Abstract
A total of 149 lung cancer patients were recruited to receive intensity modulated radiation therapy (IMRT). The association of developing radiation pneumonitis (RP) with genetic polymorphism was evaluated. The risks of four polymorphic sites in three DNA repair related genes (ERCC1, rs116615:T354C and rs3212986:C1516A; ERCC2, rs13181:A2251C; XRCC1, rs25487:A1196G) for developing grade ≥ 2 RP were assessed respectively. It was observed that ERCC1 T354C SNP had a significant effect on the development of grade ≥ 2 RP (CT/TT vs. CC, adjusted HR = 0.517, 95% CI, 0.285–0.939; adjusted P = 0.030). It is the first time demonstrating that CT/TT genotype of ERCC1 354 was significantly associated with lower RP risk after radio therapy.
Keywords: radiation pneumonitis, ERCC1, ERCC2, XRCC1, lung cancer
INTRODUCTION
Radiation therapy currently plays a crucial role in the treatment of lung cancer, primarily because of both its radical and palliative cure [1]. However, radiation pneumonitis (RP), which has been identified as the most significant dose-limiting toxicity with 5%–36% of patients experiencing serve symptom (grade 2–5 RP) [2–3] is one of major obstacles for clinical application of radio therapy, and radiation pneumonitis even has been suggested by previous studies as an independent negative prognostic factor for the survival of lung cancer patients [4]. Many factors, including age, sex, smoking, radiation dose, irradiated lung volume, surgery and chemotherapy, are often used to estimate RP risk [5–7]. Currently in clinical use, dosimetric parameters, such as the volume of the lung that received 20 Gy radiation dose (V20) and median lung dose (MLD) are important determinants of radiotherapy-induced lung toxicity [8–10]. Unfortunately, consideration of only these factors is definitely insufficient to evaluate the risk of developing RP, and several relative studies have suggested that RP has a genetic basis [11–13]. Currently, there is an increasing consensus that RP is considered as a so-called complex, inherited polygenic trait. Single-nucleotide polymorphisms (SNPs) have been proposed as a potential predictive biomarker for the development of RP. SNPs in inflammation, DNA repair, stress response and angiogenesis-related genes were proved to be associated with RP, with different underlying mechanisms [14].
Radiotherapy exerts its effects on damaging cells primarily either directly, by attacking cellular macromolecules, including genomic DNA, or indirectly, by generating reactive oxygen species and subsequent byproducts. Both types of mechanisms result in extensive DNA damage and cell-death [15]. Hence, repair of radiation-induced DNA damage is considered as one of the most important cellular defense mechanisms against radiation pressure [16]. SNPs may change protein total level and/or functional activities and thereby impact DNA repair capacity [17]. To date, there are four major pathways have been discovered for repairing DNA lesion, as follows: base excision repair (BER); nucleotide excision repair (NER); DNA double-strand break repair by homologous recombination (HR) and non-homologous end joining (NHEJ).
Nucleotide excision repair (NER) as one of the most important mechanisms for repairing has been used by cells to repair a wide variety of DNA structural lesions, including bulky adducts, cross-links, oxidative DNA damages, thymidine dimmers and alkylating damages [18]. Excision repair cross-complimentary group 1 and group 2 (ERCC1 and ERCC2), both located in the chromosome 19q13.2-13.3, are two key rate-limiting enzymes and are indispensable for NER. X-ray repair cross-complementing group 1 (XRCC1) belongs to BER system, which is responsible for the removal of single damaged DNA bases and for efficient repair of DNA single-strand breaks generated extensively by radiation therapy [19].
The aim of this study was to evaluate the possible relationship between several common genetic polymorphisms in DNA repair genes (ERCC1, ERCC2 and XRCC1) and the risk of developing RP in lung cancer patients receiving intensity modulation radiated therapy.
RESULTS
Patient characteristics and clinical outcomes
Table 1 displays the characteristics of 149 lung cancer patients including 127 (85.2%) males and 22 (14.8%) females. The median age was 60 years (range from 24 to 84 years old). Histological distribution was 30.2% squamous cell carcinoma (45 cases), 18.8% adenocarcinoma (28 cases), 38.5% small cell cancer (57 cases) and 12.8% others (19 cases). The majority of patients (65.1%) appeared stage III disease, 28.2% presented with stage IV disease, and 6.7% had stage I–II disease. Smokers accounted for 71.1% (106 cases) of the total. 25 (16.8%) patients were concomitant with chronic obstructive pulmonary disease. Notably, 13 (9.4%) patients had experienced surgery before radiation. Most of patients (89.9%) were treated with a combination of chemotherapy or target drugs therapy with radiotherapy. 84.6% patients received radiation with dose above 60Gy (median : 60Gy; range from 30 to 72Gy). The median values for MLD, V20 and V5 were 13.8Gy (range from 4.2 to 34.9), 22% (range from 5.0 to 35.5) and 58% (range from 15.0 to 92.7), respectively. The median follow-up time was 9 months (range from 3 to 24 months) after radiotherapy. Of the 149 patients, 10 cases (6.7%) developed RP of grade 3 or higher level, 57 (38.3%) developed RP of grade ≥ 2 RP, and 90 (62.7%) of grade 1 or lower level. The median time of grade ≥ 2 RP was 2.6 months (range from 1 to 6 months).
Table 1. Patient demographics and clinical information (N = 149).
