Abstract
Background
Exercise is recommended as a cornerstone of treatment for type 2 diabetes mellitus (T2DM), however, it is often poorly adopted by patients. Even in the absence of apparent cardiovascular disease, persons with T2DM have an impaired ability to carry out maximal and submaximal exercise and these impairments are correlated with cardiac and endothelial dysfunction. Glucagon-like pepetide-1 (GLP-1) augments endothelial and cardiac function in T2DM. We hypothesized that administration of a GLP-1 agonist (exenatide) would improve exercise capacity in T2DM.
Methods and Results
Twenty-three participants (64±4 years; mean±SE) with uncomplicated T2DM were randomized in a double-blinded manner to receive either 10mcg BID of exenatide or matching placebo after baseline measurements. Treatment with exenatide did not improve VO2peak (P=0.1464) or VO2 kinetics (P=0.2775). Diastolic function, assessed via resting lateral E:E’, was improved with administration of exenatide compared with placebo (Placebo Pre: 7.6±1.0 vs. Post: 8.4±1.2 vs. Exenatide Pre: 8.1±0.7 vs. Post: 6.7±0.6; P=0.0127). Additionally, arterial stiffness measured by pulse wave velocity, was reduced with exenatide treatment compared with placebo (Placebo Pre: 10.5±0.8 vs. Post: 11.5±1.1 seconds vs. Exenatide Pre: 11.4±1.8 vs. Post: 10.2±1.4 seconds; P=0.0373). Exenatide treatment did not improve endothelial function (P=0.1793).
Conclusions
Administration of exenatide improved cardiac function and reduced arterial stiffness, however, these changes were not accompanied by improved functional exercise capacity. In order to realize the benefits of this drug on exercise capacity, combining exenatide with aerobic exercise training in participants with T2DM may be warranted.
Clinical Trials Registration
Keywords: cardiovascular diseases, exercise, diabetes mellitus
1. Introduction
Despite overwhelming evidence to support aerobic exercise training as part of the treatment for type 2 diabetes mellitus (T2DM), patients with T2DM are commonly sedentary. Even in the absence of cardiovascular disease (CVD), persons with T2DM have reduced functional exercise capacity assessed via peak oxygen consumption (VO2peak) compared with healthy controls matched for age and physical activity level (Schneider et al., 1984; Kjaer et al., 1990; Regensteiner et al., 1995; Bauer et al., 2007; Regensteiner et al., 2015). This impairment in functional exercise capacity increases risk for both all cause and CVD mortality (Wei et al., 1999). Patients with T2DM also have reduced submaximal exercise capacity during constant workloads accompanied by slowed oxygen uptake kinetics (VO2 kinetics). Impairments in either maximal or submaximal responses to exercise may partly explain our previous findings that submaximal ambulatory activities require a greater relative effort for persons with T2DM compared with controls (Regensteiner et al., 1995; 1998; Huebschmann et al., 2009; 2015). The decreased submaximal VO2 and slowed VO2 kinetics in T2DM are similar to impairments observed in disease states with diminished oxygen delivery (Hansen et al., 1987) suggesting the impairment in aerobic exercise tolerance in T2DM is associated with a decrease in oxygen delivery to skeletal muscle. This concept is supported by an increase in skeletal muscle deoxygenation at the onset of exercise in patients with T2DM (Bauer et al., 2007). Further, our group has previously reported strong correlations between endothelial and diastolic function and exercise capacity in patients with T2DM (Regensteiner et al., 1998; Brandenburg et al., 1999; Bauer et al., 2007) supporting the notion that oxygen delivery is impaired in patients with T2DM with low exercise capacity. Development of a pharmacological strategy that would improve oxygen delivery to skeletal muscle during aerobic exercise may increase functional exercise capacity in patients with T2DM.
Glucagon-like peptide-1 (GLP-1), an insulin secretagogue, improves glycemic control by lowering circulating glucose concentrations (both fasting and postprandial) and glycated hemoglobin (HbA1C) (Mafong & Henry, 2009; Monami et al., 2009). Independent of the impact on glucose control, GLP-1 may enhance oxygen delivery to skeletal muscle via augmentation of endothelial and cardiac function in T2DM (Ceriello et al., 2006; Mafong & Henry, 2009; Koska et al., 2015). Previous investigations of GLP-1 have demonstrated improvement of left ventricular systolic and diastolic function in humans, rodents, and canines (Luque, 2002; Nikolaidis et al., 2004b; 2004a; Nikolaidis, 2005; Zhao et al., 2006; Sokos et al., 2007; Mafong & Henry, 2009). Further, studies in rodents, both in vivo and ex vivo, suggest a direct effect of GLP-1 on endothelial function via endothelial nitric oxide synthase activation (Nyström, 2008; Keller et al., 2014). In humans, GLP-1 recruits muscle microvasculature and lowers blood pressure in clinical trials (Mafong & Henry, 2009; Chai et al., 2014). The impact of GLP-1 on cardiac and endothelial function thus addresses two key contributors of oxygen delivery to skeletal muscle and potentially the exercise impairment observed in patients with T2DM. Accordingly, we hypothesized that exenatide, a GLP-1 receptor agonist, would improve functional exercise capacity in people with T2DM.
