Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease that causes dry skin and functional disruption of the skin barrier. AD is often accompanied by allergic inflammation. AD patient suffer from heavy itching, and their quality of life is severely affected. Some pharmaceuticals for AD have some side effects such as skin atrophy. So it is necessary to develop mild solutions such as food ingredients without side effects. There are various causes of AD. It is especially induced by immunological imbalances such as IFN-γ reduction. IFN-γ has an important role in regulating IgE, which can cause an allergy reaction. NC/Nga mice develop AD and IgE hyperproduction. In a previous study, we revealed that administration of polysaccharide from black currant (R. nigrum) has an effect on immunomodulation. It induces IFN-γ production from myeloid dendritic cells. We named this polysaccharide cassis polysaccharide (CAPS). In this report, we studied the effect of administering CAPS on atopic dermatitis in NC/Nga mice. Thirty NC/Nga mice that developed symptoms of atopic dermatitis were used. We divided them into three groups (control, CAPS administration 12 mg/kg/day, CAPS administration 60 mg/kg/day). For 4 weeks, we evaluated clinical score, serum IgE levels, gene expression of spleen, and skin pathology. We revealed that CAPS administration improves atopic dermatitis symptoms. We also found that CAPS administration suppresses IgE hyperproduction and induces IFN-γ gene transcription in the spleen. Finally, we confirmed that CAPS administration suppresses mast cell migration to epidermal skin. These results indicated that CAPS has an effect on AD.
Keywords: black currant, polysaccharide, atopic dermatitis, NC/Nga mice
INTRODUCTION
Atopic dermatitis (AD) is one of the serious skin diseases in childhood [1], and most patients have a family history of atopy or IgE hyperproduction [2, 3]. IgE activates mast cells, which release various inflammatory mediators [4]. They disrupt skin tissues, which are therfore unable to prevent inflammatory cells from invading into skin lesions [5]. As a result, AD becomes a chronic inflammatory skin disease, which causes dry skin, functional disruption of the skin barrier, and serious itching [6]. AD elicits thickening of the epidermis and accumulation of inflammatory cells like mast cells in the epidermis, and it induces chronic inflammation [7]. AD is induced by an immunological imbalance of Th-1/Th-2 type cytokines such as IFN-γ [8]. IFN-γ regulates inflammation, and mast cells and Th2 type T cells can produce the cytokines related to IgE production [9].
There are some AD pharmaceuticals, such as steroid [10], that have been found to be occasionally associated with anthropogenic side effects [11]. So it is very important to find efficient component to improve atopic dermatitis with no side effect.
Black currant is well known as a healthy fruit and is used to make various foods and beverages, such as jams and juices. There are various reports about the beneficial effects of black currant on human health [12], vasodilatation [13], and eyestrain [14] and also its effect as an antivirus agent [15]. These properties are mainly due to the anthocyanins in black currant. In previous studies, we found immunostimulating effects of polysaccharides derived from black currant [16,17,18] and revealed its antitumor activity and effect on IFN-γ production in a mouse study [16, 17]. We named this polysaccharide as cassis polysaccharide (CAPS). We also investigated whether CAPS stimulated myeloid dendritic cells through myeloid differentiation primary response gene 88 (Myd88) depending on TLR4 signaling (data not shown). In this report, we investigated whether CAPS administration has an effect on atopic dermatitis and IgE hyperproduction.
MATERIALS AND METHODS
Sample preparation
Ribes nigrum was purchased from Central Chemical Co., Ltd. (Tokyo, Japan). Crude black currant juice was obtained, and glucanase enzymes were added: 0.01% Sclase S (Mitsubishi-chmical Foods Corporation, Tokyo, Japan) and 0.01% Viscozyme (Novozymes Japan Ltd., Chiba, Japan). After incubation at 48°C for 5 hr, inactivation was carried out at 80°C for 10 min. Then, the supernatant was recovered by centrifugation (10,000 g for 10 min). Ethanol precipitation was performed with a double volume of black currant juice, and the precipitant was collected by centrifugation (10,000 g for 10 min) and finally dissolved in a moderate volume of phosphate-buffered saline (PBS). Ethanol precipitation was repeated three times before lyophilization. Finally, the precipitant was dried with a freeze dryer. We obtained 13.5 g of freeze-dried CAPS powder from 3,454 g black currant juice; its average molecular weight (MW) was 27,643. CAPS powder does not contain endotoxins. The dietary fiber content was 828 mg in 1 g of CAPS powder.
