Abstract
Current treatments for rheumatoid arthritis (RA) have long-term side effects such that new treatments are needed that can safely help manage the disease. There is a growing appreciation that GABA receptors (GABA-Rs) on immune cells provide new targets that can be used to modulate immune cell activity. Here, we show for the first time that activation of peripheral GABA-Rs can inhibit the development of disease in the collagen-induced arthritis (CIA) mouse model of RA. Mice that received oral GABA had a reduced incidence of CIA, and those mice that did develop CIA had milder symptoms. T cells from GABA-treated mice displayed reduced proliferative responses to collagen and their APC had a reduced ability to promote the proliferation of collagen-reactive T cells. Thus, GABA downregulated both T-cell autoimmunity and APC activity. Collagen-reactive T cells from GABA-treated mice displayed reduced recall responses in the presence of GABA ex vivo, indicating that GABA consumption did not desensitize these cells to GABA. GABA-treated mice had reduced collagen-reactive IgG2a, but not IgG1 antibodies, consistent with reduced Th1 help. The levels of serum anti-collagen IgG2a antibodies were correlated significantly with the CIA disease scores of individual mice. Our results suggest that activation of peripheral GABA-Rs may provide a new modality to modulate T cell, B cell, and APC activity and help ameliorate RA and other inflammatory diseases.
Keywords: GABA receptor, GABA, rheumatoid arthritis, T cell, APC, anti-collagen antibody, autoimmune
Introduction
GABA receptors (GABA-Rs) are expressed by many neurons in the central nervous system (CNS) as well as other types of cells in the periphery [1]. Our previous studies, and those of others, have showed that GABAA-R subunits are expressed by mouse T cells and APC, as well as by human T cells and monocytes [2–7]. Treatment with GABA inhibited the development of type 1 diabetes (T1D) in nonobese diabetic mice (NOD) [3] and treatment with a GABAA-R ligand ameliorated experimental autoimmune encephalitis (EAE) [6]. Accordingly, GABAA-Rs may be a new class of drug targets for reducing chronic inflammation.
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that affects about 1% of the population [8,9]. T-cell autoimmunity plays a key role in the development and progression of RA. Anti-inflammatory agents and analgesics are useful for treating RA; however, their efficacy and safety remain a concern [10,11]. Therefore, the development of new safe therapeutic reagents that target new pathways and have synergistic beneficial effects with existing treatments will be of great significance in managing RA. While GABA-R modulation can inhibit T1D and EAE, whether it can ameliorate RA is an open question, because RA involves different immune mechanisms and is associated with specific MHC haplotypes.
The collagen-induced arthritis (CIA) model of RA in DBA/1j mice shares many immunological and pathological features with human RA and has been widely used for screening anti-RA drugs (reviewed in [12,13]). We treated collagen-immunized mice with GABA rather than other GABAA receptor agonists that are in clinical use because the latter were developed to pass through the blood–brain barrier and modulate neuronal GABAA receptors in the CNS. In contrast, GABA does not pass through the blood–brain barrier effectively and is safe for human use, making it an excellent candidate for modulating peripheral immune cell function without CNS side effects.
Materials and methods
CIA induction
All experiments were approved by UCLA’s Animal Research Committee. Male DBA/1j mice (Jackson Laboratories, Bar Harbor, ME, USA) 8–10 weeks of age were immunized with 200 µg bovine collagen II (bCII, Chondrex, Redmond, WA, USA) in 50% complete Freund’s adjuvant (CFA) containing 2mg/ml Mycobacterium tuberculosis strain H37Ra (Difco, Detroit, MI, USA) intradermally at the base of the tail and were boosted 21 days later with 100 µg of bovine collagen in incomplete Freund’s adjuvant. Their food and water consumption, body weights, as well as joint inflammation were measured longitudinally.
GABA treatment
After the initial immunization, the mice were provided with plain drinking water or water containing 2 mg/ml of GABA (Sigma Aldrich, St. Louis, MO, USA) for the entire 8-week observation period. The water bottles were changed every 7 days with fresh material. Based on our prior observations that the mice drink 4–5 ml of water per day, each experimental mouse consumed approximately 8–10 mg of GABA daily.
Proliferation assays
Groups of DBA/1j mice were immunized and placed on plain water or water + GABA, as above. Ten days after boosting, their splenic mononuclear cells (5 × 105/well) were challenged in triplicate with bCII peptide p259–273 (GIAGFKGEQGPKGEP, > 95% purity, Biosynthesis) in fetal calf serum (FCS)-free HL-1 medium (in triplicate) for 96 h in the presence or absence of 1mMof GABA. T-cell proliferation was determined by 3H-thymidine (1 µCi) incorporation, as per [2].
