Abstract
AIM
To assess the relationship between the presence of toll-like receptor 4 (TLR4) polymorphisms and bacterial infections in cirrhotic patients with ascites.
METHODS
We prospectively included consecutive patients with cirrhosis and ascites hospitalized during a 6-year period. Patients with human immunodeficiency virus (HIV) infection or any other immunodeficiency, patients with advanced hepatocellular carcinoma (beyond Milan’s criteria) or any other condition determining poor short-term prognosis, and patients with a permanent urinary catheter were excluded. The presence of D299G and/or T399I TLR4 polymorphisms was determined by sequencing and related to the incidence and probability of bacterial infections, other complications of cirrhosis, hepatocellular carcinoma, and mortality during follow-up. A multivariate analysis to identify predictive variables of mortality in the whole series was performed.
RESULTS
We included 258 patients: 28 (10.8%) were carriers of D299G and/or T399I TLR4 polymorphisms (polymorphism group) and 230 patients were not (wild-type group). The probability of developing any bacterial infection at one-year follow-up was 78% in the polymorphism group and 69% in the wild-type group (P = 0.54). The one-year probability of presenting infections caused by gram-negative bacilli (51% vs 44%, P = 0.68), infections caused by gram-positive cocci (49% vs 40%, P = 0.53), and spontaneous bacterial peritonitis (29% vs 34%, respectively, P = 0.99) did not differ between the two groups. The one-year probability of transplant-free survival was 55% in the polymorphism group and 66% in the wild-type group (P = 0.15). Multivariate analysis confirmed that age, Child-Pugh score, active alcohol intake, previous hepatic encephalopathy, hepatocellular carcinoma and serum creatinine were associated with a higher risk of death during follow-up.
CONCLUSION
Genetic polymorphisms D299G and/or T399I of TLR4 do not seem to play a relevant role in the predisposition of cirrhotic patients with ascites to bacterial infections.
Keywords: Cirrhosis, Genetic polymorphisms, Toll-like receptor 4, Bacterial infections, Ascites
Core tip: Patients with cirrhosis present a high incidence of bacterial infections. Toll-like receptor (TLR) 4 genetic polymorphisms, particularly D299G, have been previously associated with an increased predisposition to infection in several populations. In the present study, genetic polymorphisms D299G and/or T399I of TLR4 do not seem to play a relevant role in the predisposition to develop bacterial infections or in the prognosis of cirrhotic patients with ascites. Age, serum creatinine, Child-Pugh score, active alcohol intake, previous hepatic encephalopathy and the presence of hepatocellular carcinoma were independent predictive factors of mortality during follow-up.
INTRODUCTION
Cirrhotic patients, particularly those with decompensated disease, are at high risk to develop bacterial infections. Such infections may in turn precipitate other decompensations of cirrhosis, including renal failure, hepatic encephalopathy, variceal bleeding and acute-on-chronic liver failure (ACLF). As a consequence, bacterial infections have a significant impact on survival in these patients[1-3].
The most common bacteria causing spontaneous bacterial peritonitis (SBP), spontaneous bacteremia and urinary tract infections are enteric gram-negative bacteria (mainly Escherichia coli), while gram-positive bacteria are more likely to be the causative agent in cases of pneumonia (Streptococcus) and instrumentation-related infection (Staphylococcus)[1-3]. The relative importance of gram-positive pathogens has increased in recent years due to norfloxacin prophylaxis and invasive procedures. However, bacterial translocation of gram-negative gut bacteria continues to be an important step in the pathogenesis of infections in cirrhotic patients, mainly in those with advanced liver insufficiency and portal hypertension[1-4] because of a failure in their local and systemic immune defenses[5].
Toll-like receptors (TLR) are a family of transmembrane receptors found on monocytes, macrophages and neutrophils, and play a key role in the innate immune response. Their main function is the recognition of pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), lipoproteins and peptidoglycans[6].
TLR4 in particular recognizes LPS from gram-negative bacilli. The presence of the genetic polymorphisms D299G (rs4986790) and/or T399I (rs4986791) of TLR4 is believed to increase susceptibility to developing infections in some populations[7-10] including cirrhotic patients[10-14]. In a previous retrospective study, an association between the presence of the D299G TLR4 polymorphism and a predisposition to developing bacterial infections in cirrhosis patients was observed[14]. However, this association has yet to be evaluated prospectively.
The aim of this study was to prospectively assess the relationship between the presence of D299G and/or T399I TLR4 polymorphisms and the incidence of bacterial infections in cirrhotic patients with ascites.