| Parameter (Variable) |
All Patients (n = 149) |
Grade 0-1RP (n = 92) |
Grade ≥ 2RP (n = 57) |
|---|---|---|---|
| Sex | |||
| Male | 127 (85.2%) | 82 (89.1%) | 45 (78.9%) |
| Female | 22 (14.8%) | 10 (10.9%) | 12 (21.1%) |
| KPS | |||
| < 80 | 24 (16.1%) | 13 (14.1%) | 11 (19.3%) |
| ≥ 80 | 125 (83.9%) | 79 (85.9%) | 46 (80.7%) |
| Smoking History | |||
| No | 43 (28.9%) | 26 (28.3%) | 17 (29.8%) |
| Yes | 106 (71.1%) | 66 (71.7%) | 40 (70.2%) |
| COPD | |||
| No | 124 (83.2%) | 80 (87.0%) | 44 (77.2%) |
| Yes | 25 (16.6%) | 12 (13.0%) | 13 (12.8%) |
| Tumor Histology | |||
| Squamous cell carcinoma | 45 (30.2%) | 27 (29.3%) | 18 (31.6%) |
| Adenocarcinoma | 28 (18.8%) | 20 (21.7%) | 8 (14.0%) |
| Small cell lung cancer | 57 (38.2%) | 33 (35.9%) | 24 (42.1%) |
| Others | 19 (12.8%) | 12 (13.1%) | 7 (12.3%) |
| Stage | |||
| I–II | 10 (6.7%) | 6 (6.5%) | 4 (7.0%) |
| III | 97 (65.1%) | 59 (64.1%) | 38 (66.7%) |
| IV | 42 (28.2 %) | 27 (29.4%) | 15 (26.3%) |
| Surgery | |||
| NO | 136 (90.6%) | 85 (92.4%) | 51 (89.5%) |
| YES | 13 (9.4%) | 7 (7.6%) | 6 (10.5%) |
| Chemotherapy | |||
| No | 15 | 7 (7.6%) | 8 (14.0%) |
| Yes | 134 | 85 (92.4%) | 49 (86.0%) |
| Median age(range)(y) | 60 (24–84) | 59 (24–84) | 62 (35–80) |
| Median Radiation dose(range)(Gy) | 60 (30–72Gy) | 60 (32–70) | 61.6 (30–72) |
| Bilateral Lung dose-volumehistogram | |||
| Median V5 (range) | 58.0% (15.0–92.7%) | 58.0% (15.0–92.7%) | 58.5% (29.0–91.9%) |
| Median V20 (range) | 22.0% (5.0–35.5%) | 21.0% (5.0–29.6%) | 23.5% (6.0–35.5%) |
| Median MLD (range) | 13.8Gy (4.2–34.9Gy) | 13.6Gy (5.3–22.4Gy) | 14.2Gy (4.2–34.9Gy) |
Abbreviations: RP = radiation pneumonitis; KPS = Karnofsky performance status; COPD = chronic obstructive pulmonary disease; V5 = volume of normal lung receiving 5Gy or more radiation; V20 = volume of normal lung receiving radiation of 20 Gy or more; MLD = mean lung dose.
Table 2 shows genotype distribution of the four detected SNPs. The rs11615 site (ERCC1, T354C) consisted of 57.0% cases of CC genotype, 40.3% cases of CT and 2.7% cases of TT genotypes respectively. Meanwhile, the rs3212986 (ERCC1, C1516A) locus, CC, CA and AA genotype carriers were 69 cases (45.6%), 65 cases (44.3%) and 15 cases (10.1%), respectively. Rs25487 (XRCC1, A1196G) comprised 58.4% GG, 34.9% GA and 6.7% AA genotype, respectively. In terms of rs13181 (ERCC2, A2251C), 124 (83.2%) were AA, and 25 (16.8%) were AC genotype carriers, but no any CC genotype were detected due to the extremely low frequency (almost lower than 1% of homozygous variants) of genotype in Chinese population, in consistent with the previous literature data [20–22]. All the other allele frequencies observed in the study were similar to those previously reported in Chinese population [23–26].
Table 2. Genotype frequency of gene polymorphisms in this study.
| Single-Nucleotide Polymorphism |
All Patients (n = 149) |
Grade 0-1RP (n = 92) |
Grade ≥ 2RP (n = 57) |
|---|---|---|---|
| ERCC1 rs11615 (T354C; Asn118Asn) | |||
| CC | 85 (57.0%) | 48 (52.2%) | 37 (64.9%) |
| CT | 59 (40.3%) | 42 (45.6%) | 17 (29.8%) |
| TT | 5 (2.7%) | 2 (2.2%) | 3 (5.3%) |
| CT or TT | 64 (43.0%) | 44 (47.8%) | 20 (35.1%) |
| ERCC1 rs3212986 (C1516A; Gln504Lys) | |||
| CC | 69 (46.3%) | 42 (46.7%) | 27 (47.4%) |
| CA | 65 (43.6%) | 39 (42.4%) | 26 (45.6%) |
| AA | 15 (10.1%) | 11 (11.9%) | 4 (7.0%) |
| CA or AA | 80 (53.7%) | 50 (53.3%) | 30 (52.6%) |
| ERCC2 rs13181 (A2251C; Lys751Gln) | |||
| AA | 124 (83.2%) | 78 (84.8%) | 46 (80.7%) |
| AC | 25 (16.8%) | 14 (15.2%) | 11 (19.3%) |
| CC | 0 | NC | NC |
| AC or CC | 25 (16.8%) | 14 (15.2%) | 11 (19.3%) |
| XRCC1 rs25487 (A1196G; Arg1196Gln) | |||
| GG | 87 (58.4%) | 51 (55.4%) | 36 (63.2%) |
| GA | 52 (34.9%) | 34 (37.0%) | 18 (31.6%) |
| AA | 10 (6.7%) | 7 (7.6%) | 3 (5.2%) |
| GA or AA | 62 (41.6%) | 41 (44.6%) | 21 (36.8%) |
Abbreviations: ERCC1 =excision repair cross-complementing group 1; ERCC2 = excision repair cross-complementing group 2; XPD = xeroderma pigmentosum group D; XRCC1 = X-ray repair cross-complementing group 1; Ile = isoleucine; Val = valine; Ala = alanine; Gln = glutamine; Lys =lysine; Arg = arginine.
Association of clinical variables and RP
We assessed the associations of developing grade ≥ 2 RP with parameters such a as therapy-related factors, including age, sex , races and Karnofsky Performance Score (KPS), tumor histology, disease stage, smoking history, COPD history, surgery history, usage of chemotherapy, radiation dose, V20, V5 and MLD by univariate and multivariate Cox regress analyses as presented in Table 3. We found that only V20 had statistically significant association with RP (grade ≥ 2) via both univariate and multivariate analyses (Table 3. bottom two lines), concordant with the results from a recent meta-analysis [27]. No other clinical factors were observed to be associated with RP risk in the study population.
