (Ctl) or packed red blood cell supernatant after 1 day or 42 days of storage (D1 or D42), with leukoreduction (LR) or without leukoreduction (NLR) ± anti-CD3 stimulation. Compiled data from 10 independent experiments expressed as mean ± SEM. *p < 0.05 versus Ctl + anti-CD3 group.
PRBC supernatant does not alter T-cell activation
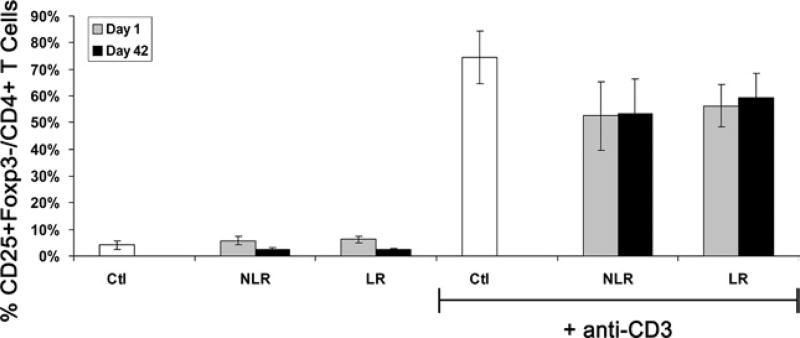
Because anti-CD3 stimulation itself has been reported to cause nonspecific Foxp3 expression,30 the ability of PRBC supernatant to nonspecifically activate T cells was determined. PBMCs were exposed to Ctl or one of the four PRBC supernatants used previously (1-day NLR, 42-day NLR, 1-day LR, and 42-day LR) with or without anti-CD3 stimulation. PBMCs were then analyzed by flow cytometry for the percentage of activated CD25+ T cells among all CD4+ T cells. Because most Tregs also express CD25, an attempt was made to exclude this population by gating on the population of CD25+Foxp3−CD4+ T cells as activated, non-Treg, T cells. Without stimulation, the percentage of activated T cells was low in all groups (4.1% ± 1.7%, 5.8% ± 1.7%, 2.6% ± 0.5%, 6.2% ± 1.1%, and 2.3%±0.6% CD25+Foxp3− activated T cells per CD4+ T cells for Ctl, 1-day NLR, 42-day NLR, 1-day LR, and 42-day LR, respectively; Fig. 2). With anti-CD3 stimulation, the percentage of activated T cells increased in all groups. PRBC supernatant did not activate more T cells than Ctl and even nonsubstantially activated slightly less than control (52% ± 13%, 53% ± 13%, 56% ± 8%, and 59% ± 9% for 1-day NLR, 42-day NLR, 1-day LR, and 42-day LR, respectively, versus 75% ± 10% for Ctl; n = 4; Fig. 2). Taken together with the Treg results, these data suggest PRBC supernatant specifically induces Tregs, but does not result in global T-cell activation, and this induction is not altered by storage or LR.
Figure 2.
T-cell activation is not altered by packed red blood cell supernatant. Peripheral blood mononuclear cells were exposed to control media (Ctl) or packed red blood cell supernatant after 1 day or 42 days of storage, with leukoreduction (LR) or without leukoreduction (NLR) ± anti-CD3 stimulation. Compiled data from four independent experiments expressed as mean ± SEM.