MATERIALS AND METHODS
Patients
We prospectively included all cirrhotic patients with ascites hospitalized in the Department of Gastroenterology at Hospital de la Santa Creu i Sant Pau, a tertiary hospital in Barcelona, Spain, from May 2005 to May 2011. We included the patients to the study the first day of admission at the hospital. Biopsy and clinical, analytical and ultrasonographic data were used to diagnose cirrhosis. With the aim of minimizing the effects of confounding factors in the development of bacterial infections, the course of cirrhosis and survival, patients with human immunodeficiency virus (HIV) infection or any other immunodeficiency, patients with advanced hepatocellular carcinoma (beyond Milan’s criteria) or any other condition determining poor short-term prognosis, and patients with a permanent urinary catheter were excluded. At admission, we recorded demographic, clinical and analytical characteristics (etiology of cirrhosis, degree of liver insufficiency, renal function), and previous infections and decompensations of cirrhosis.
We obtained blood samples for a posterior genetic analysis of TLR4 polymorphisms on the first day of admission. Patients were assigned to two groups: Subjects with D299G and/or T399I TLR4 polymorphisms (polymorphism group) and subjects without (wild-type group).
The study was approved by the Research Ethics Committee at Hospital de la Santa Creu i Sant Pau. All patients gave consent to be included in the study after receiving appropriate verbal and written information.
Follow-up evaluation
We prospectively determined the incidence and the probability to present infections, other complications of cirrhosis, hepatocellular carcinoma, and mortality during follow-up; and we compared patients from the two groups. Follow-up was performed through regular outpatients visits with a frequency according to patient’s clinical condition but at least twice a year, and hospitalizations. A multivariate analysis to identify predictive variables of bacterial infection and mortality in the whole series was performed.
Spontaneous bacterial peritonitis (SBP) was diagnosed on the basis of an ascitic fluid neutrophil polymorphonuclear cell count ≥ 250/mm3 with or without positive culture[4]. Bacterascites was defined as the presence of a positive culture with a neutrophil polymorphonuclear count < 250/mm3 in ascitic fluid[4]. Bacteremia was diagnosed when blood cultures were positive. Conventional criteria were applied for the diagnosis of urinary tract infections, pneumonia, cellulitis and other infections[1]. Secondary bacterial peritonitis and postoperative wound infections were excluded from this analysis, since TLR polymorphisms are unlikely to influence the bacteria responsible for these infections and including them would possibly have biased the analysis of the results.
Genomic DNA extraction and polymorphism genotyping
Genomic DNA was extracted from buffy-coat fraction by using QIAmp DNA blood minikit (Qiagen Inc., Valencia, CA, United States). Sequencing was performed by Macrogen Inc, South Korea using BigDye (Applied Biosystem) chemistry after the PCR-amplified DNA fragment was confirmed. The sequencing primer used for TLR4 Asp299Gly (D299G, rs4986790) was 5’-TGGAATGCTGGAAATCCAGA-3’, and for Thr399Ile (T399I, rs4986791) was 5’-CTCTAGAGGGCCTGTGCA-3’.
Statistical analysis
Data are expressed as mean ± SD or frequencies. Results were analysed using the Fisher exact test for qualitative variables. For quantitative parameters the normal distribution was confirmed with Kolmogorov-Smirnov or Shapiro-Wilk tests. We used the nonparametric Mann-Whitney test for non-normally distributed data, and the Student’s “t” test for normally distributed data. The probabilities of bacterial infections and survival were calculated using the Kaplan Meier method and compared with the log rank test. A multivariate analysis including the variables with a P value < 0.05 in the univariate analysis was performed using Cox proportional hazards regression to identify independent predictive factors of bacterial infection and survival. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using the IBM Corp. Released 2013 IBM SPSS Statistics for Windows, Version 22.0; IBM Corp, Armonk, NY, United States.
RESULTS
Patients’ characteristics
From May 2005 until May 2011, 332 cirrhotic patients with ascites were admitted to the Department of Gastroenterology and assessed for inclusion in the study. All patients presented ascites with or without other current complications of cirrhosis, such as bacterial infection, variceal bleeding, hepatic encephalopathy, hepatorenal syndrome, or other admission causes. We evaluated patients with and without previous decompensations of cirrhosis. Seventy-four of the 332 patients were excluded for the following reasons: 9 patients due to HIV infection, one due to variable common immunodeficiency, 45 due to advanced hepatocellular carcinoma, 12 due to other conditions associated with a poor short-term prognosis (two lung neoplasia, one ovarian neoplasia, one bladder neoplasia, one lymphoma, one brain neoplasia, four severe cardiac failure, and two advanced respiratory insufficiency), and 7 due to permanent urinary catheter. Finally, 258 patients were included in the study. The retrospective data of the first 111 patients was previously published in a preliminary study from our group[14].