Other groups of DBA/1j mice were immunized with 100 µg of bCII peptide in 50% CFA at the base of the tail and given plain water or water + GABA. Nine days later, their popliteal lymph node and splenic mononuclear cells were prepared independently. Splenic APC and lymph node T cells were purified from control and experimental mice by negative selection using anti-CD3 or anti-CD220, anti-Mac1, and anti-CD11c-conjugated magnetic beads (Miltenyi Biotech, San Diego, CA, USA), respectively. Lymph node T cells (1.5 × 105/well) were cultured in triplicate with splenic APCs (5 × 105/well) from control or GABA-treated mice and challenged with the bCII peptide for testing T-cell proliferation. Cells cultured in medium alone or stimulated with anti-CD3 (1 µg/ml) were used as negative and positive controls, respectively.
ELISA analysis of collagen-specific antibodies
The levels of serum collagen-specific IgG, IgG1, and IgG2a antibodies in individual control and experimental mice 8 weeks after the final immunization were characterized by ELISA, as described previously [14,15] using 10 µg/ml bCII as antigen for coating the plates.
Results
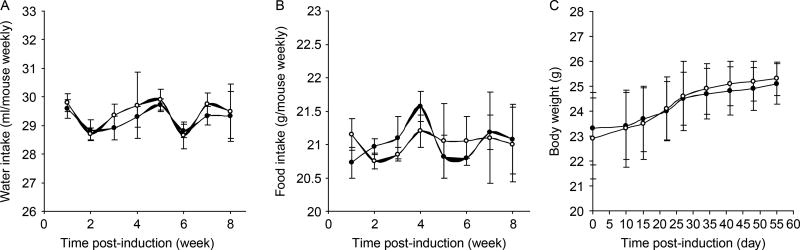
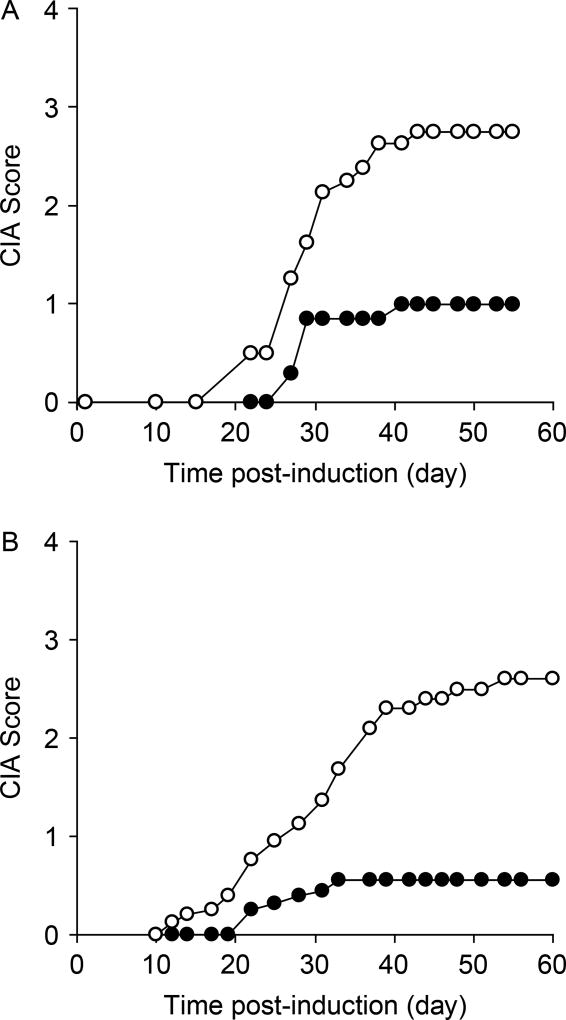
There was no significant difference in food and water consumption, or body weights between the control and GABA groups over the 8-week observation period (Figure 1). Although 7 out of 8 control mice developed CIA, only 4 out of 7 GABA-treated mice developed CIA symptoms (Figure 2(A)). Similarly, in a second independent study, 11 out of 12 control mice developed CIA, but only 6 out of 10 GABA-treated mice displayed CIA symptoms (Figure 2(B), p = 0.03, for combined results by Fisher exact analysis). Importantly, the mean clinical scores in GABA-treated mice were significantly reduced compared with that in controls (Figure 2(A) and (B), p < 0.01 for each study by Student’s t-test). Thus, oral treatment with GABA inhibited the incidence and severity of CIA in mice.