Endotoxin measurement
Endotoxin were analyzed with an Endozyme Endotoxin Test Kit (Hyglos GmbH, Bernried am Starnberger See, Germany), according to the manufacturer’s instructions.
Molecular weight distribution
The MW of CAPS was analyzed by gel-filtration chromatography on an HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with a Shodex OHpak SB-805 HQ column (Showa Denko, Tokyo, Japan) equilibrated with PBS at a flow rate of 1 ml/min. The detection was performed with an RID-20A Refractive Index Detector (Shimadzu Corporation, Kyoto, Japan). A calibration curve was prepared with a dextran standard solution consisting of T-2000 (MW: 2,000,000), T-500 (MW: 473,000), T-70 (MW: 67,200), T-40 (MW: 43,000), T-10 (MW: 10,000), saccharose (MW: 342), and glucose (MW: 180). These markers were purchased from Pharmacosmos (Holbaek, Denmark).
Dietary fiber quantification
The dietary fiber content of CAPS was measured with the Proskey method by Japan Food Research Laboratories.
Mice
Specific pathogen-free male NC/Nga mice (4 weeks) were purchased from Japan SLC, Inc. (Shizuoka, Japan). The mice were housed under a 12:12-h light/dark cycle in a thermoregulated room maintained at 23 ± 3.0°C. Humidity was maintained in the range of 45 ± 10%, and food (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and water were provided. The animals were treated in accordance with LSI Medience Corporation’s ethical guidelines for animal care, handling, and termination.
Experimental design
Mice (7 weeks) were allocated to three groups of 10 mice and fed normal diets after confirmation of atopic dermatitis symptoms. The test samples were administered daily via oral administration. Water was administered to the control group. CAPS dissolved in distilled water was orally administered once a day throughout the experimental period at a dose of 12 or 60 mg/kg. Dermatitis symptoms were evaluated by the clinical severity score at 0, 7, 14, 21, and 28 days after the first administration of test samples. The clinical severity score was evaluated by a previous method [19]. The score was defined as the sum of the individual scores graded as 0 (normal), 1 (mild), 2 (moderate), 3 (severe), or 4 (much severe). After 28 days, the animals were anesthetized with pentobarbital, and blood was collected via the abdominal aorta with heparinized syringes. To obtain splenocytes from the spleen of NC/Nga mice, spleens were pressed between two sterile glass microscope slides to release cells. Cells (1.0 × 106 cells/ml) were ultimately cultured at 37°C in 5% CO2 in air for 2 days in RPMI1640 medium supplemented with 1 mM sodium pyruvate (Thermo Fisher Scientific, Yokohama, Japan), 2.5 mM HEPES (Thermo Fisher Scientific, Yokohama, Japan), 100 unit/ml penicillin (Thermo Fisher Scientific, Yokohama, Japan), 100 µg/ml streptomycin (Thermo Fisher Scientific, Yokohama, Japan), and 10% fetal calf serum.
Measurement of IgE level
The levels of serum IgE were measured with a Ready-SET-Go!® ELISA kit (eBiosciences Inc., San Diego, CA, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was purified with a QIAshredder homogenizer and an RNeasy Mini Kit (QIAGEN Japan, Tokyo, Japan) according to the manufacturer’s instructions. cDNA was synthesized with a PrimeScript II 1st strand cDNA Synthesis kit (Takara Bio Inc., Kusatsu, Shiga, Japan). The expression levels were monitored using SYBR Premix Ex Taq II (Perfect Real Time; Takara Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. The cDNA mixture (250 ng) was added to 20 µl reaction mixture. The primers were as follows: GAPDH (tctgctgatgcccccatgttcg and tgggtggcagtgatggcatgga), TNF-α (ctgtagcccacgtcgtagc and tgagatccatgccgttg), IFN-γ (tggaggaactggcaaaaggatggtg and cgctggacctgtgggttgttgacct), IL-12p40 (tctgctgatgcccccatgttcg and tgggtggcagtgatggcatgga), and IL-4 (acgccatgcacggagatggatg and ccaggcatcgaaaagcccgaaa). Reactions were performed using a LightCycler 480 (Roche Diagnostics, Mannheim, Germany). Samples were quantified by comparison with a standard curve generated by cDNA templates using the respective primers. The value for the target gene was divided by the value for the GAPDH gene, and relative gene expressions were normalized by the value for the control.