Analysis of TLR4 polymorphisms showed 28 patients (10.8%) were carriers of D229G and/or T399I polymorphisms. All patients were heterozygous and no homozygous patients were detected. Twenty-five patients (9.7%) had both polymorphisms, one patient was a carrier of the D299G polymorphism only and two were carriers of the T399I polymorphism only. Patients were then separated into two groups: 28 patients (10.8%) were D299G and/or T399I TLR4 polymorphism (polymorphism group) carriers and 230 who were not (wild-type group).
Table 1 summarizes the clinical and analytical characteristics of the both groups at inclusion in the study. We found no statistical differences between the two groups regarding demographics, etiology of cirrhosis, degree of liver insufficiency assessed by the Child-Pugh and MELD scores, previous decompensations of cirrhosis, current medications, type of first decompensation or cause of current admission. However, previous hepatic encephalopathy was more frequent and serum creatinine had a more elevated level in the polymorphism group than in the wild-type group.
Table 1.
Baseline characteristics of patients from the polymorphism group and the wild-type group
| Polymorphism group (n = 28) | Wild-type group (n = 230) | P value | |
| Age (yr) | 65.93 ± 12.1 | 64.90 ± 12.7 | 0.74 |
| Gender (male/female) | 17 (60.7)/ 11 (39.3) | 128 (55.7)/ 102 (44.3) | 0.69 |
| Cause of cirrhosis (%) | 0.07 | ||
| Alcohol | 14 (50) | 125 (54.3) | |
| Hepatitis C virus | 11 (39.3) | 82 (35.7) | |
| Hepatitis B virus | 0 (0) | 8 (3.5) | |
| Alcohol + HCV/HVB | 6 (21.4) | 18 (7.8) | |
| Others | 3 (10.7) | 22 (9.6) | |
| Active alcoholism (%) | 9/20 (45) | 80/143 (55.9) | 0.84 |
| Diabetes mellitus (%) | 9 (32.1) | 75 (32.6) | 1.00 |
| Child-Pugh score | 8.25 ± 1 | 8.31 ± 1.6 | 0.95 |
| MELD score | 15.29 ± 4.8 | 15.35 ± 6.2 | 0.69 |
| Previous decompensations (%) | 20 (71.4) | 160 (69.6) | 1.00 |
| Previous ascites (%) | 19 (67.9) | 151 (65.7) | 1.00 |
| Previous encephalopathy (%) | 13 (46.4) | 51 (22.2) | 0.009 |
| Previous variceal bleeding (%) | 7 (25) | 54 (23.5) | 0.82 |
| Previous spontaneous bacterial peritonitis (%) | 3 (10.7) | 16 (7) | 0.44 |
| Hepatocellular carcinoma (%) | 4 (14.3) | 26 (11.3) | 0.55 |
| Norfloxacin prophylaxis (%) | 4 (14.3) | 18 (7.8) | 0.28 |
| Beta-blockers (%) | 10 (35.7) | 74 (32.2) | 0.68 |
| Diuretics (%) | 15 (53.6) | 135 (58.7) | 0.69 |
| Serum sodium (mmol/L) | 135.14 ± 6.3 | 135.14 ± 9.8 | 0.78 |
| Serum urea (mmol/L) | 9.6 ± 4.1 | 9.6 ± 9.1 | 0.18 |
| Serum creatinine (μmol/L) | 117.29 ± 59.6 | 100.6 ± 59.9 | 0.01 |
| Serum bilirubin (μmol/L) | 54.7 ± 59.7 | 43 ± 34.1 | 0.64 |
| Serum albumin (g/L) | 26.6 ± 5.5 | 27.9 ± 5.5 | 0.43 |
| Prothrombin time ratio | 1.51 ± 0.24 | 1.57 ± 0.67 | 0.65 |
| Ascitic fluid total protein (g/L) | 13.9 ± 6.6 | 15.6 ± 10.7 | 0.30 |
| First decompensation1 | |||
| Ascites (%) | 19 (67.8) | 165 (71.7) | 0.66 |
| Encephalopathy (%) | 3 (10.7) | 6 (2.6) | 0.06 |
| Variceal bleeding (%) | 4 (14.3) | 44 (19.1) | 0.79 |
| Infection (%) | 2 (7.1) | 12 (5.2) | 0.65 |
| Cause of current admission2 | |||
| Ascites (%) | 8 (28.6) | 101 (43.9) | 0.15 |
| Encephalopathy (%) | 5 (17.9) | 22 (9.6) | 0.18 |
| Variceal bleeding (%) | 3 (10.7) | 31 (13.5) | 1.00 |
| Spontaneous bacterial peritonitis (%) | 6 (21.4) | 33 (14.3) | 0.39 |
| Other infection (%) | 3 (10.7) | 13 (5.7) | 0.39 |
| Other (%) | 3 (10.7) | 30 (13) | 1.00 |
Refers to the first decompensation that patients presented in the past (in patients with previous decompensation) or at present admission (in patients without previous decompensation);
Main cause of the hospitalization in which the patient was included to the study. Data are presented as mean ± SD or frequencies (%). MELD: Model for end-stage liver disease; HCV: Hepatitis C virus; HBV: Hepatitis B virus.