Figure 1.
GABA treatment does not alter mouse consumption of water or food, or their weight. DBA/1j mice were immunized with collagen and fed water (control, open circles) or water containing GABA (black circles). Their (A) water, (B) food consumption, and (C) weights were measured longitudinally. Data shown are mean ± SEM. N = 8 mice/group.
Figure 2.
GABA treatment inhibits the development of CIA. The development of CIA in collagen-immunized mice that were given plain water (open circles), or water containing GABA (black circles), was monitored longitudinally. The CIA scores were recorded as mean of four paw joints of individual mice at each time point. 0, no evidence of erythema or swelling; 1, erythema or mild swelling in the midfoot (tarsals) or ankle joint; 2, erythema and mild swelling extending from ankle to the midfoot; 3, erythema and moderate swelling extending from the ankle to metatarsal joints; and 4, erythema and severe swelling encompassing the ankle, foot, and digits. Graphs show longitudinal mean disease severity scores from two separate studies (N = 8 mice/group for panel A, N = 10–12 mice/group for panel B).
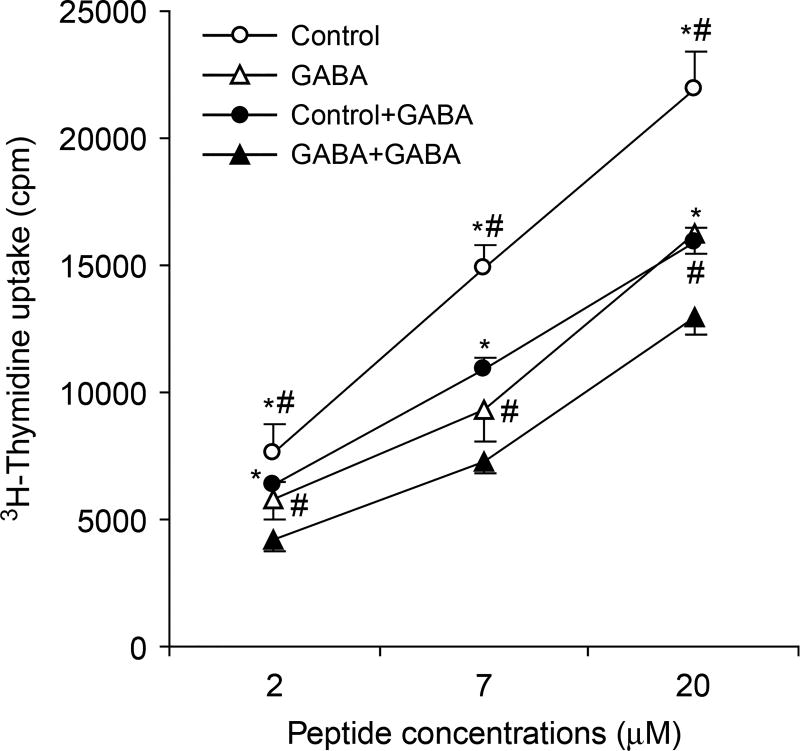
To obtain insights into the mechanism(s) by which GABA treatment inhibited the development of CIA in mice, we tested whether the treatment reduced the development of T-cell responses to collagen. We immunized mice with bCII, provided them with plain water or water + GABA, reboosted them 21 days later, and 10 days later we tested their splenic mononuclear cell responses to the bCII peptide in the presence or absence of GABA (1 mM). We observed that splenic T cells from GABA-treated mice displayed significantly less proliferative responses to the bCII peptide compared to T cells from control mice (Figure 3, p < 0.05 by Student t-test). We observed that inclusion of GABA in the media inhibited the proliferation of bCII-reactive T cells from both control and GABA-treated mice (compared to cultures without GABA) to similar extents (Figure 3). Thus, continual GABA consumption did not desensitize activated T cells to GABA.
Figure 3.
T cells from GABA-treated mice display reduced proliferative responses to collagen and are still sensitive to GABA-mediated inhibition in vitro. Splenic mononuclear cells from control (circles) and GABA-treated (triangles) mice were challenged in triplicate with the indicated concentrations of bCII peptide in the absence (open symbols) or presence (black symbols) of 1mM of GABA. Antigen-stimulated T-cell proliferation was determined by 3H-thymidine incorporation. Data are expressed as mean CPM ± SEM of each group of mice (N = 5 mice per group). *p < 0.05 vs. GABA-treated mice, #p < 0.05 vs. GABA-treated in vitro by Student’s t-test.