2. MATERIALS AND METHODS
2.1 Participants
Twenty-three adults with uncomplicated T2DM between the ages of 45 and 70 years were recruited through general advertising and local clinics to participate in the current investigation. The Institutional Review Board of the University of Colorado School of Medicine (UCSOM) approved the experimental protocol, and the nature, purpose and risks of the study were explained before written informed consent was obtained from each participant. This investigation is registered at clinicaltrials.gov (NCT01364584).
Presence of T2DM was documented by chart review and presence of treatment for T2DM. To make this investigation as clinically relevant as possible, persons with T2DM were included if their diabetes was treated by diet alone and/or oral antidiabetic medications and demonstrated glycemic control with total HbA1C of less than 9% on therapy. All participants were sedentary (defined as exercising one hour per week or less) and inclusion in the study was only permitted if participants did not plan to alter their exercise or diet efforts during the study.
History, physical examination and laboratory testing confirmed absence of comorbid conditions. Exclusion criteria included: use of GLP-1 receptor agonists or dipeptidyl peptidase 4 inhibitors, cigarette use within one year prior to study, evidence of acute liver disease, evidence of distal symmetrical neuropathy (by evaluation of symptoms [numbness, paresthesia] and signs [elicited by vibration, pinprick, light touch, ankle jerks]), autonomic dysfunction (>20 mmHg fall in upright blood pressure without a change in heart rate), proteinuria (urine protein >200 mg/dl) or creatinine (≥ 2 mg/dl), evidence of heart disease by history, echocardiography, or abnormal resting or exercise electrocardiogram (≥ 1 mm ST segment depression), angina, or other cardiac or pulmonary symptoms potentially limiting exercise performance. Systolic blood pressure >190 mmHg at rest or >250 mmHg with exercise or diastolic pressure >95 mmHg at rest or >105 mmHg with exercise were also grounds for exclusion. All exclusions were made for reasons of participant safety or potential effects on exercise performance.
2.2 Study Protocol
Participants came to the Vascular Research Laboratory at the UCSOM for seven visits over a four-month period. During visit one, a history and physical examination was completed as well as venous blood drawn for measuring several circulating metabolic factors. Sedentary lifestyle was confirmed with the Low-Level Physical Activity Recall survey (LoPAR) (Sallis et al., 1985). In addition, a resting electrocardiogram and urinalysis were performed. During the next visit, participants completed a graded cycle exercise test for habituation purposes and body composition was assessed via dual-energy x-ray absorptiometry (DEXA). The following visit included a second graded cycle exercise test using a metabolic cart to determine VO2peak, echocardiography to determine cardiac function at rest, and brachial artery ultrasound measurements (flow-mediated dilation [FMD]) for assessment of endothelial function. During the final visit prior to participant randomization, arterial stiffness was measured at rest by determining pulse wave velocity. Additionally, participants completed three bouts of constant workload exercise for assessment of VO2 kinetics. Following the conclusion of these visits, participants were randomized in a double blind design to receive either 10 mcg twice-daily exenatide, subcutaneously, 30–60 minutes prior to meals (n = 11) or a matched placebo (n = 12) for three months. Prior to the beginning of placebo/exenatide administration, participants received training from study staff regarding injection techniques. The entry procedures were repeated three months after treatment initiation.
2.3 Blood Collection and Preparation
Blood was drawn from participants after a 12-hour fast via standard venipuncture technique for the measurement of glucose, insulin, HbA1C, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglyceride, glycerol and free fatty acid concentrations. Parameters were assayed according to previously reported methods (Regensteiner et al., 1995; 1998). The homeostasis model assessment for insulin resistance (HOMA-IR) and beta cell function (HOMA-β) were calculated using previously described methods (Matthews et al., 1985).
2.4 Graded Exercise Test
VO2peak was determined via graded exercise to exhaustion as previously described (Regensteiner et al., 1995; 1998; Brandenburg et al., 1999) using a stationary cycle ergometer (Lode Bike, Groningen, The Netherlands) and a metabolic cart (Medgraphics Ultima CPX, Medical Graphics Corp., St. Paul, MN, USA). After the start of exercise, the work rate was increased in 10–20 watt/minute increments (depending on age and sex) in order to allow each participant to reach maximum within 7–12 minutes. Peak VO2 was confirmed by a respiratory exchange ratio (RER) greater than 1.1. During incremental exercise testing, the highest VO2 and heart rate averaged over 20 seconds were defined as the maximum values.
2.5 Constant Work Rate (CWR) Exercise Testing
Participants performed three identical exercise transitions from rest to CWR exercise (85% of anaerobic threshold) on a cycle ergometer as previously described (Regensteiner et al., 1998; Brandenburg et al., 1999; Bauer et al., 2007). During each transition, data were collected during two minutes of rest, then four minutes of pedaling with no resistance, and six minutes at 85% of anaerobic threshold. Transitions were separated by a minimum of 10 minutes of rest. RER measurements and heart rate data were recorded throughout each CWR bout.
2.6 VO2 Kinetic Methods
Gas exchange and heart rate data for kinetic analysis were processed using a software program developed in our laboratory as previously described (Regensteiner et al., 1998; Brandenburg et al., 1999). The data for each exercise transition were time interpolated to 1-second intervals. The three CWR exercise transitions were time-aligned and averaged to provide a single, averaged exercise response for each subject.