Histopathological study
The skins of the animals were fixed in 10% buffered formalin solution and decalcified in 10% formic acid. A block of skin was removed and embedded in paraffin by the conventional method, and it was then stained with hematoxylin-eosin and toluidine blue. We counted the number of mast cells that were stained by toluidine blue per 1 mm2 with a microscope.
Statistics
Data were expressed as the mean ± standard deviation. Statistical significance was assessed by Steel’s test. The statistical software used was SAS 9.1.3. (SAS Institute Japan, Tokyo, Japan) or Ekuseru-Toukei 2010 (Social Survey Research Information Co., Ltd., Tokyo, Japan). P values less than 0.05 were considered statistically significant.
RESULTS
Effects of CAPS administration on atopic dermatitis
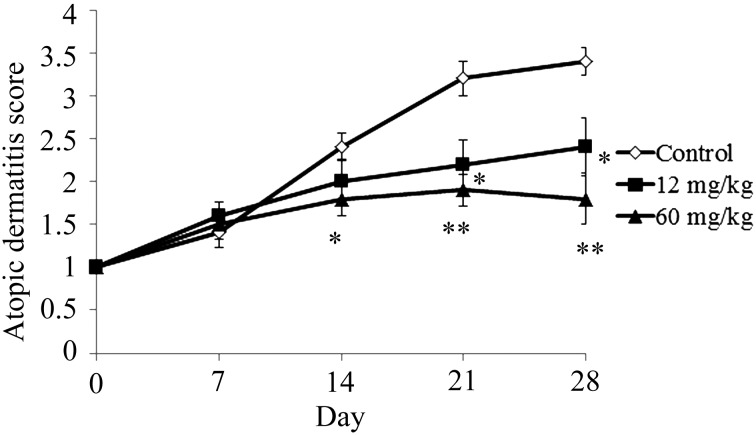
NC/Nga mice with no superficial dermatitis (male, 7 weeks) were used in this study. In the control group, the atopic dermatitis score increased gradually after commencement of the study (Fig. 1). The atopic dermatitis scores of the 12 mg/kg CAPS administration group were significantly lower than those of the control group at 21 days and 28 days (Fig. 1). The atopic dermatitis scores of the 60 mg/kg CAPS administration group were significantly lower than control group at 14, 21, and 28 days.
Fig. 1.
Effect of CAPS on atopic dermatitis score.
The results are expressed as mean ± standard error (n=10). The differences between the control and the CAPS administration groups were statistically significant by the Steel test (*p<0.05, **p<0.01).
Effect of CAPS administration on serum IgE level
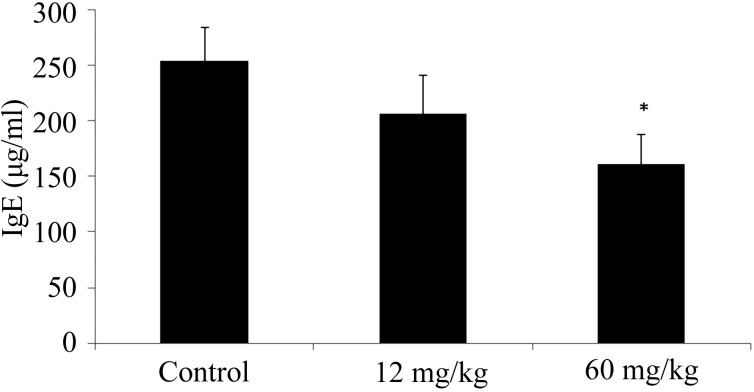
The serum IgE level of the 12 mg/kg CAPS administration group was lower compared with that of the control group (Fig. 2). The serum IgE level of the 60 mg/kg CAPS administration group was significantly lower compared with that of the control group (Fig. 2).
Fig. 2.
Effect of CAPS on serum IgE levels.
The results are expressed as mean ± standard error (n=10). The differences between the control and the CAPS administration groups were statistically significant by the Steel test (p<0.05).
RT-PCR of splenocytes
The IFN-γ and IL-12p40 transcription level of the 12 and 60 mg/kg groups were significantly increased compared with those of the control (Fig. 3). The TNF-α transcription level of the 60 mg/kg group was significantly increased compared with that of the control (Fig. 3). The IL-4 transcription level of the 12 and 60 mg/kg group were significantly decreased compared to control (Fig. 3).