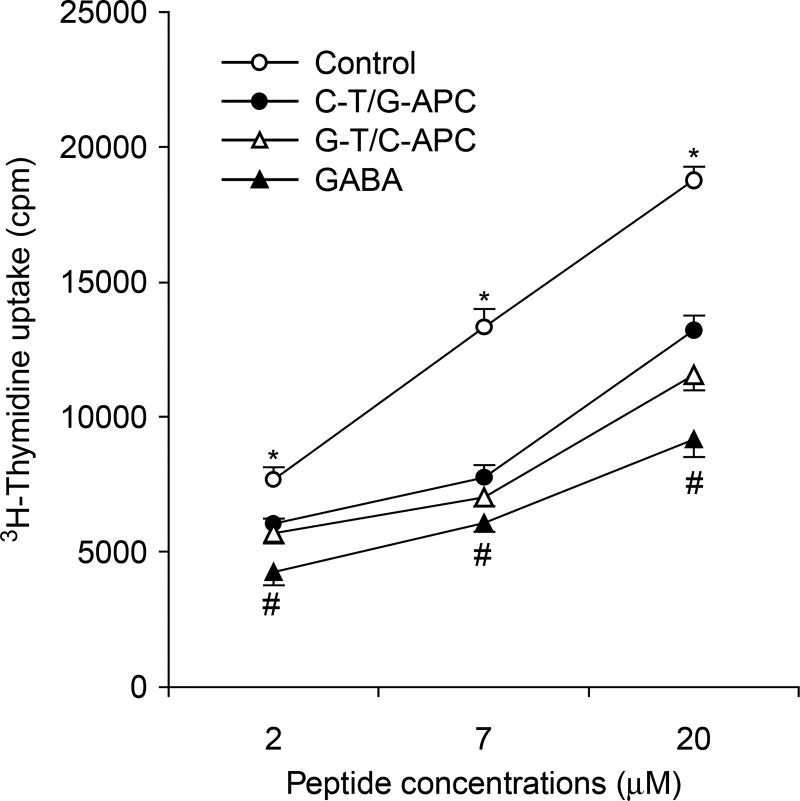
Because T-cell activation and expansion depends on antigen presentation by APCs, we examined whether oral GABA treatment could also affect APC activity. Nine days after immunization with bCII peptide in CFA, T cells were purified from the popliteal lymph nodes of control or GABA-treated mice and were co-cultured with APCs that were purified from the spleens of control or GABA-treated mice and incubated with a dose range of bCII peptide in vitro to assess their proliferative responses. We found that co-cultures of T cells from control mice and APCs from GABA-treated mice displayed significantly less proliferation than control cultures (Figure 4, p < 0.05). Co-cultures of T cells from GABA-treated mice and APCs from control mice displayed even less proliferation, suggesting that GABA mainly affected T cells in vivo. Co-cultures of both T cells and APC from GABA-treated mice displayed the lowest proliferative responses. Together, these data indicate that GABA consumption inhibited both the antigen-presenting activity of APC and autoreactive T-cell responses.
Figure 4.
GABA treatment modulates APC function. Mice were immunized with bCII peptide and given plain water or water with GABA. Nine days later, their popliteal lymph node T cells and splenic APC were purified. The purified T cells from control mice (C-T), or GABA treated (G-T), were co-cultured with purified splenic APC from control (C-APC) or GABA-treated (G-APC) mice and challenged with the indicated concentrations of bCII peptide to assess T-cell proliferation. Data shown are mean CPM ± SEM for each group of mice (N = 5 per group). The cells in medium alone had 580–820 CPM and stimulated with anti-CD3 had 22,000–28,000 CPM. *p < 0.05 vs. the C-T/G-APC group, #p < 0.05 vs. the G-T/C-APC group by Student’s t-test.