PRBC-induced Tregs are immunosuppressive
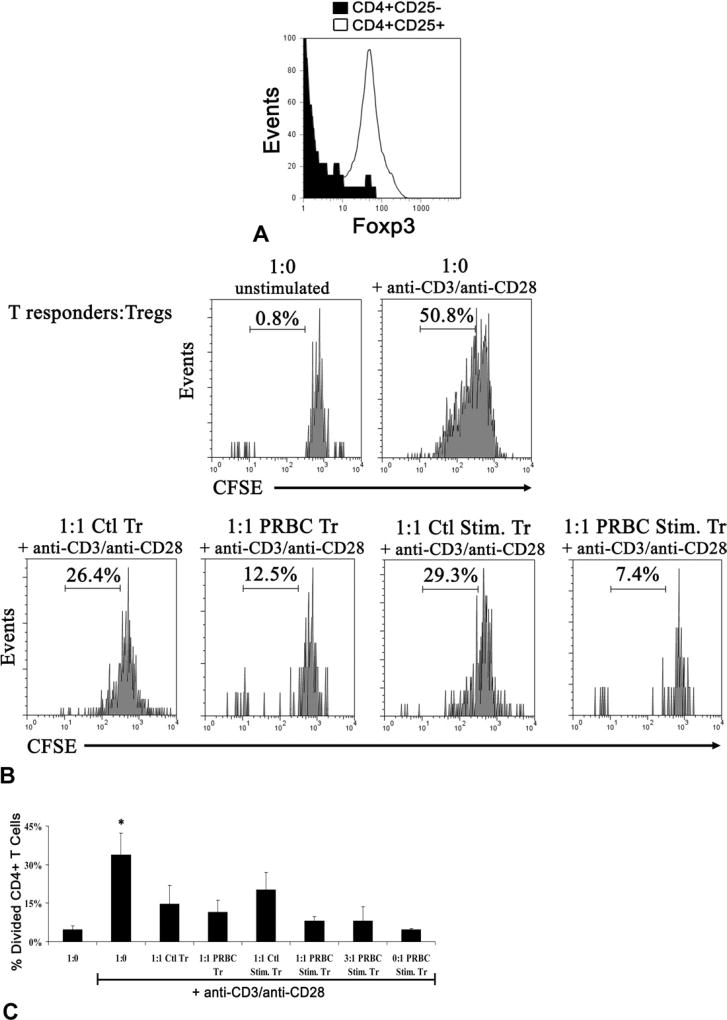
To characterize the suppressive capacity of PRBC-induced Tregs, the ability of these Tregs to suppress the proliferation of CD4+CD25− T-responder cells was assessed. After exposure to Ctl or one of the PRBC supernatants (42-day LR) with or without anti-CD3 stimulation for 5 days, CD4+CD25+ Tregs and CD4+CD25− T-responder cells were sorted by magnetic bead immunosorting. Tregs were sorted based on their CD25 expression rather than by Foxp3 as only surface markers can be used to retain viable cells. Figure 3A confirms what others have previously shown, that sorting CD4+CD25+ T cells enriches for Foxp3+ cells.31 Tregs induced by PRBC or PRBC + anti-CD3 stimulation (PRBC Tregs or PRBC + Stim. Tregs) suppressed proliferation of isolated CD4+CD25− T-responder cells to a similar degree as control or control + anti-CD3 stimulation Tregs (Ctl Tregs or Ctl + Stim. Tregs) harvested from Ctl (resulting in 15% ± 7%, 11% ± 5%, 20% ± 7%, and 8% ± 2% divided CD4+ T cells for Ctl, PRBC, Ctl + Stim. and PRBC + Stim. Tregs, respectively, versus 34% ± 9% in wells without Tregs, p < 0.05, n = 4; Fig. 3C). PRBC + Stim. Tregs maintained their suppressive capacity when diluted to a 3:1 T-responder cell to Treg ratio, resulting in only 8% ± 6% of divided CD4+ T cells (Fig. 3C). Tregs induced by PRBC suppressed proliferation of T-responder cells. PRBC + Stim. Tregs also were anergic when cultured alone with stimulation (Fig. 3C) and 74% ± 6% express CD25, consistent with the reported in vitro characterization of Tregs.
Figure 3.
Regulatory T cells (Tregs) induced by packed red blood cell (PRBC) supernatant are immunosuppressive. Peripheral blood mononuclear cells were exposed to control media (Ctl) or PRBC supernatant ± anti-CD3 stimulation. (A) Peripheral blood mononuclear cells were then sorted by magnetic beads into Tregs (CD4+CD25+) or T-responder cells (CD4+CD25−). CD4+CD25+ sorted T cells are enriched for Foxp3+ cells as depicted in the flow plot. Sorted cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and T-responder cells from Ctl were then cocultured with Tregs from control media (Ctl Tr), PRBC supernatant (PRBC Tr), control media with anti-CD3 (Ctl Stim. Tr) or PRBC supernatant with anti-CD3 (PRBC Stim Tr) in various ratios ± anti-CD3/anti-CD28 stimulation for 5 days. (B) Representative flow plots depict the percentage of divided CD4+ T cells as the CFSE diluted population. (C) Compiled data from four independent experiments expressed as mean ± SEM. *p < 0.05 versus all other groups.