Table 3. Association between clinical factors and RP (grade ≥ 2) incidence.
| Parameter | Patients | Number of | Percent of | Univariate Analysis | Multivariate Analysisa | ||||
|---|---|---|---|---|---|---|---|---|---|
| (Variable) | RPs (n = 57) | Patients with RP | HR | 95% CI | p | AHR | 95% CI | p | |
| Sex | |||||||||
| Male | 127 | 45 | 35.4% | 1.0 | 1.0 | ||||
| Female | 22 | 12 | 54.5% | 1.370 | 0.725–2.590 | 0.333 | 1.888 | 0.605–5.891 | 0.274 |
| Age (years) | |||||||||
| < 60 | 71 | 24 | 33.8% | 1.0 | 1.0 | ||||
| ≥ 60 | 78 | 33 | 42.3% | 1.339 | 00.791–2.266 | 0.277 | 2.517 | 1.167–5.430 | 0.019 |
| KPS | |||||||||
| < 80 | 24 | 11 | 45.8% | 1.0 | 1.0 | ||||
| ≥ 80 | 125 | 46 | 36.8% | 0.622 | 0.343–1.279 | 0.220 | 0.935 | 0.393–2.222 | 0.879 |
| Smoking Status | |||||||||
| NO | 43 | 17 | 39.5% | 1.0 | 1.0 | ||||
| YES | 106 | 40 | 37.7% | 1.092 | 0.619–1.927 | 0.761 | 1.690 | 0.622–4.590 | 0.304 |
| COPD | |||||||||
| No | 124 | 44 | 35.5% | 1.0 | 1.0 | ||||
| Yes | 25 | 13 | 52.0% | 1.621 | 0.872–3.013 | 0.127 | 1.043 | 0.400–2.724 | 0.931 |
| Tumor characteristics | |||||||||
| Histology | |||||||||
| Squamous cell carcinoma | 45 | 18 | 40.0% | 1.0 | 1.0 | ||||
| Adenocarcinoma | 28 | 8 | 28.6% | 0.643 | 0.279–1.478 | 0.298 | 0.800 | 0.264–2.420 | 0.800 |
| Small cell lung cancer | 57 | 24 | 42.1% | 0.978 | 0.531–1.803 | 0.943 | 1.244 | 0.560–2.765 | 0.591 |
| Others | 19 | 7 | 36.8% | 0.898 | 0.375–2.151 | 0.810 | 1.368 | 0.465–4.02 | 0.569 |
| Stage | |||||||||
| I–II | 10 | 4 | 40.0% | 1.0 | 1.0 | ||||
| III | 97 | 38 | 39.2% | 1.101 | 0.393–3.087 | 0.854 | 0.909 | 0.232–3.558 | 0.891 |
| IV | 42 | 15 | 35.7% | 0.941 | 0.312–2.838 | 0.914 | 0.967 | 0.233–4.008 | 0.963 |
| Surgery | |||||||||
| NO | 136 | 51 | 37.5% | 1.0 | 1.0 | ||||
| YES | 13 | 6 | 46.2% | 1.193 | 0.512–2.781 | 0.683 | 3.048 | 0.959–9.686 | 0.059 |
| Chemotherapy | |||||||||
| NO | 15 | 8 | 53.3% | 1.0 | 1.0 | ||||
| YES | 134 | 49 | 36.6% | 0.729 | 0.345–1.541 | 0.408 | 0.549 | 0.204–1.475 | 0.234 |
| Radiation dose | |||||||||
| < 60 | 23 | 7 | 30.4% | 1.0 | 1.0 | ||||
| ≥ 60 | 126 | 50 | 39.7% | 1.435 | 0.650–3.186 | 0.372 | 1.080 | 0.283–4.114 | 0.910 |
| Bilateral Lung dose-volume histogram | |||||||||
| V5 < 58% | 75 | 28 | 37.3% | 1.0 | 1.0 | ||||
| V5 ≥ 58% | 74 | 29 | 39.2% | 1.161 | 0.691–1.952 | 0.573 | 1.133 | 0.403–3.186 | 0.813 |
| V20 < 20% | 56 | 14 | 25.0% | 1.0 | 1.0 | ||||
| V20 ≥ 20%<25% | 53 | 24 | 45.3% | 2.043 | 1.057–3.950 | 0.034 | 2.971 | 1.085–8.133 | 0.034 |
| V20 ≥ 25% | 40 | 19 | 47.5% | 2.182 | 1.094–4.354 | 0.027 | 5.810 | 1.391–24.27 | 0.016 |
| MLD < 13.8Gy | 74 | 24 | 32.4% | 1.0 | 1.0 | ||||
| MLD ≥ 13.8Gy | 75 | 33 | 44.0% | 1.372 | 0.811–2.321 | 0.239 | 1.605 | 0.666–3.872 | 0.292 |
Abbreviations: RP = radiation pneumonitis; HR = hazard ratio; KPS = Karnofsky performance status; COPD = chronic obstructive pulmonary disease; V5 = volume of normal lung receiving 5Gy or more radiation; V20 = volume of normal lung receiving 20 Gy or more radiation; MLD = mean lung dose.
a: Multivariate analyses were adjusted for all factors listed in this table.
RP and genotype association
Table 4 shows the results of univariate and multivariate analyses of the correlation between the genetic polymorphisms and grade ≥ 2 RP via the Cox proportional hazards regression model. We observed that rs11615 SNP (ERCC1, T354C) was significantly associated with RP risk. Compared with CC genotype, the variant CT and TT genotypes were associated with decreased hazards of RP in the multivariate model (CT and TT vs. CC; HR = 0.465 & 0.517; 95% CI = 0.261–0.921 & 0.285–0.939; adjusted P = 0.027 & 0.030, respectively). Meanwhile, in case of rs25487 (XRCC1, A1196G), compared with the GG genotype, patients carrying GA or AA variants did not show significantly decreased RP risk (HR, 0.670, 95% CI, 0.367–1.225; adjusted P = 0.194; or HR, 0.649, 95% CI, 0.167–2.517; adjusted P = 0.531) respectively. When combination of GA or GG genotypes were considered, comparing with GG carriers, a slightly reduced but with no statistical significance risk was observed (HR, 0.667, 95% CI, 0.377–1.182; adjusted P = 0.166). There was no significant relationship between RP and other genetic polymorphisms were discovered.