Fig. 3.
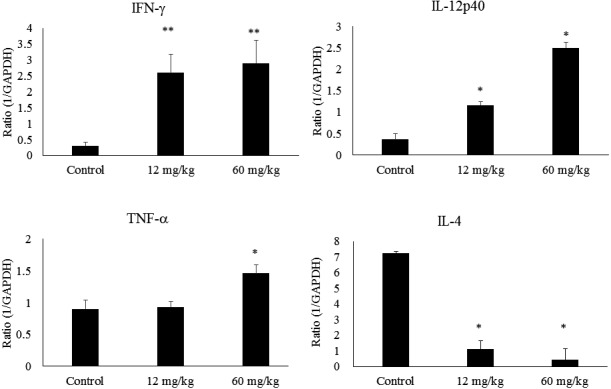
Transcription levels of INF-γ, IL-12p40, TNF-α, and IL-4.
The results are expressed as mean ± standard error (n=10). The differences between the control and the CAPS administration groups were statistically significant by the Steel test (*p<0.05, **p<0.01).
Histopathological study
In the control group, skin ulcer thickening of the epidermis and infiltration of many kinds of cells into the dermis were observed (Fig. 4). In the sections stained by toluidine blue for mast cells detection, infiltration of mast cells into the dermis was clearly observed in the control group (Fig. 5). The control group had the highest number of mast cells in the epidermis, and the 60 mg/kg CAPS administration group showed a significantly decreased number of mast cells in the epidemis (Fig. 6).
Fig. 4.
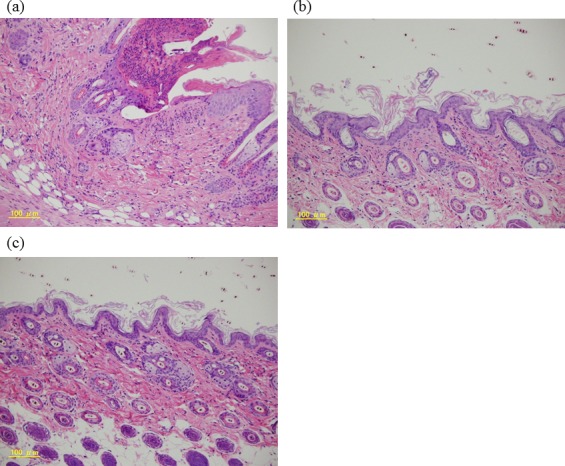
Skin lesion analysis.
Histological features of skin lesions stained with hematoxylin-eosin; (a) control, (b) 12-mg/kg CAPS, (c) 60-mg/kg CAPS.
Fig. 5.
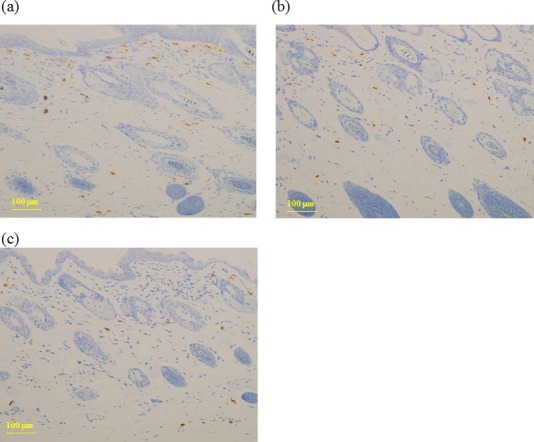
Mast cell detection.
Histological features of skin lesions stained with toluidine blue; (a) control, (b) 12-mg/kg CAPS, (c) 60-mg/kg CAPS.
Fig. 6.
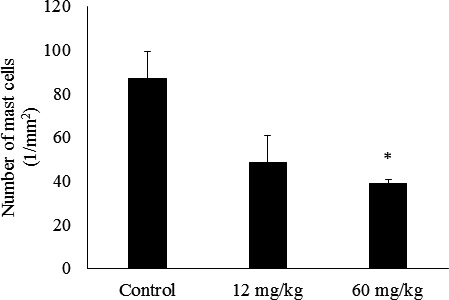
Effect of CAPS administration on mast cells in the dermis of NC mice (n=4).
The results are expressed as mean ± standard error (n=4). The differences between the control and the CAPS administration groups were statistically significant by the Steel test (p<0.05).