PRBC supernatant does not substantially alter cytokine response
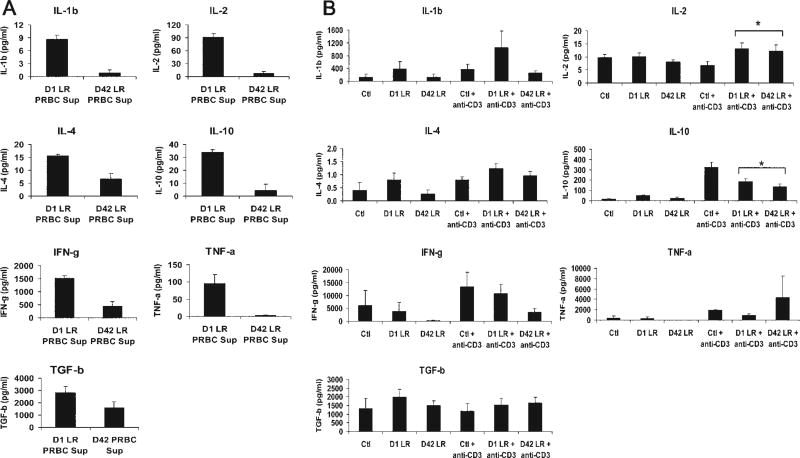
To identify possible cytokines in PRBC supernatant that can play a role in Treg induction or immune modulation, a cytokine profile including IL-1β, IL-2, IL-4, IL-10, interferon-γ, tumor necrosis factor–α, and transforming growth factor–β1 levels were evaluated in the PRBC supernatant by cytokine bead array or standard ELISA. Because other investigators have reported that LR diminishes the cytokine content of PRBCs,7,11 and because both LR and NLR supernatants induced Tregs to a similar degree, only LR PRBC supernatants from 1-day and 42-day were evaluated for cytokines still present after prestorage leukocyte filtration. Levels of all seven cytokines were detectable in the 1-day LR PRBC supernatant, but were reduced after prolonged storage of 42 days, reaching statistical significance for all cytokines except transforming growth factor –β for the small number of supernatants assayed (n = 3; p < 0.05; Fig. 4A).
Figure 4.
Cytokine levels in leukoreduction (LR) packed red blood cell (PRBC) supernatant decreases with storage and exposure of peripheral blood mononuclear cells to PRBC supernatant does not result in substantial cytokine alterations. (A) LR PRBC supernatant (n = 3) was analyzed by bead array or standard ELISA (for transforming growth factor [TGF]–β1) for cytokine concentrations. D42 LR PRBC supernatants had statistically significant decreases in all cytokines except for TGF-β. (B) Peripheral blood mononuclear cells were exposed to control media (Ctl) or PRBC supernatant from leukoreduced units after 1 day or 42 days of storage (D1 LR or D42 LR) ± anti-CD3 stimulation. The supernatant was collected and cytokines were measured. Compiled data from three independent experiments expressed as mean ± SEM. *p < 0.05 versus Ctl + anti-CD3 group.