Table 4. Associations between genotypes and grade ≥ 2 RP.
| Parameter | Patients | Number of | Percent of | Univariate Analysis | Multivariate Analysisa | ||||
|---|---|---|---|---|---|---|---|---|---|
| (Variable) | RPs (n = 57) | Patients with RP | HR | 95% CI | p | AHR | 95% CI | p | |
| ERCC1 rs11615 (T354C; Asn118Asn)b | |||||||||
| CC | 85 | 37 | 43.5% | 1.0 | 1.0 | ||||
| CT | 59 | 17 | 28.8% | 0.531 | 0.299–0.943 | 0.031 | 0.465 | 0.261–0.921 | 0.027 |
| TT | 5 | 3 | 60.0% | 1.130 | 0.348–3.665 | 0.839 | 0.759 | 0.186–3.096 | 0.701 |
| CT or TT | 64 | 20 | 31.2% | 0.576 | 0.334–0.994 | 0.048 | 0.517 | 0.285–0.939 | 0.030 |
| ERCC1 rs3212986 (C1516A; Gln504Lys)b | |||||||||
| CC | 69 | 27 | 39.1% | 1.0 | 1.0 | ||||
| AC | 65 | 26 | 40.0% | 1.049 | 0.612–1.798 | 0.861 | 0.906 | 0.481–1.708 | 0.761 |
| CC | 15 | 4 | 36.7% | 0.639 | 0.224–1.827 | 0.403 | 0.774 | 0.251–2.388 | 0.656 |
| AC or CC | 80 | 30 | 37.5% | 0.966 | 0.574–1.626 | 0.897 | 0.869 | 0.422–1.789 | 0.703 |
| ERCC2 (XPD) rs13181 (A2251C; Lys751Gln)b | |||||||||
| AA | 124 | 46 | 37.1% | 1.0 | 1.0 | ||||
| AC | 25 | 11 | 44.0% | 1.241 | 0.643–2.397 | 0.520 | 1.151 | 0.559–2.371 | 0.703 |
| CC | 0 | NC | NC | NC | NC | NC | NC | NC | NC |
| AC or CC | 25 | 11 | 44.0% | 1.241 | 0.643–2.397 | 0.520 | 1.151 | 0.559–2.371 | 0.703 |
| XRCC1 rs25487 (A1196G; Gln399Arg)b | |||||||||
| GG | 87 | 36 | 41.4% | 1.0 | 1.0 | ||||
| GA | 52 | 18 | 34.6% | 0.732 | 0.416–1.290 | 0.281 | 0.670 | 0.367–1.225 | 0.194 |
| AA | 10 | 3 | 30.0% | 0.653 | 0.201–2.122 | 0.479 | 0.649 | 0.167–2.517 | 0.531 |
| GA or AA | 62 | 21 | 33.9% | 0.720 | 0.420–1.233 | 0.231 | 0.667 | 0.377–1.182 | 0.166 |
Abbreviations: RP = radiation pneumonitis; HR = hazard ratio; ERCC1 = excision repair cross-complementing group 1; ERCC2 = excision repair cross- complementing group 2; XPD = xeroderma pigmentosum group D; XRCC1 = X-ray repair cross- complementing group 1; Lys = lysine; Gln = glutamine; Arg = arginine; NC = not calculated.
a: Multivariate analyses in the table were adjusted for all factors listed in Table 3.
b: ERCC1 rs11615 or ERCC1 rs3212986 or ERCC2 rs13181 or XRCC1 rs25487 was independently entered into a multivariate model that was adjusted for all factors listed in Table 3.
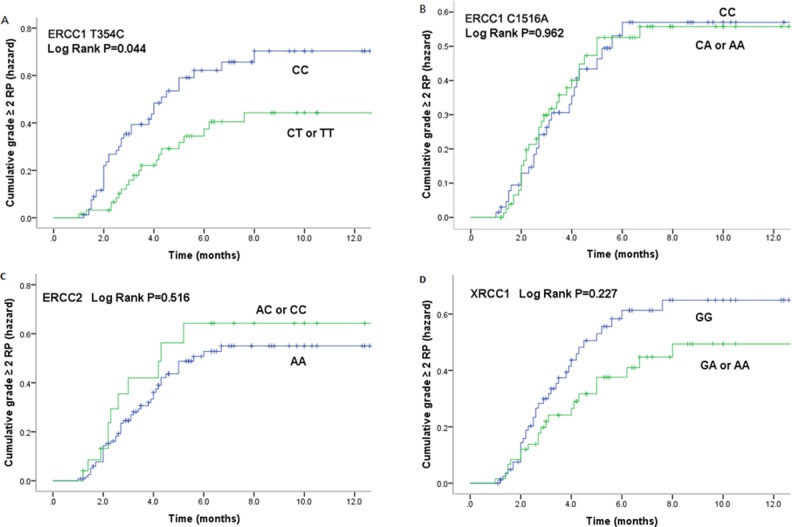
Kaplan-Meier estimates indicated that patients with rs11615 CT/TT variants had significantly lower risk of grade ≥ 2 RP (P = 0.044) (Figure 1A). The cumulative incidence of grade ≥ 2 RP at 3 months for patients with either of these two variants and the CC genotype, which is more common in east Asian population, were 16.4% and 32.5% respectively, and those at 6 months were 33.3% and 48.1% respectively.
Figure 1. Cumulative probability of grade≥2RP as a function of time from the start of radiation therapy by genotypes.
(A). ERCC1 T354C; (B). ERCC1 C1516A; (C). ERCC2 A2251C; (D). XRCC1 A1196G . The CT/TT genotypes of ERCC1 354 were statistically significant associated with a lower incidence of RP compared with CC genotype.
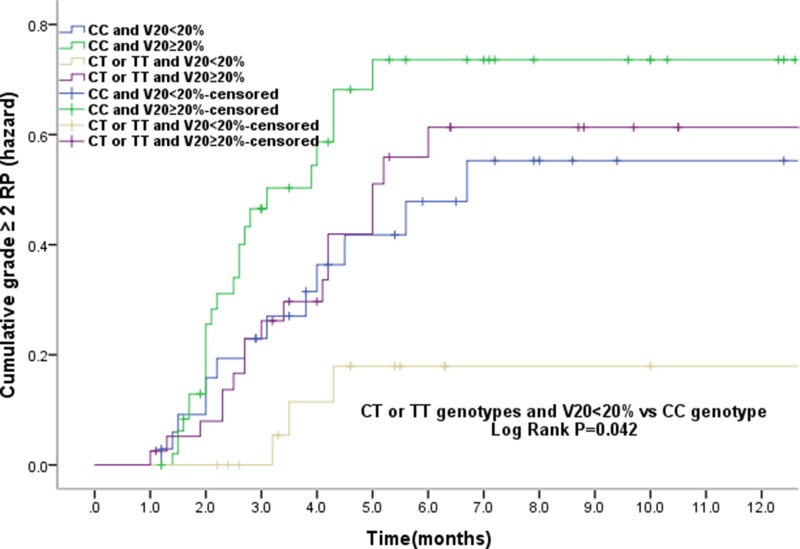
In the study population, V20 was also observed as a significant risk factor of grade ≥ 2 RP. Therefore, we further analyzed the cumulative grade ≥ 2 RP events and the relationship between genotype and V20. The results showed that of all patients who received V20 < 20%, those with rs11615 CT/TT genotypes had a lower RP incidence than CC carriers (P = 0.042). However, in patients with V20 ≥ 20%, no difference was found to be of statistical significance (Figure 2), suggesting that as far as grade ≥ 2 RP risk concerned, the parameter V20 may be independent of genetic susceptibility.