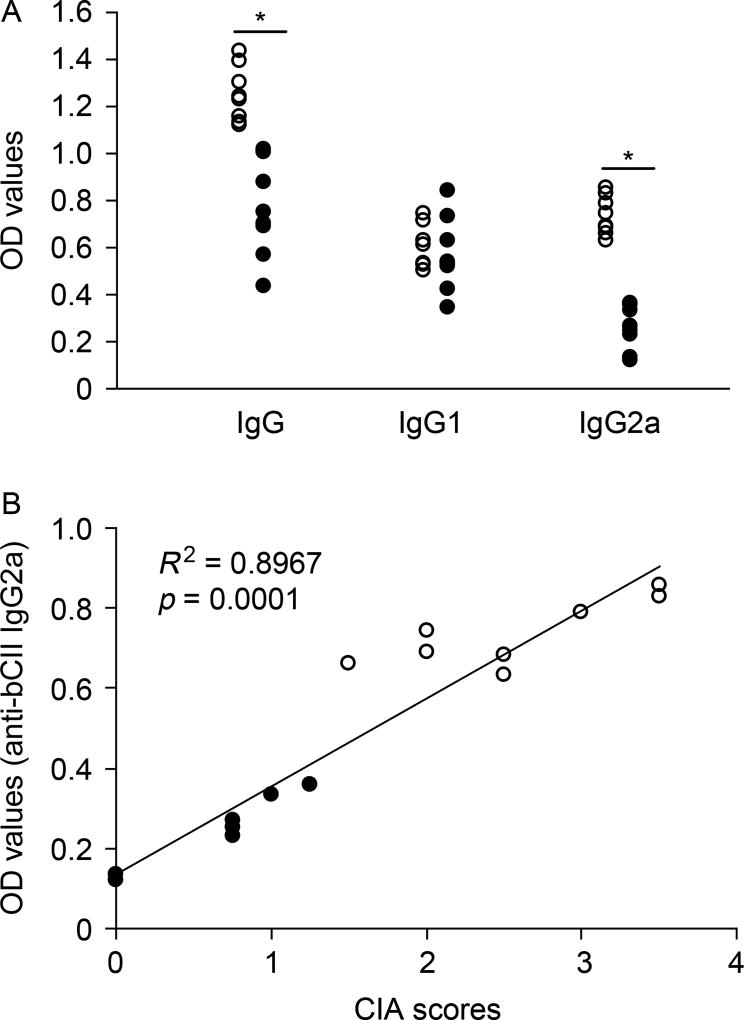
Since B cells also contribute to the pathogenesis of RA in mice and humans, we examined whether GABA treatment could modulate the production of autoantibodies against collagen. We found that GABA-treated mice had significantly reduced levels of IgG responses against bCII (Figure 5(A)). In particular, GABA consumption greatly reduced the levels of IgG2a responses to bCII, but had no discernable effect on IgG1 responses to bCII. More importantly, further analysis revealed that the levels of serum IgG2a were correlated significantly with the CIA disease scores of individual control and experimental mice at the end of the 8-week study (R2 = 0.8967, p < 0.0001 by Spearman’s correlation test, Figure 5(B)).
Figure 5.
Analysis of serum antibodies against collagen. (A) Eight weeks after the initial immunization, the levels of serum IgG, IgG1, and IgG2a responses to bCII in individual control (open circles) and GABA-treated (black circles) mice were determined by ELISA. Data shown are the mean values from individual mice. The background values of OD arranged from 0.102 to 0.114. *p < 0.001. (B) The levels of serum anti-bCII IgG2a were plotted against the CIA scores at the end of the 8-week observation period for individual control and GABA-treated mice. The association was determined by Spearman’s correlation test.
Discussion
We show for the first time that activation of peripheral GABA-Rs can reduce disease incidence and severity in an animal model of RA. Spleen cells from GABA-treated mice displayed reduced proliferative responses to the collagen peptide ex vivo. These proliferative responses were reduced by the inclusion of GABA in the media, demonstrating that long-term consumption of GABA did not desensitize cognate T cells to GABA. APCs are known to infiltrate the synovium and play an important role in RA [16,17]. Using co-cultures of purified T cells or APC from collagen peptide-immunized mice that were fed plain water or water + GABA, we showed that GABA treatment downregulated APC function, but that the major effect of treatment was on T cells. We previously reported that GABA treatment inhibits T-cell responses to β-cell antigens in NOD mice, which was associated with the inhibition of T-cell cycling, but not the depletion of immunocompetent cells [3], and Steinman and colleagues have reported that GABA-R activation inhibits MAPK signaling in APC [6]. The ability of GABA treatment to inhibit autoantigen-specific T-cell activation and proliferation and to modulate APC’s activity in vivo may underlie GABA’s ability to inhibit CIA.
B cells also play a major role in the pathogenesis of RA in mice and humans by producing cytokines, presenting antigens and by synthesizing autoantibodies that can target self-tissues or form immune complexes with autoantigens [18–20].