Oxygen uptake kinetic responses were evaluated using a 2-component exponential model allowing individual components of the VO2 kinetic response to be evaluated as previously reported (Bauer et al., 1999; 2007).
2.7 Endothelial Function
Endothelial function was assessed via measurement of brachial artery flow-mediated dilation (FMD) as previously described (Sorensen et al., 1995). Measurements were made after a four-hour fast and prior to any other exercise testing performed in the same study visit. B-mode ultrasound images of brachial artery diameter were acquired at baseline with the use of a 10.0-mHz linear-array transducer as previously reported in our laboratory (Regensteiner et al., 2003; 2005; 2015) (GE vivid 7 Dimension, Milwaukee, WI, USA). Reactive hyperemia was produced by inflating the cuff to 250 mmHg of pressure for five minutes followed by rapid deflation. After the release of the arterial occlusion, B-mode ultrasound brachial artery diameter images were measured continuously for two minutes. Brachial artery diameter were acquired and analyzed using a commercially available software package (Vascular Analysis Tools MIA, Coralville, IA) by the same individual. All images were coded by number and blinded to group assignment.
2.8 Cardiac Echocardiography
Standard two dimensional and Doppler echocardiography (Henry et al., 1980) (GE Vivid 7 Dimension, Milwaukee, WI, USA) were performed using standard methods at rest. A cardiologist blinded to the treatment allocation of the participants supervised the acquisition of these echocardiographic data, as well as performed all of the measurements and interpretation. Participants were examined in the left lateral decubitus position using standard parasternal, short-axis, and apical views. All recordings and measurements were obtained by the same observer according to the recommendations of the American Society of Echocardiography and were be performed at the same time of day for each subject to avoid the possible influence of circadian rhythm on left ventricular diastolic function. Circumferential and longitudinal strain were recorded as measures of systolic function while mitral valve E and A waves, and lateral and septal E’ were assessments of diastolic function.
2.9 Pulse Wave Velocity
Arterial stiffness was assessed via measurement of pulse-wave velocity (SphygmaCor CP system, AtCor Medical, Itasca, IL, USA) according to methods previously described (Cecelja & Chowienczyk, 2009). Measurements were made after a four-hour fast and prior to any other exercise testing performed in the same study visit.
2.10 Statistical Analysis
Statistical power was estimated from VO2peak data obtained in a previous study (Regensteiner et al., 2005) where patients were screened and evaluated identically to the current study. With 12 evaluable subjects per group a 2-group 2-sided t test would have 89% power to detect a between group difference of 2.95 at the alpha=0.05 level, assuming conservatively that the standard deviation is 2.16.
Repeated measures analysis of variance was used to determine the influence of treatment (placebo vs. exenatide) using Graphpad Prism version 6. Pairwise comparisons were performed using the Tukey test. The level of statistical significance was set at P < 0.05. Data are expressed as mean ± standard error.
3. RESULTS
3.1 Participants
T2DM was confirmed in all participants during the enrollment screening procedures. The length of time from T2DM diagnosis to the beginning of the study did not differ between the groups (Placebo: 7 ± 1 vs. Exenatide: 6 ± 2 years; P = 0.593). Pharmacological management of the participants in this study aligned with usual care for T2DM and was similar between groups; all participants were prescribed metformin and five participants in each group were prescribed a sulfonylurea. Participants randomized to the exenatide group tolerated the treatment well. Baseline physical characteristics and circulating metabolic factors from research participants are presented in Table 1. There were no baseline (i.e. pre-treatment) differences between the groups with the exception of body mass index (BMI), which was greater in the exenatide group (P = 0.021).
Table 1.
Participant characteristics at baseline and three months
| Placebo (n = 12) | Exenatide (n = 11) | |||
|---|---|---|---|---|
| Baseline | 3 Month | Baseline | 3 Month | |
| Age | 64 ± 1 | - | 64 ± 7 | - |
| Sex (m/f) | 5/7 | - | 7/4 | - |
| Body Mass (kg) | 87.9 ± 3.3 | 87.5 ± 3.1 | 101.7 ± 5.3† | 98.7 ± 5.5* |
| Body Mass Index (kg/m2) | 31.3 ± 1.2 | 31.2 ± 1.0 | 33.9 ± 1.0 | 32.9 ± 1.1* |
| Body Fat (%) | 35.7 ± 2.2 | 35.5 ± 2.5 | 37.7 ± 2.3 | 37.1 ± 2.6 |
| Lean Mass (kg) | 55.8 ± 2.8 | 56.0 ± 3.1 | 61.9 ± 3.9 | 61.3 ± 4.2 |
| Fasting Glucose (mg/dl) | 127.9 ± 8.0 | 153.5 ± 17.6 | 170.5 ± 24.2 | 117.8 ± 9.3* |
| Fasting Insulin (µU/ml) | 34.6 ± 6.4 | 28.2 ± 4.9 | 29.0 ± 3.7 | 26.3 ± 4.4 |
| HbA1C (%) | 7.2 ± 0.4 | 7.0 ± 0.4 | 7.3 ± 1.1 | 6.5 ± 0.2* |
| HbA1C (mmol/mol) | 55 ± 4 | 53 ± 4 | 56 ± 12 | 48 ± 2* |
| HOMA-IR | 11.3 ± 2.3 | 10.5 ± 1.9 | 11.5 ± 1.9 | 7.3 ± 1.1 |
| HOMA-β | 208.7 ± 38.6 | 148.4 ± 31.7 | 117.1 ± 16.4 | 236.8 ± 51.9* |
| Total Cholesterol (mg/dl) | 175.3 ± 11.7 | 177.1 ± 20.0 | 156.3 ± 9.8 | 139.4 ± 8.2 |
| LDL Cholesterol (mg/dl) | 97.6 ± 7.4 | 91.7 ± 7.9 | 93.8 ± 6.2 | 81.9 ± 7.9 |
| Triglycerides (mg/dl) | 217.8 ± 58.0 | 255.1 ± 108.0 | 156.8 ± 18.3 | 170.0 ± 16.7 |
| Glycerol (mg/dL) | 11.3 ± 2.3 | 10.5 ± 1.9 | 11.5 ± 1.9 | 7.3 ± 1.1* |
| Free Fatty Acids (mmol/L) | 500.5 ± 67.8 | 596.0 ± 53.0 | 580.8 ± 76.3 | 547.6 ± 59.3* |
Data are Mean ± Standard Error.