Figure 2. The effects of single nucleotide polymorphism at ERCC1 T354C with volume of normal lung receiving 20Gy or more radiation (V20) < 20% the cumulative incidence of grade ≥ 2RP.
Patients with the CT or TT genotypes of ERCC1 T354C and V20 < 20% had a statistically significant lower incidence of grade ≥ 2RP compared with CC genotype. The associations between ERCC1 T354C and the risk grade ≥ 2RP is independent of the V20.
DISCUSSION
In the study, we assessed the associations of grade ≥ 2 RP events with clinical factors and genetic factors among lung cancer patients underwent IMRT. Our results showed that a genetic polymorphic site, rs11615, which is a functional variant of DNA repair gene ERCC1, may be an independent indicator for the RP risk. Therefore, taking genetic factors into account in clinical practice probably allow us to tailor customized radiotherapy protocols. NER is a versatile and important DNA repair system, and is responsible for repairing bulky DNA damage that includes drug-bound DNA adducts and UV-induced DNA changes [18, 28]. ERCC1, as the rate-limiting and highly conserved key enzyme in the multistep NER process, plays a critical role in the NER system. The function of ERCC1 is essential for maintaining the integrity and stability of genome, removing the copy mistakes, preventing gene mutation and tumorigenesis, and also influencing the level of whole BER repair activity [18]. Evidence suggests polymorphisms in ERCC1, may serve as a useful predictor for response to chemotherapies [28]. Previous reports illustrated that increased expression of ERCC1 might be related to better DNA repair capacity and a worse response to chemotherapy or vice versa [29, 30].
SNPs of ERCC1could affect mRNA expression and have attracted increasing interests as potential predictors of cancer therapy outcome and patient prognosis in lung cancer patients [18–19]. Two most common ERCC1 SNPs are rs11615 and rs3212986. Rs11615 is T→C substitution at exon 4 and this substitution is a synonymous silent polymorphism since resulting no amino acid residue change (Asn→Asn,) [31, 32]. However this variant does affect the transcription of ERCC1 and hence changes mRNA and protein levels. Therefore it also alters sensitivity to platinum-based therapeutics and may impact clinical outcome of patients receiving platinum-based chemotherapy [23, 28, 33]. In this study, we found that patients bearing rs11615 T (TT or CT) variant had a significantly reduced risk to develop RP compared to those carrying CC genotype (adjusted HR = 0.517; 95% CI, 0.285–0.939; adjusted P = 0.030), in consistent with the association in chemotherapeutic response previously reported. The possible reason may be that T allele carriers had elevated ERCC1 mRNA and thereby increased DNA repair activity. To our knowledge, this study is the first time that demonstrated that ERCC1 T354CSNP was significantly associated with RP risk among Chinese patients of ethnic Han with lung cancer administrated with radiotherapy, and implied this SNP as a promising biomarker for predicting normal tissue sensitivity to radiation. In addition, it is interesting that CT/TT genotypes carriers with V20 < 20% had lower risk of RP than those with CC genotype, but no significant genotype association was observed if V20 ≥ 20%. This finding suggests that genetic factors may have played an even more important role in RP susceptibility when a limited volume of lung was exposed to high dose of radiation. In terms of rs3212986 polymorphism, a C→A change in the 3’untranslated region, no functional difference had yet been reported [31]. It was implied to impact the ERCC1 mRNA stability but not influence the mRNA expression [34]. The polymorphism was found not to be related to the chemotherapeutic response but the outcome [20, 35]. The data in this study revealed that rs3212986 SNP had no statistical significant impact on RP risk, similar to that in chemotherapeutic response in previous report [20, 35].
As regarding to ERCC2, it is one of the most important DNA repair proteins and has dual functions in cells: nucleotide excision repair and cell cycle regulation through actions on the Cdk-activating kinase. It plays a crucial role in the NER pathway by recognizing and repairing a wide range of structurally unrelated lesions and oxidative damage. Several reports indicated that ERCC2 was also implicated in repairing ionizing radiation-induced DNA damage [36–37]. One common ERCC2 polymorphism is rs13181 variant, which changes codon at position 751 (a A→C substitution in exon 23) resulting Lys→Gln residue change. The C variant has been identified with a higher DNA adducts or lower DNA repair capacity. Only limited studies revealed no clear association of rs13181 polymorphism with acute or later side effects caused by radiation in breast cancer [38–39]. Instead, a recent study by Zhang et al. concluded that C variant was significantly associated with the risk of acute radiation-induced esophageal toxicity in lung cancer patients [40]. Based on the above facts, in this study we also explored whether rs13181SNP is related to RP risk in lung cancer, but no significant association was observed.
As for XRCC1, it is the first gene that was revealed to affect cell sensitivity to ionizing radiation, oxidative stress, and DNA alkylating agents [41]. The XRCC1 rs25487 variant is one of the most common polymorphism. Recently, Growing attentions have been paid to the association of rs25487 with the risk of normal tissue injuries after radiotherapy. However, the conclusion was ambiguous and even divergent results were reported [38, 39, 42, 43]. Two recent reports revealed the association of rs25487 with the risk of RP. A retrospective study reported by Yin et al, indicated that the G variant of rs25487, after adjusted for potential confounding factors, was associated with a reduced risk of RP in patients with non-small-cell lung cancer patients (HR = 0.48, P = 0.041) [42]. Then Kelsey et al. performed a prospective study to examine the correlation between XRCC1 and radiation-induced lung injuries in lung cancer patients receiving definitive radiotherapy. The result demonstrated that patients with the ancestral allele A were more radiosensitive to radiation (P = 0.01) [43]. In this study, we also found that patients carrying G variant had a lower risk of developing RP (HR, 0.653, 95% CI, 0.342–1.245), which is consistent with previous studies [42–43]. However as far as the population of this study, there was no statistical significance was reached, which agrees with the findings reported by Cheuk et al [44]. The possible reason for this discrepancy may be partly attributed to large ethnic frequency differences of rs25487 alleles.
In conclusion, the study provides the first clinical data that the rs11615 polymorphism in ERCC1 gene may have a predictive value for RP. The results suggest that in addition to the measures of clinical and radiation dosimetric factors, the naturally occurring genetic variants in ERCC1 gene may also be useful to predict the risk of RP. However, the results should be interpreted with caution in clinical practice since multi factors can contribute to the risk of RP, and moreover, further investigations are warranted to confirm these findings.