To characterize additionally the immune response of PBMCs to PRBC exposure, the same cytokines were examined in the supernatants of the PRBC supernatant exposure experiments. Namely, we sought to determine if PRBC supernatant resulted in an immunosuppressive cytokine response to correlate with Treg induction. Supernatants from the previous PRBC supernatant exposure experiments were collected and analyzed for IL-1β, IL-2, IL-4, IL-10, interferon-γ, tumor necrosis factor–α, and transforming growth factor–β1 levels. Anti-CD3 stimulation increased the levels of most cytokines in the supernatant (Fig. 4). IL-1β, IL-4, interferon-γ, tumor necrosis factor–α, and transforming growth factor–β levels were no different in supernatants from PRBC-exposed PBMCs compared with control PBMCs (Fig. 4). IL-2 levels were slightly higher from anti–CD3-stimulated PBMCs exposed to PRBC supernatants versus Ctl (13 ± 2 and 12 ± 2 for 1-day LR and 42-day LR, respectively, versus 7 ± 1 pg/mL for Ctl; p < 0.05, n = 3; Fig. 4), but had slightly lower IL-10 levels (186 ± 24 and 137 ± 23 for 1-day LR and 42-day LR, respectively, versus 322 ± 53 pg/mL for Ctl; p < 0.05, n = 3; Fig. 4). Taken together, PRBC supernatant did not substantially alter levels of IL-1β, IL-4, interferon-γ, tumor necrosis factor–α, and transforming growth factor–β secreted from PBMCs and only slightly increased IL-2 levels and decreased IL-10 levels.
PRBC storage solution does not induce Tregs but washed PRBC supernatant abrogates Treg induction
Because all PRBC supernatants induced Tregs regardless of storage time or LR, a common mediator of Treg induction in all supernatants was sought. One component present in all supernatants is the storage solution of the PRBCs. This contains the additive solution AS-5, which contains dextrose, adenine, mannitol, and sodium chloride; and the anticoagulant-preservative solution CPD, which contains citrate, dextrose and monobasic sodium phosphate.26 PBMCs were exposed to various concentrations of storage solution or Ctl, with or without anti-CD3 stimulation for 5 days. PBMCs were analyzed for Foxp3+ Tregs and CD25+Foxp3− activated T cells as in previous experiments. Concentrations of storage solution > 0.5% appeared to globally reduce cell numbers after 5 days, and concentrations > 5% did not result in sufficient numbers of cells to analyze by flow cytometry. At 0.5% concentration, the storage solution did not induce Tregs with (4.8% ± 2.9% Foxp3+ Tregs among all CD4+ T cells) or without (1.3% ± 0.1%) anti-CD3 stimulation compared with Ctl (3.4% ± 2.0% and 1.2% ± 0.2%, for Tregs with or without anti-CD3, respectively). The storage solution also did not alter the percentage of activated T cells with and without stimulation versus control. Storage solution alone does not reproduce the effects of the whole PRBC supernatant in terms of Treg induction.
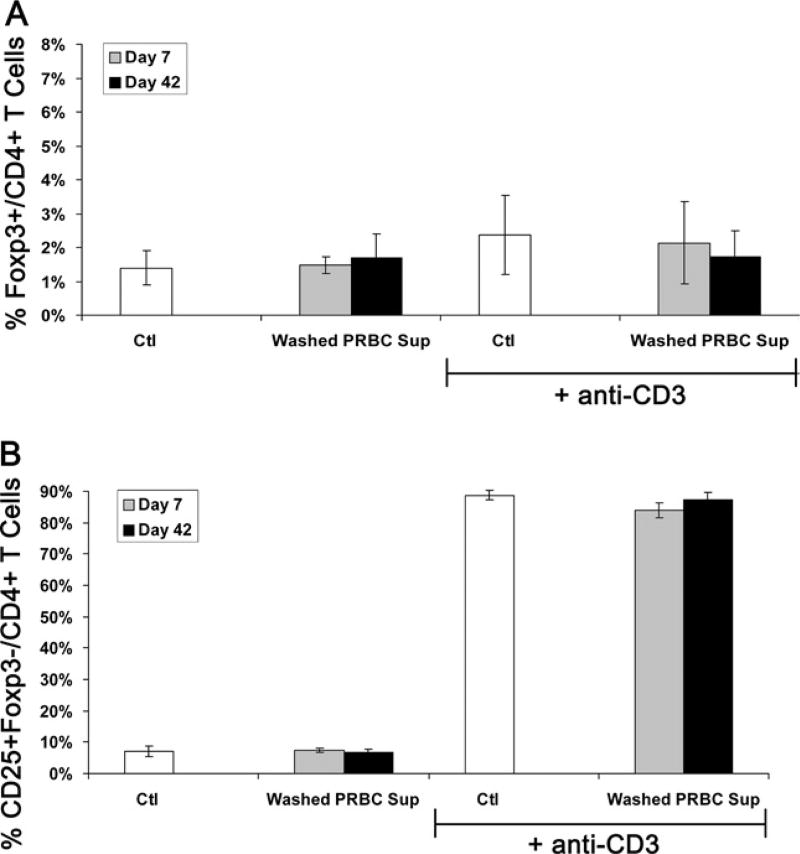
To evaluate the effects of the plasma fraction of PRBC supernatant, units were washed poststorage with saline before supernatant collection to reduce plasma content and exposed to PBMCs as in previous experiments. Using washed PRBC supernatant from fresh units (day 7) or old units (day 42), induction of Tregs in PBMCs stimulated with anti-CD3 was abrogated and was no different than in Ctl (2.1% ± 1.2% and 1.7% ± 0.8% versus 2.4% ± 1.4% for 7 days and 42 days washed PRBC supernatant versus Ctl; Fig. 5A).Washed PRBC supernatants also did not alter T-cell activation versus Ctl (Fig. 5B). Removal of the plasma fraction of PRBCs abrogates the inductive effect on Tregs. In addition, levels of transforming growth factor–β1 were undetectable in fresh and stored washed PRBC supernatant.