P < 0.05 Different compared with placebo at same time point;
P < 0.05 Different compared with baseline within group.
3.2 Body Composition
Select body composition parameters from baseline measurements and following three months of placebo/exenatide are presented in Table 1. There was a significant reduction of body mass (P = 0.021) and BMI (P = 0.025) with exenatide administration compared with placebo. Lean mass (P = 0.445) and body fat percentage (P = 0.673) were unaltered by administration of exenatide or placebo.
3.3 Circulating Metabolic Factors
Circulating metabolic factors at baseline and following three months of placebo/exenatide are presented in Table 1. Fasting blood glucose concentration was significantly decreased following three months of exenatide treatment compared with placebo (P = 0.001). The decrease in blood glucose was accompanied by a significant reduction in HbA1C in the exenatide group compared with placebo (P = 0.005) and an improvement in HOMA-β (P = 0.011). Additionally, glycerol (P = 0.001) and free fatty acids (P = 0.006) were lowered with exenatide treatment compared with placebo. There was no impact of exenatide administration on fasting insulin, HOMA-IR, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides (all P > 0.075).
3.4 Resting Heart Rate and Blood Pressure
Resting heart rate (Placebo Pre: 78 ± 4 vs. Post: 79 ± 4 vs. Exenatide Pre: 76 ± 4 vs. Post: 73 ±0 4 beats per minute) and blood pressure (Systolic blood pressure: Placebo Pre: 122 ± 5 vs. Post: 122 ± 3 vs. Exenatide Pre: 119 ± 4 vs. Post: 114 ± 5 mmHg; Diastolic blood pressure: Placebo Pre: 77 ± 3 vs. Post: 77 ± 2 vs. Exenatide Pre: 71 ± 2 vs. Post: 70 ± 2 mmHg) were not affected by exenatide administration (all P > 0.2). Diastolic blood pressure was lower in the exenatide group at baseline compared with the placebo group (P = 0.025).
3.5 VO2peak and VO2 kinetics
There were no baseline differences in VO2peak or VO2 kinetics between the exenatide or placebo groups (Table 2). VO2peak did not change with administration of exenatide or placebo (P = 0.146) nor did VO2 kinetics (P = 0.278).
Table 2.
Measures of maximal and submaximal exercise capacity at baseline and three months
| Placebo (n =12) |
Exenatide (n = 11) |
|||
|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | |
| VO2peak (ml/kg/min) | 15.4 ± 0.9 | 16.1 ± 1.1 | 17.5 ± 1.0 | 16.6 ± 1.1 |
| VO2peak (ml/min) | 1363 ± 95 | 1419 ± 119 | 1783 ± 129 | 1647 ± 120 |
| RERpeak | 1.22 ± 0.3 | 1.25 ± 0.4 | 1.19 ± 0.3 | 1.25 ± 0.2 |
| Peak heart rate (bpm) | 134 ± 5 | 137 ± 5 | 140 ± 4 | 138 ± 5 |
| Peak workload (watts) | 108 ± 9 | 110 ± 11 | 135 ± 12 | 132 ± 12 |
| VO2 kinetics τ (sec) | 63.3 ± 5.9 | 67.2 ± 2.7 | 78.2 ± 6.8 | 71.4 ± 8.5 |
Data are Mean ± Standard Error. Peak oxygen consumption: VO2peak. Respiratory exchange ratio: RER. Beats per minute: bpm.
3.6 Cardiac Function
Table 3 contains echocardiography variables measured at baseline and following three months of placebo/exenatide administration. There were no baseline differences between the groups except for mitral valve E velocity, which was slower in the exenatide group compared with the placebo group. This difference between the groups remained at the three-month follow up but was not affected by exenatide administration (P = 0.0846). Measures of diastolic function (lateral and septal E:E’) decreased (improved) with exenatide administration while these measures worsened in the placebo group during the three-month study (Lateral E:E’: P = 0.013; Septal E:E’: P = 0.038). Exenatide administration did not alter, (P = 0.461), mitral valve E:A ratio (P = 0.086), mitral valve deceleration time (P = 0.086), septal E’ (P = 0.373), or lateral E’ (P = 0.398). Measures of systolic function: circumferential strain (P = 0.453), longitudinal strain (P = 0.126), and stroke volume (P = 0.371) did not change in either group.