MATERIALS AND METHODS
Patients
In this prospective study, a group of 149 patients newly diagnosed with lung cancer were treated with definitive radiation between April 2014 and March 2016 at Chinese PLA General Hospital (Beijing, China). The eligible criteria were as follows: histologically or cytologically confirmed lung cancer, including non-small cell lung cancer or small cell lung cancer; no previous and co-existent thoracic radiotherapy; no severe radiotherapy contraindications; Karnofsky performance status (KPS) ≥ 60 scores. Symptom evaluation for each patient was required in advance. Patients’ characteristics and their outcomes were unknown to investigators performing genetic analyses. The results of genotyping were disclosed to clinical investigators after data analyses. This prospective research protocol was approved by the Internal Review Board of Chinese PLA General Hospital (Beijing, China), and conducted in agreement with the Helsinki Declaration. Written informed consent was obtained from all patients before undergoing radiotherapy.
Radiation treatment
All patients received image-guided intensity-modulated radiation therapy (IMRT) with 6-MV photo beam. Patients underwent computed tomography (CT)-based treatment simulation in the supine position and immobilized in an upper body. Then, the images were transmitted to the Pinnacle planning system (version 9.2, Philips), and the target volume and critical normal organs were delineated. The total lung volume was defined as a total lung volume minus tumor. The IMRT plans were generated and optimized via the Pinnacle version 9.2 planning system, and the doses were calculated via the collapsed cone convolution dose algorithm. The plans for each patient were optimized by direct machine parameter optimization according to the target volume and the location of the organs at risk, and V20 was limited to 25–35%. The image-guided IMRT was performed via the two predefined linear accelerators (Elekta Synergy and Varian clinac ix). A total of 30–72Gy dose with the intention of radical or palliative treatment was applied once per day, five times per week. The baseline clinical characteristics and treatment details of the patients are shown in Table 1.
Pulmonary toxicity assessment and follow-up
All patients included in the investigation were examined and evaluated prospectively by their radiation oncologists weekly during radiotherapy and 4–6 weeks after completion of treatment. Patients were then followed up every 3 months for the first 2 years and thereafter every 6 months. Additional visits were required if symptoms appeared. Radiographic examination by chest X-ray or computed tomography was performed at each follow-up visit after completion of radiotherapy. RP was evaluated by at least two radiation oncologists based on clinical symptoms and imaging information of each patient, and graded according to the Common Toxicity Criteria for Adverse Events (CTCAE) version 4 as follows: (1) Grade 0, no change; (2) Grade 1, asymptomatic and only observed by radiographic findings with no intervention indicated; (3) Grade 2, having symptoms limiting instrumental activities of daily living (ADL) and with medical intervention indicated; (4) Grade 3, having severe symptoms limiting self-care ADL and oxygen indicated; (5) Grade 4, having life-threatening respiratory compromise with urgent intervention indicated (e.g. tracheotomy or intubation); (6) Grade 5, resulting in death form severe RP. If the symptoms were present at baseline, worsening of symptoms of at least one grade was considered as RP. The following situations should be excluded when diagnosing the RP: (1) pulmonary infection; (2) thoracic disease progression (PD). The development of grade ≥ 2 RP was defined as the primary end point. The time to endpoint development was calculated from the start of radiation. Patients were censored at the time of last follow-up or death.
DNA extraction and genotyping
The SNPs selected and their dbSNP ID (rs) were as follows: ERCC1 T354C (rs11615), ERCC1 C1516A (rs3212986), ERCC2 A2251C (rs13181) and XRCC1 1196A1196G (rs25487). Blood sample was collected from each patient before radiotherapy. Total genomic DNA from peripheral blood leukocytes was extracted with the Maxwell system (Promega, Madison, WI, USA). The SNP status was analyzed with the SurPlexTM-xTAG platform (Surexam, Guangzhou, China) as described by Zhu et al. including five major steps [45]. Generally, target genes were amplified with PCR and cleaned up with Exonuclease I and shrimp alkaline phosphatase (EXO-SAP) to remove excess nucleotides and primers. Then, the target genes were amplified again with 70 allele-specific primers linked to 70 universal tags. Using these tags, the amplified alleles were hybridized onto beads and analyzed with Luminex. The median fluorescence intensity was measured and analyzed. As to quality control, samples were randomly selected and sent to independent services for DNA sequencing analyses.
Multiplex PCR was performed in a 50 μl volume containing: 1× Ex Taq polymerase buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.2 mM of each primer, 1.0 U Ex Taq HS DNA polymerase (TaKaRa) and 10 ul supernatant of boiling DBS as template DNA. Thermo cycling was performed using 30 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s. The reaction was concluded with a final extension step of 72°C for 10 min and the product was kept at 4°C until use. A similar approach was used to enrich DNA fragments of MTHFR and XPD genes using 3 mM MgCl2. Exonuclease I and shrimp alkaline phosphatase (EXO-SAP) reaction was performed in a 25 μl volume containing: 1× SAP buffer, 1 U shrimp alkaline phosphatase (TaKaRa), 10 U exonuclease I (degrading any remaining PCR primers) (New England Biolabs, Ipswich, USA) and 7.5 μl PCR product. Samples were then incubated at 37°C for 30 min, followed by 20 min incubation at 80°C to inactivate the enzymes. Multiplex ASPE was carried out via 5 μl treated PCR product in a final volume of 20 μl. Each reaction consisted of SAP buffer, 1.25 mM MgCl2, 5 mM biotin-dCTP, 5 mM of dATP, dTTP and dGTP respectively; 0.75 U of Platinum Tsp; and 25 nM ASPE primer pool. The ASPE reactions were incubated at 96°C for 2 min and then subject to 40 cycles at 94°C for 30 s, 52°C for 1 min, and 74°C for 2 min.
Statistical analysis
Patients were categorized according to their genotypes. Cox proportional hazard models were carried out to identify clinical variables and genotypes associated with the risk of RP on univariate and multivariate analyses. Kaplan-Meier analysis was performed to assess the effects of different genotypes on cumulative probability of RP and comparisons were made with the log-rank test. All statistical tests were 2-sided, and P values < 0.05 were of statistically significance. Data analyses was performed via the IBM SPSS Statistics platform (Version 20, SPSS Science, IL, USA).
Acknowledgments
We thank the staff of the participating clinics for their contribution to the data collection, and all the patients who participated in the study.