Figure 5.
Washed PRBC supernatant does not induce regulatory T cells (Tregs) or activate T cells. Peripheral blood mononuclear cells (PRBC) were exposed to control media (Ctl) or washed packed red blood cell supernatant after 7 days or 42 days of storage ± anti-CD3 stimulation. Compiled data from three independent experiments expressed as mean % (A) Foxp3+ Tregs or (B) CD25+Foxp3− activated T cells of all CD4+ T cells ± SEM.
DISCUSSION
Transfusion-related immunosuppression is potentially mediated by any component of a PRBC transfusion, including erythrocytes and transfused leukocytes, platelets, and soluble factors derived from these cells or originally present in the donor plasma. The current study was undertaken to investigate the role of soluble factors in transfusion-related immunosuppression. Namely, induction of Tregs was examined as a component of immunosuppression from an ABT. We found that PRBC supernatant was able to induce Tregs from stimulated PBMCs. This induction occurred, somewhat surprisingly, to a similar degree regardless of LR or duration of storage. The PRBC supernatant did not globally activate T cells, and the supernatant specifically upregulates Tregs. Tregs induced by the PRBC supernatant are immunosuppressive, as they suppress proliferation of responder T cells. PRBC-induced Tregs are at least as suppressive as control Tregs and even showed a trend of greater suppression. All cytokines evaluated decreased with storage in LR PRBC supernatants, and none were persistently elevated after 42 days of storage. In addition, cytokine levels from PBMCs exposed to the supernatant were not substantially different than from control, eliminating the possibility that a proinflammatory cytokine response can counterbalance Treg induction, and also reducing the possibility that the activity of the induced Treg is IL-10– or transforming growth factor-β–dependent, as neither of these immunosuppressive cytokines were elevated in the cellular supernatants. The PRBC storage solution itself did not induce Tregs and washed PRBC supernatant abrogated the inductive capacity of PRBC supernatant on Tregs, implicating the plasma fraction.
The mechanism of immunosuppression after ABT remains uncertain. Ghio and colleagues12 have described functional immunologic defects, including attenuation of cytotoxicity and decreased mixed lymphocyte reaction after PRBC supernatant exposure. These investigators found the immunosuppression of PRBC supernatant was ameliorated with LR and that the degree of immunosuppression correlated with the concentration of soluble HLA class I molecules and Fas ligand, which both increased with storage duration and were reduced with LR. In the present study, storage duration and LR did not alter induction of Tregs, so Fas ligand or HLA class I molecules are likely not involved in PRBC-mediated Treg induction. In addition, Tregs are known to be particularly susceptible to Fas ligand–mediated apoptosis32 and yet, interestingly, they were upregulated in response to PRBC exposure. PRBC-mediated Treg induction occurred only in the presence of anti-CD3 stimulation, suggesting the “two-hit” phenomenon described in other aspects of transfusion-related immunomodulation,2 with T-cell receptor activation and PRBC exposure required for Treg induction.