Table 3.
Echocardiography measurements at rest at baseline and three months
| Placebo (n = 12) | Exenatide (n = 11) | |||
|---|---|---|---|---|
| Baseline | 3 Month | Baseline | 3 Month | |
| Circumferential Strain | −23.5 ± 0.7 | −22.6 ± 1.4 | −23.7 ± 1.7 | −25.6 ± 1.78 |
| Longitudinal Strain | −19.3 ± 0.7 | −18.3 ± 0.8 | −17.6 ± 1.2 | −18.5 ± 1.2 |
| Stroke Volume (mL/beat) | 81.0 ± 12.8 | 80.7 ± 1.9 | 88.2 ± 3.3 | 93.3 ± 2.8 |
| MV E Wave Velocity | 0.74 ± 0.06 | 0.78 ± 0.06 | 0.69 ± 0.04† | 0.61 ± 0.06† |
| MV E:A Wave Velocity | 0.83 ± 0.06 | 0.82 ± 0.06 | 1.04 ± 0.13 | 0.85 ± 0.09 |
| MV Deceleration Time | 283.3 ± 12.8 | 239.8 ± 14.6 | 236.0 ± 6.6 | 234.6 ± 17.0 |
| Septal E’ | 0.07 ± 0.01 | 0.07 ± 0.1 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| Septal E:E’ | 10.4 ± 0.8 | 12.6 ± 1.4 | 9.6 ± 0.7 | 8.3 ± 0.9* |
| Lateral E’ | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 |
| Lateral E:E’ | 7.6 ± 1.0 | 8.4 ± 1.2 | 8.1 ± 0.7 | 6.7 ± 0.6* |
Data are Mean ± Standard Error. Mitral Valve: MV.
P < 0.05 Different compared with placebo at same time point.
P < 0.05 Different compared with baseline within group.
P < 0.05 Main effect of drug.
3.7 Flow Mediated Dilation
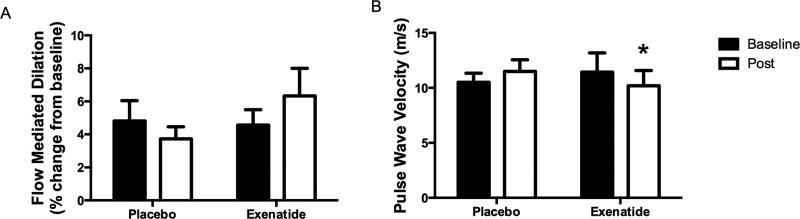
Baseline brachial artery diameters were not different between the groups at the beginning of the study (Placebo: 4.4 ± 0.4 vs. Exenatide 4.1 ± 0.2 mm; P = 0.581) and they were not changed during the treatment period (Placebo: 4.3 ± 0.4 vs. Exenatide 4.1 ± 0.3 mm; P = 0.610). Absolute change in brachial artery diameter following cuff deflation was also not different at the beginning of the study (Placebo: 0.192 ± 0.046 vs. Exenatide 0.193 ± 0.045 mm; P = 0.428) or following the treatment period (Placebo: 0.151 ± 0.026 vs. Exenatide 0.226 ± 0.057 mm; P = 0.393). Finally, there were no differences between groups in relative brachial artery FMD at the beginning of the study (P = 0.391). Three months of placebo administration decreased FMD by 22.6% from the baseline response (Figure 1), whereas it increased by 38.8% with exenatide treatment, however, these findings did not attain statistical significance (P = 0.179).
Figure 1.
Effect of exenatide on vascular outcomes. (A) Flow mediated dilation and (B) pulse wave velocity before and following three months of placebo or exenatide administration. Data are Mean ± Standard Error. *P < 0.05 Different compared with placebo at same time point.
3.8 Arterial Stiffness
Pulse wave velocity was not different at baseline between the two groups. Treatment with exenatide resulted in a decreased (improved) pulse wave velocity compared with placebo (Figure 1; P = 0.037) indicating a reduction in arterial stiffness.
4. DISCUSSION
We found that exenatide, a GLP-1 receptor agonist, treatment significantly improved the individual cardiovascular parameters of arterial stiffness and diastolic cardiac function in treated patients over a 3 month period. These statistically significant cardiac and vascular findings may have relevance to recent clinical investigations that report decreased all-cause and cardiovascular mortality and decreased incidence of myocardial infarction with other GLP-1 receptor agonists in patients with T2DM (liraglutide (Marso & Daniels, 2016) and semaglutide (Steg & Roussel, 2016)). However, in spite of improved cardiovascular parameters, we saw no significant improvement in peak VO2 or VO2 kinetics with administration exenatide. In the context of the cardiovascular improvements, the absence of change in VO2peak is unexpected in light of our current understanding of the determinants of aerobic exercise capacity.