DISCUSSION
There are no previous reports about the effect of black currant on atopic dermatitis. In the present study, we demonstrated for the first time that black currant dietary fiber has an effect on atopic dermatitis. Furthermore, we confirmed that efficacy of CAPS administration on AD-like skin lesions in NC/Nga mice (Fig. 1).
In the control group, the final clinical score was worsened compared with the initial one (Fig. 1). We ensured that atopic dermatitis development in this experiment, as NC/Nga mice are infected with bacteria under conventional breeding conditions and are affected by AD [20, 21]. In the CAPS administration groups, their clinical scores were significantly improved compared with those of the control group in a concentration-dependent manner. From these results, we determined that CAPS administration has an effect on atopic dermatitis.
Generally, AD-like skin lesions are related to serum IgE hyperproduction [6]. In the present study, the serum IgE levels of the CAPS administration groups were significantly decreased compare with those of the control group in a concentration-dependent manner (Fig. 2). IgE is related to activation of mast cells and to releases of inflammatory mediators [6]. The numbers of mast cells in the CAPS administration groups were decreased compared with that of the control group (Fig. 6). We assumed that mice in the control group had an imbalance in their immune systems and that it was caused by inflammatory mediators. The possibly resulted in disruption of the skin barrier.
We confirmed that CAPS administration induced IFN-γ and IL-12p40 mRNA in the spleen (Fig. 3). IFN-γ regulates IgE production [9], and IL-12p40 is a component of IL-12, which has a role in controlling IFN-γ production [22]. These results indicate that CAPS induces Th-1 type cytokines such as IL-12 and IFN-γ and that they might suppress IgE hyperproduction. In a previous study, we confirmed that CAPS administration stimulates Th-1 type cytokines in DCs through Myd-88 depending on TLR4 signaling [19]. TLR4 signaling induces Th-1 type cytokines such as IFN-γ and IL-12 [23]. We also confirmed that CAPS administration lowered IL-4 transcription. IL-4 is the key cytokine of Th-2 type cytokines [24]. These results support our hypothesis.
We assumed that CAPS administration stimulates Th-1 type cytokines (IFN-γ, IL-12), which causes suppression of IgE hyperproduction; as a result, it might have an effect on AD symptoms. Black currant fruits contain a large amount of CAPS, and black currant is a safe food that has been eaten by people for a long time. We strongly believe that CAPS has the potential to be a health beneficial food for AD without side effects.
We compared immunostimulating efficacies of fruits in a previous study [16]. Thirty-seven fruits were compared in an in vitro study, and CAPS was found to have the strongest immunostimulating effect. Thus, we assumed that CAPS is the most effective fruit polysaccharide for atopic dermatitis.
In the above study, we also researched the chemical components of CAPS [16]. CAPS contain a large amount of galactose, and it contains a galacto-oligosaccharide structure. Generally, galacto-oligosaccharide has effect on prebiotics [25]. Thus, CAPS might have an effect on prebiotics.
In conclusion, we investigated the inhibitory effect of oral administration of CAPS on the development of AD-like skin lesions in NC/Nga mice. Ingestion of CAPS prevented the development of dermatitis and the increase of IgE hyperproduction in a dose-dependent manner. IFN-γ and IL-12p40 transcription levels in the spleen were increased by CAPS administration. The number of mast cells in skin lesions decreased after CAPS ingestion. These results clearly demonstrate that intake of CAPS is effective in preventing and alleviating the development of AD-like skin disease.
Conflict of Interest
HA, GW, YK, YK, RY, RT, MM, and TY are employees of Kirin Co., Ltd., the study sponsor. KS is an employee of Mercian Corporation.