Other investigators have suggested a role for Tregs in transfusion-mediated immunosuppression, but this has not been studied directly.33,34 Lapierre and colleagues35 examined aspects of immunosuppression and tolerance after NLR versus LR PRBC transfusion in cancer patients. In this small, randomized, controlled trial of 35 patients, they found that the median gene expression of Foxp3 did not increase 3 days after blood transfusion and the relative changes were no different from NLR versus LR transfusions. In the present study, Treg induction was examined in vitro using Foxp3 protein expression and suppressive function with 5 days of ABT exposure, as Treg induction is time-dependent and can require 4 to 5 days for upregulation.36
The immunosuppressive capacity of ABT to induce Tregs, regardless of LR or storage duration, was unexpected. Prolonged storage has been shown to increase mortality in transfused recipients, most recently reported in a large observational study of nearly 20,000 cardiac surgery patients with higher mortality found in individuals who received blood older than 14 days.37 The mechanism of this worse prognosis remains unclear and it might be the result of proinflammatory or immunosuppressive effects of the ABT. Most investigations of the “storage lesion” of PRBCs have identified increased proinflammatory mediators with prolonged storage.38,39 Prestorage LR has been implemented universally in Canada and Western Europe and, to some degree, in the US, partly in an attempt to ameliorate the immunomodulation of transfused leukocytes or leukocyte-derived products. The only proved benefits of prestorage leukocyte filtration are the reduction of nonhemolytic febrile transfusion reactions, reduction in cytomegalovirus transmission, decreased HLA alloimmunization with platelet refractoriness, and improved short-term survival in cardiac surgery patients.40,41 Similar to the effects of stored blood transfusions on cardiac patients, it is unclear if this improved survival with LR transfusion is a result of alterations of immune upregulation or suppression. Although pretransplantation ABT improves allograft survival,42 no human studies have proved prestorage leukoreduction ameliorates this effect. Similarly, the effects of leukocyte-reduced ABT on infection risk are inconclusive.43 Although animal models have suggested that prestorage leukoreduction attenuates the protumor effect of ABT,44 no human, randomized, controlled, clinical trials have been completed to compare LR versus NLR ABT. The finding that LR does not alter PRBC induction of Tregs might be consistent with the lack of clinical evidence of a benefit of LR, and suggests that LR might not attenuate transfusion-related immunosuppression. In addition, the current study found that washing the plasma out of the PRBC supernatant abrogated its ability to induce Tregs. The plasma fraction contains one or several potential factors that can induce Tregs in the recipient. Indeed, transfusion with plasma alone in the form of fresh-frozen plasma has been shown to induce immunologic responses in the recipient, although the role of Treg induction in these responses remain unclear.45
There are some limitations of this study. Although the plasma fraction of the PRBC supernatant is required for Treg induction, the precise mediator remains unclear. Transforming growth factor–β, in conjunction with IL-2 exposure and anti-CD3 stimulation, has been shown to generate Tregs from naïve CD4+CD25− precursors.36 Transforming growth factor–β is present in the PRBC supernatants tested (in addition to IL-2), and presents a possible mechanism of transfusion-mediated induction of Tregs. Other potential inducers of Tregs that might be present in the transfused plasma fraction include thrombospondin-1, vitamin A metabolites, heme oxygenase-1, and prostaglandin E2. The degree of PRBC-mediated Treg induction appears relatively modest, with an increase from approximately 3% of all CD4+ T cells in Ctl to 6% in PRBC-exposed cells. Despite the relative rarity of Tregs, they are quite potent. Treg dysfunction or absence is associated with severe autoimmune disorders, such as multiple sclerosis, rheumatoid arthritis, and immune dysfunction, such as polyendocrinopathy, enteropathy, and X-linked (IPEX).46–48 Absolute increases of only 2% circulating Tregs per all CD4+ T cells or less is strongly correlated with increased mortality in patients with cancer and an absolute decrease of 2% circulating Tregs is correlated with the likelihood of organ rejection 1 year after transplantation.22,49,50 Although Foxp3+ Tregs were induced, CD25+ Tregs were used in the functional assay and this population might include some activated effector T cells. Unfortunately, there is no method to sort viable human Tregs based on their Foxp3 status. The CD25+ sort did enrich for Foxp3+ cells and these cells were suppressive in the present study. The precise roles of PRBC-induced Tregs in transfusion-mediated immunosuppression are also unclear. Tregs are generally activated or induced by a wide variety of antigens, including self-antigens, and by antigen-independent mechanisms.17 They act through cell-contact–dependent and cell-contact–independent methods, in part by secretion of nonspecific immunosuppressive molecules, such as IL-10 and transforming growth factor–β,17 although these cytokines were not elevated in PBMCs exposed to PRBC supernatant in the present investigation.