With regards to the management of T2DM, exenatide treatment reduced body mass, BMI, and lowered both blood fasting glucose and HbA1C. Fasting insulin was not impacted by administration of exenatide; however, HOMA-β did improve consistent with an improvement in pancreatic β-cell function. GLP-1 receptor agonists do not directly target insulin signaling but improving glucose homeostasis often results in better insulin action (Kim & Egan, 2008).
T2DM is a leading cause of CVD and mortality (Beckman et al., 2002); one factor potentially associated with the excess CVD in T2DM is the well-established impairment in functional exercise capacity in patients compared with healthy controls (Schneider et al., 1984; Kjaer et al., 1990; Regensteiner et al., 1995). Exercise capacity is a potent predictor of cardiovascular and all-cause mortality (Wei et al., 1999; 2000). The causes of the exercise abnormalities in T2DM are not fully elucidated, although recent work from our group demonstrates associations between insulin resistance, endothelial dysfunction, and cardiac dysfunction (including circumferential strain) with decreased exercise capacity in this population (Regensteiner et al., 1995; 1998; Brandenburg et al., 1999; Bauer et al., 2007; Bjornstad et al., 2016). Further, impairments in submaximal VO2 and VO2 kinetics observed in patients with T2DM are similar to impairments identified in disease states with impaired oxygen delivery (Hansen et al., 1987), suggesting the association of reduced oxygen delivery to skeletal muscle in T2DM and the impairment in aerobic exercise tolerance. The insulin secretogogue GLP-1 augments blood flow to skeletal muscle via increases in endothelial and cardiac function in T2DM (Ceriello et al., 2006; Mafong & Henry, 2009; Chai et al., 2014; Koska et al., 2015); this mechanism could theoretically lead to improved exercise capacity and reduced CVD risk.
Oxygen consumption and, therefore exercise capacity, can be described in the context of the Fick Principle, in which skeletal muscle VO2 is the product of blood flow and oxygen extraction. Considering our previous data suggesting the strong correlation between endothelial and diastolic function and exercise capacity in patients with T2DM (Regensteiner et al., 1998; Brandenburg et al., 1999; Bauer et al., 2007), we hypothesized that exenatide administration would augment exercise capacity in participants with T2DM by increasing blood flow to skeletal and cardiac muscle. It is well established that GLP-1 improves blood flow to skeletal muscle via increases in endothelial and cardiac function in T2DM (Ceriello et al., 2006; Mafong & Henry, 2009; Chai et al., 2014). Improvements in endothelial function, examined in rodents in vivo and ex vivo, suggest a direct effect of GLP-1 on endothelial function via eNOS activity (Nyström, 2008). This improvement in endothelial function observed in experimental models translates to an observed reduction in blood pressure in human clinical trials (Mafong & Henry, 2009). Our findings in the present study are in agreement with these previously reported data (Ceriello et al., 2006; Mafong & Henry, 2009; Chai et al., 2014). First, following three months of exenatide treatment, pulse wave velocity was slowed; indicating that treatment with exenatide decreases arterial stiffness. Second, brachial artery FMD, a measure of endothelial function, tended to increase with exenatide administration, although this finding did not reach statistical significance. Despite these changes, exenatide did not increase VO2peak in the current study.
Specific cardiac improvements associated with GLP-1 include the in vitro finding of increasing the cAMP concentration in cardiac myocytes (Vila Petroff et al., 2001), and improved left ventricular function in humans, rodents and dogs (Luque, 2002; Nikolaidis et al., 2004b; 2004a; Nikolaidis, 2005; Zhao et al., 2006; Sokos et al., 2007; Mafong & Henry, 2009). One study of particular interest reported that exenatide used in a porcine model of ischemia and reperfusion not only reduced infarct size but also prevented deterioration of systolic and diastolic cardiac function (Timmers et al., 2009). Data from the current study support a positive role of GLP-1 on cardiac function; resting lateral and septal E:E’ decreased with exenatide administration, indicating an improvement in left ventricular diastolic function, while these ratios worsened in the placebo group. These significant changes were small, but over an increased duration of time, they could have major clinical implications.
The improvements in arterial stiffness and diastolic function in the context of the current investigation would be expected to lead to an increase in improved blood flow to skeletal muscle augmenting the delivery of oxygen to exercising skeletal muscle. However, VO2peak was unchanged by exenatide treatment. We have generated two hypotheses that could explain the lack of improvement in VO2peak. It is possible that the primary limiting factor for peak VO2 in T2DM may be oxygen extraction; therefore, a potential augmentation of skeletal muscle blood flow alone would not improve VO2peak. Another possibility is that homeostatic adaptation to exenatide treatment occurred: i.e. improved cardiovascular function presumably increased skeletal muscle blood flow in this current investigation and oxygen extraction may have decreased because there was no demand (e.g. exercise training). Together, these possibilities suggest that changes to skeletal muscle oxygen utilization, the second component of the Fick Principle, may be needed concomitantly with the exenatide-associated augmentation of blood flow to improve exercise capacity in patients with T2DM. These possibilities will need further investigation to determine their merit.