Footnotes
CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
FUNDING
The study was funded by grants form the National Key Projects of Research and Development of China (2016 YFC 0904600) and the Central Military Commission of the Ministry of Health (16BJZ17).
REFERENCES
- 1.Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM, Tome WA, Chappell RJ, Tolakanahalli R, Mohindra P, Bentzen SM, Cannon GM. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–4348. doi: 10.1200/JCO.2013.51.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor of captopril for mitigation of radiation injury to normal lung. J Radiat Res. 2012;53:633–640. doi: 10.1093/jrr/rrs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Sun J, Zhu J, Li X, Zhen Y, Sui S. Functional dosimetric metrics for predicting radiation-induced lung injury in non-small cell lung cancer patients treated with chenoradiotherpy. Radiat Oncol. 2012;7:69. doi: 10.1186/1748-717X-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farr KP, Kallehauge JF, Moller DS, Khalil AA, Kramer S, Bluhme H, Morsing A, Grau C. Inclusion of functional information from perfusion SPECT improves predictive value of dose–volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: A prospective study. Radiother Oncol. 2015;117:9–16. doi: 10.1016/j.radonc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Das SK, Zhou S, Zhang J, Yin FF, Dewhirst MW, Marks LB. Predicting lung radiotherapy-induced pneumonitis using a model combining parametric Lyman probit with nonparametric decision trees. Int J Radiat Oncol Biol Phys. 2007;68:1212–1221. doi: 10.1016/j.ijrobp.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schallenkamp JM, Miller RC, Brinkmann DH, Foote T, Garces YI. Incidence of radiation pneumonitis after thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410–416. doi: 10.1016/j.ijrobp.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015;25:100–109. doi: 10.1016/j.semradonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J. Predicting risk of radiation-induced lung injury. J Thorac Oncol. 2007;2:864–874. doi: 10.1097/JTO.0b013e318145b2c6. [DOI] [PubMed] [Google Scholar]
- 10.Pang Q, Wei Q, Xu T, Yuan X, Lopez Guerra JL, Levy LB, Liu Z, Gomez DR, Zhuang Y, Wang LE, Mohan R, Komaki R, Liao Z. Functional promoter variant rs2868371 of HSPB1 is associated with risk of radiation pneumonitis after chemoradiation for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1332–1329. doi: 10.1016/j.ijrobp.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, Mao L, Wang XS, Martel M, Komaki R, Cox JD, Milas L, Wei Q. Single nucleotide polymorphism at rs1982073:T869C of the TGF beta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–3378. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi M, Tang Y, Liu B, Li Q, Zhou X, Yu S, Fu S, Cai Y, Yuan X. Genetic variants in the ITGB6 gene is associated with the risk of radiation pneumonitis in lung cancer patients treated with thoracic radiation therapy. Tumour Biol. 2016;37:3469–3477. doi: 10.1007/s13277-015-4171-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Yang M, Bi N, Fang M, Sun T, Ji W, Tan W, Zhao L, Yu D, Lin D, Wang L. ATM polymorphisms are associated with risk of radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 2010;77:1360–1368. doi: 10.1016/j.ijrobp.2009.07.1675. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Xie F, Ouyang X. Predictive SNPs for radiation-induced damage in lung cancer patients with radiotherapy: a potential strategy to individualize treatment. Int J Biol Markers. 2015;30:e1–11. doi: 10.5301/jbm.5000108. [DOI] [PubMed] [Google Scholar]
- 15.Edvardsen H, Kristensen VN, Grenaker Alnaes GI, Bøhn M, Erikstein B, Helland A, Borresen-Dale AL, Fossa SD. Germline glutathione S-transferase variants in breast cancer: Relation to diagnosis and cutaneous long-term adverse effects after two fractionation patterns of radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1163–1171. doi: 10.1016/j.ijrobp.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Popanda O, Marquardt JU, Chang-Claude J, Schmezer P. Genetic variation in normal tissue toxicity induced by ionizing radiation. Mutat Res. 2009;667:58–69. doi: 10.1016/j.mrfmmm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Patrono C, Sterpone S, Testa A, Cozzi R. Polymorphisms in base excision repair genes: Breast cancer risk and individual radiosensitivity. World J Clin Oncol. 2014;5:874–882. doi: 10.5306/wjco.v5.i5.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Z, Su M, Li X, Li M, Ma R, He Q, Zhou B. ERCC2, ERCC1 polymorphisms and haplotypes, cooking oil fume and lung adenocarcinoma risk in Chinese non-smoking females. J Exp Clin Cancer Res. 2009;28:153. doi: 10.1186/1756-9966-28-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isla D, Sarries C, Rosell R, Alonso G, Domine M, Taron M, Lopez-Vivanco G, Camps C, Botia M, Nuñez L, Sanchez-Ronco M, Sanchez JJ, Lopez-Brea M, et al. Single nucleotide polymorphisms and outcome in docetaxelcisplatin-treated advanced non-small-cell lung cancer. Ann Oncol. 2004;15:1194–1203. doi: 10.1093/annonc/mdh319. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Xian L. The association between the ERCC1/2 polymorphisms and the clinical outcomes of the platinum-based chemotherapy in non-small cell lung cancer (NSCLC) - a systematic review and meta- analysis. Tumour Biol. 2014;35:2905–2921. doi: 10.1007/s13277-013-1493-5. [DOI] [PubMed] [Google Scholar]
- 21.Ryu JS, Hong YC, Han HS, Lee JE, Kim S, Park YM, Kim YC, Hwang TS. Association between polymorphisms of ERCC1 and XPD and survival in non-small-cell lung cancer patients treated with cisplatin combination chemotherapy. Lung Cancer. 2004;44:311–316. doi: 10.1016/j.lungcan.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Yuan P, Wu C, Zhang X, Wang F, Guo H, Zhong R, Xu Y, Wu J, Yu D, Wu T, Zhang X, Nie S, et al. Assessment of XPD Lys751Gln and XRCC1 T-77C polymorphisms in advanced non-small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer. 2011;73:110–115. doi: 10.1016/j.lungcan.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Sun X, Sun N, Qin S, Cheng H, Feng J, Chen B, Cheng L, Lu Z, Ji J, Zhou Y. Association between polymorphisms of ERCC1 and XPD and clinical response to platinum-based chemotherapy in advanced non-small cell lung cancer. Am J Clin Oncol. 2010;33:489–494. doi: 10.1097/COC.0b013e3181b9cedc. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Xia J, Li H, Dai J, Hu Y. Association of XRCC1 Variants with Acute Skin Reaction After Radiotherapy in Breast Cancer Patients. Cancer Biother Radiopharm. 2010;25:681–685. doi: 10.1089/cbr.2010.0811. [DOI] [PubMed] [Google Scholar]