In summary, allogeneic blood transfusion induces Tregs and this induction does not appear to be attenuated by leukoreduction or altered by duration of storage. This induction is independent of exposure to transfused cells, but dependent on exposure to allogeneic plasma. These findings can provide one mechanism of transfusion-related immunosuppression seen clinically after ABT, and additional investigation into PRBC-mediated Treg induction can provide insights into attenuating this phenomenon.
Abbreviations and Acronyms
- ABT
allogeneic blood transfusion
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- Ctl
control media
- IL
interleukin
- LR
leukoreduced
- NLR
nonleukoreduced
- PBMC
peripheral blood mononuclear cell
- PRBC
packed red blood cell
- Treg
regulatory T cell
Footnotes
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Baumgartner, Banerjee, McCarter
Acquisition of data: Baumgartner
Analysis and interpretation of data: Baumgartner, Silliman, Moore, Banerjee, McCarter
Drafting of manuscript: Baumgartner
Critical revision: Baumgartner, Silliman, Moore, McCarter
References
- 1.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. discussion 624–625. [PubMed] [Google Scholar]
- 2.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- 4.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005033.pub2. CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters WR, Fry RD, Fleshman JW, Kodner IJ. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum. 1989;32:749–753. doi: 10.1007/BF02562122. [DOI] [PubMed] [Google Scholar]
- 6.Chang H, Hall GA, Geerts WH, et al. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sang. 2000;78:13–18. doi: 10.1159/000031143. [DOI] [PubMed] [Google Scholar]
- 7.Kristiansson M, Soop M, Shanwell A, Sundqvist KG. Prestorage versus bedside white blood cell filtration of red blood cell concentrates: effects on the content of cytokines and soluble tumor necrosis factor receptors. J Trauma. 1996;40:379–383. doi: 10.1097/00005373-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Mynster T, Dybkjoer E, Kronborg G, Nielsen HJ. Immunomodulating effect of blood transfusion: is storage time important? Vox Sang. 1998;74:176–181. [PubMed] [Google Scholar]
- 9.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 11.Shanwell A, Kristiansson M, Remberger M, Ringden O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–684. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–996. doi: 10.1046/j.1537-2995.2001.41080988.x. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 16.Dieckmann D, Plottner H, Berchtold S, et al. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303– 1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venet F, Chung CS, Monneret G, et al. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 19.MacConmara MP, Maung AA, Fujimi S, et al. Increased CD4+CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 21.Miracco C, Mourmouras V, Biagioli M, et al. Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep. 2007;18:1115–1122. [PubMed] [Google Scholar]
- 22.Griffiths RW, Elkord E, Gilham DE, et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.McCarter MD, Baumgartner J, Escobar GA, et al. Immunosuppressive dendritic and regulatory T cells are upregulated in melanoma patients. Ann Surg Oncol. 2007;14:2854–2860. doi: 10.1245/s10434-007-9488-3. [DOI] [PubMed] [Google Scholar]
- 25.Boschiero L, Nacchia F, Fior F, et al. Specific alloantigen self-control by regulatory T cells in organ transplantation: a review. Transplant Proc. 2007;39:2013–2017. doi: 10.1016/j.transproceed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Brecher ME. Technical manual, AABB. 15. Bethesda, MD: American Association of Blood Banks; 2005. [Google Scholar]