Previous data support the rationale that pharmacological targeting of impaired exercise capacity could augment exercise capacity in T2DM patients (Regensteiner et al., 2005; Kadoglou et al., 2008). In the current investigation, exenatide administration improved cardiovascular function, a limiting factor of exercise capacity in T2DM, without increasing exercise capacity. Our data suggest the possibility that exenatide treatment would be more likely to improve exercise capacity if a concomitant stimulus, such as aerobic exercise training. Several previous investigations have determined that independently mimicking exercise adaptation without an actual training stimulus (i.e. pharmacological manipulation of blood flow or skeletal muscle protein upregulation) does not augment functional exercise capacity. For example, administration of a peroxisome proliferator-activated receptor delta (PPARδ) agonist increased several indicators of skeletal muscle adaptation to endurance training in mice, however, time to fatigue and distance at fatigue were no different than for controls (Narkar et al., 2008). Similarly, acute treatment with a beta-2 adrenergic receptor agonist did not improve maximal exercise capacity in humans despite increasing heart rate and stroke volume (Beloka et al., 2011). Finally, muscle-specific overexpression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), an important regulator of mitochondrial biogenesis and adaptation to endurance exercise training, did not augment functional exercise capacity in response to endurance training (Wong et al., 2015). Our own data in rodents using saxagliptin, a pharmaceutical that potentiates circulating GLP-1 by preventing its degradation, demonstrated no change in running distance with saxagliptin alone but a >50% augmentation of the exercise training response (Keller et al., 2014), highlighting the potential for GLP-1 to target cardiovascular function and augment the response to an exercise stimulus. These previous reports, and the data from the current investigation, suggest that in order to improve exercise capacity, the additional stimulus of exercise training could be needed to potentiate the impact of pharmacologic effects of this type of agent on exercise capacity. Future investigations of exenatide and functional exercise capacity should include an aerobic exercise intervention.
Additional components of the study design warrant further discussion. As mentioned previously, administration of exenatide reduced arterial stiffness and improved diastolic function although exercise capacity did not change. This investigation would have been complemented by a measure of in vivo and/or ex vivo mitochondrial function to better determine the root of the unaffected exercise capacity in light of improved cardiovascular function. Further, measures of leg blood flow and/or skeletal muscle blood flow might have better linked the cardiovascular improvements with skeletal muscle function. Finally, the current investigation was not powered to determine sex-specific responses to exenatide. Consideration of sex-specific responses in future experimental designs could provide valuable insight regarding exenatide treatment.
In conclusion, administration of exenatide improved glycemic control and two assessments of diastolic cardiac function and reduced arterial stiffness, however, these beneficial changes were not accompanied by improvements in VO2peak in participants with T2DM. Lowered exercise capacity and increased mortality are closely related in the diabetic population, indicating a need to develop strategies to improve exercise tolerance. It is intriguing to hypothesize, based on the improvements in cardiac and vascular function observed, that the combination of exenatide with aerobic exercise training in participants with T2DM is warranted.
Acknowledgments
JGR, JEBR, AGH, TAB, SM, LH, SG, JD conducted the study. RLS analyzed data and wrote the manuscript. LH, SM, and SG collected data. KLM, CO, AGH, TAB, and JD collected and analyzed data. JEBR and JGR initiated the project, analyzed data, and wrote the manuscript. All authors contributed to the revision of the manuscript for important intellectual content and approved the final version of the manuscript for publication. JGR and JEBR are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We would like to thank the participants in the study who played such an important role. We thank Ian Leavitt, MS and Katie Rogers, MS (Department of Medicine, University of Colorado School of Medicine, Division of General Internal Medicine) for their role in helping to finalize the data analysis. Amylin Pharmaceuticals, a wholly owned subsidiary of AstraZeneca, supported this investigator-initiated study. Other sources of funding for this study were provided by NIH-K23HL118133 (AGH), NIH-5T32HL007171 (RLS), NIH-T32ST63009794 (CO), VA Merit, Denver Research Institute, CCTSI-UL1RR025780 (JEBR, JGR), ADA 1-12-CT-64 (JGR), the Center for Women’s Health Research (JGR, JEBR).