References
- 1.Uehara M, Kimura C. 1993. Descendant family history of atopic dermatitis. Acta Derm Venereol 73: 62–63. [DOI] [PubMed] [Google Scholar]
- 2.Van Bever HP. 1992. Recent advances in the pathogenesis of atopic dermatitis. Eur J Pediatr 151: 870–873. [DOI] [PubMed] [Google Scholar]
- 3.Amin K. 2012. The role of mast cells in allergic inflammation. Respir Med 106: 9–14. [DOI] [PubMed] [Google Scholar]
- 4.Gaspari AA. 2005. Multiple pathways driving IgE production and chronic dermatitis in mice: a model for atopic dermatitis? J Invest Dermatol 124: xi–xii. [DOI] [PubMed] [Google Scholar]
- 5.Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. 2000. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol 142: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY. 2000. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol 105: 860–876. [DOI] [PubMed] [Google Scholar]
- 7.Alenius H, Laouini D, Woodward A, Mizoguchi E, Bhan AK, Castigli E, Oettgen HC, Geha RS. 2002. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol 109: 106–113. [DOI] [PubMed] [Google Scholar]
- 8.Reinhold U, Pawelec G, Wehrmann W, Herold M, Wernet P, Kreysel HW. 1988. Immunoglobulin E and immunoglobulin G subclass distribution in vivo and relationship to in vitro generation of interferon-gamma and neopterin in patients with severe atopic dermatitis. Int Arch Allergy Appl Immunol 87: 120–126. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski TF, Fitch FW. 1988. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol 140: 4245–4252. [PubMed] [Google Scholar]
- 10.Smith EW. 1995. Four decades of topical corticosteroid assessment. Curr Probl Dermatol 22: 124–131. [PubMed] [Google Scholar]
- 11.Assmann T, Homey B, Ruzicka T. 2001. Topical tacrolimus for the treatment of inflammatory skin diseases. Expert Opin Pharmacother 2: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 12.Gopalan A, Reuben SC, Ahmed S, Darvesh AS, Hohmann J, Bishayee A. 2012. The health benefits of blackcurrants. Food Funct 3: 795–809. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Matsumoto H, Todoki K. 2002. Endothelium-dependent vasorelaxation induced by black currant concentrate in rat thoracic aorta. Jpn J Pharmacol 89: 29–35. [DOI] [PubMed] [Google Scholar]
- 14.Knox YM, Suzutani T, Yosida I, Azuma M. 2003. Anti-influenza virus activity of crude extract of Ribes nigrum L. Phytother Res 17: 120–122. [DOI] [PubMed] [Google Scholar]
- 15.Iida H, Nakamura Y, Matsumoto H, Kawahata K, Koga J, Katsumi O. 2013. Differential effects of black currant anthocyanins on diffuser- or negative lens-induced ocular elongation in chicks. J Ocul Pharmacol Ther 29: 604–609. [DOI] [PubMed] [Google Scholar]
- 16.Takata R, Yamamoto R, Yanai T, Konno T, Okubo T. 2005. Immunostimulatory effects of a polysaccharide-rich substance with antitumor activity isolated from black currant (Ribes nigrum L.). Biosci Biotechnol Biochem 69: 2042–2050. [DOI] [PubMed] [Google Scholar]
- 17.Takata R, Yanai T, Yamamoto R, Konno T. 2007. Improvement of the antitumor activity of black currant polysaccharide by an enzymatic treatment. Biosci Biotechnol Biochem 71: 1342–1344. [DOI] [PubMed] [Google Scholar]
- 18.Dejima K, Ohshima A, Yanai T, Yamamoto R, Takata R, Yoshikawa T. 2007. Effects of polysaccharide derived from black currant on relieving clinical symptoms of Japanese cedar pollinosis: a randomized double-blind, placebo-controlled trial. Biosci Biotechnol Biochem 71: 3019–3025. [DOI] [PubMed] [Google Scholar]
- 19.Ashigai H, Komano Y, Wang G, Kawachi Y, Sunaga K, Yamamoto R, Takata R, Miyake M, Yanai T. 2017. Polysaccharide from black currant (Ribes nigrum L.) stimulates dendritic cells through TLR4 signaling. Biosci Microb Food Health 36: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. 1999. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol 120Suppl 1: 70–75. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C. 1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9: 461–466. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Itakura A, Tanaka A, Fujisawa C, Matsuda H. 2001. Inability of IL-12 to down-regulate IgE synthesis due to defective production of IFN-gamma in atopic NC/Nga mice. J Immunol 167: 5955–5962. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Chung DH. 2012. TLR4-mediated IL-12 production enhances IFN-γ and IL-1β production, which inhibits TGF-β production and promotes antibody-induced joint inflammation. Arthritis Res Ther 14: R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totsuka A, Omori-Miyake M, Kawashima M, Yagi J, Tsunemi Y. 2017. Expression of keratin 1, keratin 10, desmoglein 1 and desmocollin 1 in the epidermis: possible downregulation by interleukin-4 and interleukin-13 in atopic dermatitis. Eur J Dermatol 27: 247–253. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane GT, Steed H, Macfarlane S. 2008. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104: 305–344. [DOI] [PubMed] [Google Scholar]