- 25.Hao B, Wang H, Zhou K, Li Y, Chen X, Zhou G, Zhu Y, Miao X, Tan W, Wei Q, Lin D, He F. Identification of genetic variants in base excision repair pathway and their associations with risk of esophageal squamous cell carcinoma. Cancer Res. 2004;64:4378–4384. doi: 10.1158/0008-5472.CAN-04-0372. [DOI] [PubMed] [Google Scholar]
- 26.Shen MR, Jones IM, Mohrenweiser H. Non-conservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 27.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting Radiation Pneumonitis after Chemoradiotherapy for Lung Cancer-An International Individual Patient Data Meta- analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Xie X, Ren X, Zhang J. A meta-analysis identifies ERCC1 gene polymorphism as a predictor of better patient response to treatment with radiochemotherapy. Cancer Chemother Pharmacol. 2016;77:1183–91. doi: 10.1007/s00280-016-3015-9. [DOI] [PubMed] [Google Scholar]
- 29.Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, et al. IALT Bio Investigators DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 30.Woelfelschneider A, Popanda O, Lilla C, Linseisen J, Mayer C, Celebi O, Debus J, Bartsch H, Chang-Claude J, Schmezer P. A distinct ERCC1 haplotype is associated with mRNA expression levels in prostate cancer patients. Carcinogenesis. 2008;29:1758–1764. doi: 10.1093/carcin/bgn067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chistiakov DA, Voronova NV, Chistiakov PA. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809–824. doi: 10.1080/02841860801885969. [DOI] [PubMed] [Google Scholar]
- 32.Benhamou S, Sarasin A. ERCC2 /XPD gene polymorphisms and lung cancer: a HuGE review. Am J Epidemiol. 2005;161:1–14. doi: 10.1093/aje/kwi018. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Liu J, Sun M, Zhang Z, Liu C, Sun Y. A Significant Statistical Advancement on the Predictive Values of ERCC1 Polymorphisms for Clinical Outcomes of Platinum-Based Chemotherapy in Non-Small Cell Lung Cancer: An Updated Meta-Analysis. Dis Markers. 2016;2016:7643981. doi: 10.1155/2016/7643981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Wiencke J, Aldape K, Kesler-Diaz A, Miike R, Kelsey K, Lee M, Liu J, Wrensch M. Association of an ERCC1 polymorphism with adult-onset glioma. Cancer Epidemiol Biomarkers Prev. 2000;9:843–847. [PubMed] [Google Scholar]
- 35.Kalikaki A, Kanaki M, Vassalou H, Souglakos J, Voutsina A, Georgoulias V, Mavroudis D. DNA repair gene polymorphisms predict favorable clinical outcome in advanced non-small-cell lung cancer. Clin Lung Cancer. 2009;10:118–23. doi: 10.3816/CLC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- 36.Chang-Claude J, Popanda O, Tan XL, Kropp S, Helmbold I, von Fournier D, Haase W, Sautter-Bihl ML, Wenz F, Schmezer P, Ambrosone CB. Association between polymorphisms in the DNA Repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res. 2005;11:4802–4809. doi: 10.1158/1078-0432.CCR-04-2657. [DOI] [PubMed] [Google Scholar]
- 37.Rzeszowska-Wolny J, Polanska J, Pietrowska M, Palyvoda O, Jaworska J, Butkiewicz D, Hancock R. Influence of polymorphisms in DNA repair genes XPD, XRCC1 and MGMT on DNA damage induced by gamma radiation and its repair in lymphocytes in vitro. Radiat Res. 2005;164:132–140. doi: 10.1667/rr3400. [DOI] [PubMed] [Google Scholar]
- 38.Mangoni M, Bisanzi S, Carozzi F, Sani C, Biti G, Livi L, Barletta E, Costantini AS, Gorini G. Association Between Genetic Polymorphisms in the XRCC1, XRCC3, XPD, GSTM1, GSTT1, MSH2, MLH1, MSH3, and MGMT Genes and Radiosensitivity in Breast Cancer Patients. Int J Radiat Oncol Biol Phys. 2011;81:52–58. doi: 10.1016/j.ijrobp.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Zschenker O, Raabe A, Boeckelmann IK, Borstelmann S, Szymczak S, Wellek S, Rades D, Hoeller U, Ziegler A, Dikomey E, Borgmann K. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol. 2010;97:26–32. doi: 10.1016/j.radonc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Yang M, Bi N, Ji W, Wu C, Tan W, Zhao L, Yu D, Lin D, Wang L. Aasociation of TGF-β1 and XPD polymorphisms with severe acute radiation-induced esophageal toxicity in locally advanced lung cancer patients treated with radiotherapy. Radiother Oncol. 2010;97:19–25. doi: 10.1016/j.radonc.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Luo Z, Yang L, Chen S, Chen C, Lin Z. The association between four SNPs of X-ray repair cross complementing protein 1 and the sensitivity to radiotherapy in patients with esophageal squamous cell carcinoma. Oncol Lett. 2016;11:3508–3514. doi: 10.3892/ol.2016.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin M, Liao Z, Liu Z, Wang LE, Gomez D, Komaki R, Wei Q. Functional polymorphisms of base excision repair genes XRCC1 and APEX1 predict risk of radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:e67–73. doi: 10.1016/j.ijrobp.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelsey CR, Jackson IL, Langdon S, Owzar K, Hubbs J, Vujaskovic Z, Das S, Marks LB. Analysis of single nucleotide polymorphisms and radiation sensitivity of the lung Assessed with an objective radiologic endpoint. Clin Lung Cancer. 2013;14:267–274. doi: 10.1016/j.cllc.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheuk IW, Yip SP, Kwong DL, Wu VW. Association of XRCC1 and XRCC3 gene haplotypes with the development of radiation-induced fibrosis in patients with nasopharyngeal carcinoma. Mol Clin Oncol. 2014;2:553–558. doi: 10.3892/mco.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z, He J, Zeng T, Qin H, Xu J, Ren-Heidenreich L. A multiplexer liquidchip technology for detecting single nucleotide polymorphisms from metabolism of anti-thrombotic drugs in dried blood spots on filter paper. Acta Biochim Biophys Sin (Shanghai) 2014;46:522–525. doi: 10.1093/abbs/gmu028. [DOI] [PubMed] [Google Scholar]