We showed that GABA treatment reduced IgG and IgG2a (but not IgG1) responses to bCII. Notably, the levels of serum IgG2a response to bCII were correlated with the CIA disease scores in individual mice. Given that IgG2a antibodies are associated with Th1 help, the significantly reduced anti-collagen IgG2a responses may reflect the effect of GABA on inhibiting Th1 responses in vivo. It will be of interest to further test GABA’s ability to ameliorate inflammatory responses after the establishment of CIA.
Oral GABA was tested in the 1950s–1980s in hundreds of patients for its ability to reduce epileptic seizures and treat cerebrovascular disorders [21–23]. Because GABA has very little ability to cross the blood–brain barrier, several grams of GABA per day were administered to subjects. GABA was found to be safe, but of little clinical benefit and pharmaceutical interests have focused on GABA-R ligands that efficiently cross the blood–brain barrier. Although the inability of GABA to pass through the blood–brain barrier makes it ill-suited for modulating CNS neurons, it makes it an excellent candidate for modulating immune cell function in the periphery without unwanted CNS side effects.
Metabolic studies in humans have observed that after oral consumption of GABA, serum GABA levels peak about 90 min later and decline to baseline about 180 min after consumption [23]. In the present study, GABA was administered through drinking water. This modality may have not elicited a maximal therapeutic effect because the mice drank water intermittently. The development of oral sustained-release formulations of GABA, or safe GABA-R agonists that have a long circulation period and do not pass through the blood–brain barrier, may provide even more efficacious therapies for RA and other inflammatory diseases.
Our findings complement previous findings that GABA can inhibit the spontaneous development of T1D in mice [3], and that a GABA-R agonist can ameliorate EAE [6]. The ability of GABA-R modulation to inhibit the disease process in T1D, EAE, and CIA in mice with different genetic backgrounds seems remarkable for a substance that has little, or no, side effects. Human PBMC populations, including lymphocytes, express functional GABA-Rs [7]. Conceivably, GABA and GABA-R agonists that are unable to pass through the brain–blood barrier may provide a new class of safe therapeutic reagents to modulate T cell and APC activity in ways that help ameliorate RA and other inflammatory diseases.
Acknowledgments
This work was supported, in part, by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to DLK. The authors have a patent on the use of GABA for inflammation.
Footnotes
Declaration of interest: The authors report no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Erdo SL, Wolff JR. Gamma-aminobutyric acid outside the mammalian brain. J Neurochem. 1990;54:363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 2.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 4.Bergeret M, Khrestchatisky M, Tremblay E, Bernard A, Gregoire A, Chany C. GABA modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits. Biomed Pharmacother. 1998;52:214–219. doi: 10.1016/S0753-3322(98)80019-X. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA(A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam S, Laughton DL, Walding A, Wolstenholme AJ. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 2006;43:1432–1442. doi: 10.1016/j.molimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Majithia V, Geraci SA. Rheumatoid arthritis: Diagnosis and management. Am J Med. 2007;120:936–939. doi: 10.1016/j.amjmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol. 2009;4:417–434. doi: 10.1146/annurev.pathol.4.110807.092254. [DOI] [PubMed] [Google Scholar]
- 10.O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 11.Hasler P. Biological therapies directed against cells in autoimmune disease. Springer Semin Immunopathol. 2006;27:443–456. doi: 10.1007/s00281-006-0013-8. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 13.Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, Arita S, Evans C, Atkinson MA, Mullen Y, Sarvetnick N, Tobin AJ, Lehmann PV, Kaufman DL. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med. 1996;2:1348–1353. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- 15.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 17.Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997;40:5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- 18.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 19.Stuart JM, Dixon FJ. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983;158:378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 21.Otomo E, Araki G, Mori A, Kurihara M. Clinical evaluation of GABA in the treatment of cerebrovascular disorders. Multi-center double-blind study in comparison with pyrithioxine and placebo. Arzneimittelforschung. 1981;31:1511–1523. [PubMed] [Google Scholar]
- 22.Loeb C, Benassi E, Bo GP, Cocito L, Maffini M, Scotto P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epilepsy Res. 1987;1:209–212. doi: 10.1016/0920-1211(87)90043-x. [DOI] [PubMed] [Google Scholar]
- 23.Tower DB, Roberts E, editors. Inhibition in the nervous system and GABA. New York: Pergamon Press; 1960. [Google Scholar]