Footnotes
Clinicaltrials.gov identifier: NCT01364584
References
- Bauer TA, Regensteiner JG, Brass EP, Hiatt WR. Oxygen uptake kinetics during exercise are slowed in patients with peripheral arterial disease. J Appl Physiol. 1999;87:809–816. doi: 10.1152/jappl.1999.87.2.809. [DOI] [PubMed] [Google Scholar]
- Bauer TA, Reusch JEB, Levi M, Regensteiner JG. Skeletal Muscle Deoxygenation After the Onset of Moderate Exercise Suggests Slowed Microvascular Blood Flow Kinetics in Type 2 Diabetes. Diabetes Care. 2007;30:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Beloka SP, Janssen C, Woff E, Brassine E, Deboeck G, Randria J, Philippart de Foy V, van de Borne P, Naeije R. Effects of β2-adrenergic stimulation on exercise capacity in normal subjects. Eur J Appl Physiol. 2011;11:2239–2247. doi: 10.1007/s00421-011-1856-9. [DOI] [PubMed] [Google Scholar]
- Bjornstad P, Truong U, Dorosz JL, Cree Green M, Baumgartner A, Coe G, Pyle L, Regensteiner JG, Reusch JEB, Nadeau KJ. Cardiopulmonary Dysfunction and Adiponectin in Adolescents With Type 2 Diabetes. J Am Heart Assoc. 2016;5:e002804. doi: 10.1161/JAHA.115.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22:1640–1646. doi: 10.2337/diacare.22.10.1640. [DOI] [PubMed] [Google Scholar]
- Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Davidson J, Hanefeld M, Leiter L, Monnier L, Owens D, Tajima N, Tuomilehto JInternational Prandial Glucose Regulation Study Group Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis. 2006;16:453–456. doi: 10.1016/j.numecd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Chai W, Zhang X, Barrett EJ, Liu Z. Glucagon-Like Peptide 1 Recruits Muscle Microvasculature and Improves Insulin’s Metabolic Action in the Presence of Insulin Resistance. Diabetes. 2014;63:2788–2799. doi: 10.2337/db13-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JE, Sue DY, Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol. 1987;59:669–674. doi: 10.1016/0002-9149(87)91190-8. [DOI] [PubMed] [Google Scholar]
- Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, Sahn DJ, Schiller NB, Tajik A, Teichholz LE, Weyman AE. Report of the American Society of Echocardiography Committee on Nomenclature and Standards in Two-dimensional Echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- Huebschmann AG, Kohrt WM, Herlache L, Wolfe P, Daugherty S, Reusch JE, Bauer TA, Regensteiner JG. Type 2 diabetes exaggerates exercise effort and impairs exercise performance in older women. BMJ Open Diabetes Res Care. 2015;3:e000124. doi: 10.1136/bmjdrc-2015-000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebschmann AG, Reis EN, Emsermann C, Dickinson LM, Reusch JEB, Bauer TA, Regensteiner JG. Women with type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl Physiol Nutr Metab. 2009;34:851–857. doi: 10.1139/H09-074. [DOI] [PubMed] [Google Scholar]
- Kadoglou NPE, Iliadis F, Angelopoulou N, Perrea D, Liapis CD, Alevizos M. Beneficial effects of rosiglitazone on novel cardiovascular risk factors in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25:333–340. doi: 10.1111/j.1464-5491.2007.02375.x. [DOI] [PubMed] [Google Scholar]
- Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JEB. Saxagliptin Restores Vascular Mitochondrial Exercise Response in the Goto-Kakizaki Rat. J Cardiovasc Pharmacol. 2014;65:134–147. doi: 10.1097/FJC.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M, Hollenbeck CB, Frey-Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. Journal of Applied Physiology. 1990;68:2067–2074. doi: 10.1152/jappl.1990.68.5.2067. [DOI] [PubMed] [Google Scholar]
- Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, Truran S, Franco DA, Schwartz EA, Schwenke DC, D’Alessio D, Migrino RQ, Reaven PD. Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes. 2015;64:2624–2635. doi: 10.2337/db14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque M. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J Endocrinol. 2002;173:465–473. doi: 10.1677/joe.0.1730465. [DOI] [PubMed] [Google Scholar]
- Mafong DD, Henry RR. The role of incretins in cardiovascular control. Curr Hypertens Rep. 2009;11:18–22. doi: 10.1007/s11906-009-0005-x. [DOI] [PubMed] [Google Scholar]
- Marso SP, Daniels GH. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. European Journal of Endocrinology. 2009;160:909–917. doi: 10.1530/EJE-09-0101. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang Y-X, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis LA. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen Y-T, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004a;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004b;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- Nyström T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res. 2008;40:593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JEB. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care. 2005;28:2877–2883. doi: 10.2337/diacare.28.12.2877. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Huebschmann AG, Herlache L, Weinberger HD, Wolfel EE, Reusch JEB. Sex differences in the effects of type 2 diabetes on exercise performance. Medicine & Science in Sports & Exercise. 2015;47:58–65. doi: 10.1249/MSS.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol. 1998;85:310–317. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, Oliver-Pickett CK, Reusch JE, Weil JV. Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vascular Medicine. 2003;8:169–175. doi: 10.1191/1358863x03vm489oa. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Medicine & Science in Sports & Exercise. 1995;27:875–881. [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1984;26:355–360. doi: 10.1007/BF00266036. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH, Elahi D, Shannon RP. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg PG, Roussel R. Randomized Trials to Evaluate Cardiovascular Safety of Antihyperglycemic Medications. Circulation. 2016;134:571–573. doi: 10.1161/CIRCULATIONAHA.116.021914. [DOI] [PubMed] [Google Scholar]
- Timmers L, Henriques JPS, de Kleijn DPV, Devries JH, Kemperman H, Steendijk P, Verlaan CWJ, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–452. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low Cardiorespiratory Fitness and Physical Inactivity as Predictors of Mortality in Men with Type 2 Diabetes. Ann Intern Med. 2000;132:605. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Ralph S, Paffenbarger J, Blair SN. Relationship Between Low Cardiorespiratory Fitness and Mortality in Normal-Weight, Overweight, and Obese Men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- Wong KE, Mikus CR, Slentz DH, Seiler SE, DeBalsi KL, Ilkayeva OR, Crain KI, Kinter MT, Kien CL, Stevens RD, Muoio DM. Muscle-Specific Overexpression of PGC-1α Does Not Augment Metabolic Improvements in Response to Exercise and Caloric Restriction. Diabetes. 2015;64:1532–1543. doi: 10.2337/db14-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]