- 27.Hughes A, Mijovic V, Brozovic B, Davies TD. Leucocyte-depleted blood: a comparison of cell-washing techniques. Vox Sang. 1982;42:145–150. doi: 10.1111/j.1423-0410.1982.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 28.Rock G, Baxter A, Gray E. Leukocyte-depleted blood: a comparison of available preparations. Can Med Assoc J. 1984;130:1566–1568. [PMC free article] [PubMed] [Google Scholar]
- 29.Uda M, Naito S, Yamamoto K, et al. Optimal protocol for preparation of leukocyte-poor red cells with a blood cell processor. Transfusion. 1984;24:120–123. doi: 10.1046/j.1537-2995.1984.24284173341.x. [DOI] [PubMed] [Google Scholar]
- 30.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 32.Fritzsching B, Oberle N, Eberhardt N, et al. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand— but not to TCR-mediated cell death. J Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Roelen D, Brand A, Claas FH. Pretransplant blood transfusions revisited: a role for CD(4+) regulatory T cells? Transplantation. 2004;77(Suppl):S26–S28. doi: 10.1097/01.TP.0000106469.12073.01. [DOI] [PubMed] [Google Scholar]
- 34.Claas FH, Roelen DL, van Rood JJ, Brand A. Modulation of the alloimmune response by blood transfusions. Transfus Clin Biol. 2001;8:315–317. doi: 10.1016/s1246-7820(01)00122-7. [DOI] [PubMed] [Google Scholar]
- 35.Lapierre V, Auperin A, Robinet E, et al. Immune modulation and microchimerism after unmodified versus leukoreduced allogeneic red blood cell transfusion in cancer patients: results of a randomized study. Transfusion. 2007;47:1691–1699. doi: 10.1111/j.1537-2995.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 36.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3+ T-regulatory cells from CD4+CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 38.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 39.Wadhwa M, Seghatchian MJ, Dilger P, et al. Cytokine accumulation in stored red cell concentrates: effect of buffy-coat removal and leucoreduction. Transfus Sci. 2000;23:7–16. doi: 10.1016/s0955-3886(00)00049-7. [DOI] [PubMed] [Google Scholar]
- 40.Blajchman MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006;60(Suppl):S83–S90. doi: 10.1097/01.ta.0000199537.09201.7b. [DOI] [PubMed] [Google Scholar]
- 41.Bilgin YM, van de Watering LM, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109:2755–2760. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]
- 42.Opelz G, Vanrenterghem Y, Kirste G, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–967. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 43.Vamvakas EC. Meta-analysis of randomized controlled trials investigating the risk of postoperative infection in association with white blood cell-containing allogeneic blood transfusion: the effects of the type of transfused red blood cell product and surgical setting. Transfus Med Rev. 2002;16:304–314. doi: 10.1053/tmrv.2002.35209. [DOI] [PubMed] [Google Scholar]
- 44.Bordin JO, Bardossy L, Blajchman MA. Growth enhancement of established tumors by allogeneic blood transfusion in experimental animals and its amelioration by leukodepletion: the importance of the timing of the leukodepletion. Blood. 1994;84:344–348. [PubMed] [Google Scholar]
- 45.MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma. 2006;60(Suppl):S46–S50. doi: 10.1097/01.ta.0000199546.22925.31. [DOI] [PubMed] [Google Scholar]
- 46.Haas J, Fritzsching B, Trubswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–1330. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 47.Sarkar S, Fox DA. Regulatory T cell defects in rheumatoid arthritis. Arthritis Rheum. 2007;56:710–713. doi: 10.1002/art.22415. [DOI] [PubMed] [Google Scholar]
- 48.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Perez SA, Karamouzis MV, Skarlos DV, et al. CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13:2714–2721. doi: 10.1158/1078-0432.CCR-06-2347. [DOI] [PubMed] [Google Scholar]
- 50.Demirkiran A, Kok A, Kwekkeboom J, et al. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12:277–284. doi: 10.1002/lt.20612. [DOI] [PubMed] [Google Scholar]