The mean follow-up in all patients was 26.6 ± 31.7 mo, 16.9 ± 25.4 mo in the polymorphism group and 27.8 ± 32.2 mo in the wild-type group (P = 0.04). Three patients (10.7%) in polymorphism group and 16 patients (7%) in wild-type group (P = 0.47) were referred to another center to be evaluated for liver transplantation, and therefore censored at that time for the present study. Within the group of patients with alcoholic cirrhosis, 86/140 (61.4%) had active alcohol use at inclusion in the study: 9/14 (64.3%) in the polymorphism group and 77/126 (61.1%) in the wild-type group (P = 1.00). Alcohol abstinence during follow-up in these patients with active alcohol consumption at inclusion was 48/86 patients (55.8%): 4/9 (44.4%) in the polymorphism group and 44/77 (57.1%) in the wild-type group (P = 0.50).
Infections during follow-up
Table 2 shows bacterial infections diagnosed in the two groups during follow-up. Considering all the infections, no statistical differences were observed between the two groups in terms of the incidence and number of infections per patient during the follow-up period, although there was a slight trend to a higher number of infections per patient in the polymorphism group than in the wild-type group. The incidence and the number of infections per patient caused by gram-negative bacilli or gram-positive cocci was similar in both groups although there was a higher, though non-statistically significant, incidence of gram-positive cocci infections in the polymorphism group compared to the wild-type group (50% vs 37.8% P = 0.22).
Table 2.
Overall incidence, number of episodes and causative bacteria of infections in patients from the polymorphism group and the wild-type group during follow-up
| Polymorphism group (n = 28) | Wild-type group (n = 230) | P value | |
| Total infections | |||
| Patients (%) | 22 (78.6) | 171 (74.3) | 0.81 |
| Number of infections | 63 | 437 | |
| Number per patient | 2.25 ± 2.40 | 1.92 ± 2.19 | 0.46 |
| Infections caused by gram-negative bacilli | |||
| Patients (%) | 11 (39.2) | 102 (44.3) | 0.84 |
| Number per patient | 0.86 ± 1.84 | 0.77 ± 1.25 | 0.75 |
| Infections caused by gram-positive cocci | |||
| Patients (%) | 14 (50) | 87 (37.8) | 0.22 |
| Number per patient | 0.64 ± 0.73 | 0.60 ± 1.009 | 0.83 |
Data are presented as mean ± SD or frequencies (%).
Table 3 shows the different types of bacterial infections caused by gram-negative bacilli or gram-positive cocci. There were not any statistical differences between both groups regarding the type of infection or the causative bacteria in the different types of infection. A trend for higher incidence of pneumonia was observed in the polymorphism group (17.9% vs 8.7%, P = 0.08), but this was not statistically significant.
Table 3.
Type of infections in patients from the polymorphism group and the wild-type group during follow-up
| Polymorphism group (n = 28) | Wild-type group (n = 230) | P value | |
| Spontaneous bacterial peritonitis | |||
| Patients (%) | 10 (35.7) | 81 (35.2) | 1.00 |
| Number of spontaneous bacterial peritonitis episodes | 15 | 109 | |
| Number per patient | 0.54 ± 0.92 | 0.47 ± 0.79 | 0.70 |
| Caused by gram-negative bacilli (%) | 0 (0) | 20 (8.7) | 0.14 |
| Caused by gram-positive cocci (%) | 3 (10.7) | 24 (10.4) | 1.00 |
| Culture negative (%) | 12 (42.8) | 57 (24.8) | 0.06 |
| Bacteremia | |||
| Patients (%) | 4 (14.3) | 47 (20.4) | 0.61 |
| Number of bacteremia episodes | 4 | 57 | |
| Number per patient | 0.14 ± 0.36 | 0.25 ± 0.55 | 0.41 |
| Caused by gram-negative bacilli (%) | 1 (3.6) | 26 (11.3) | 0.79 |
| Caused by gram-positive cocci (%) | 3 (10.7) | 31 (13.5) | 0.87 |
| Urinary infections | |||
| Patients (%) | 13 (46.4) | 111 (48.3) | 1.00 |
| Number of urinary infection episodes | 30 | 209 | |
| Number per patient | 1.07 ± 2.08 | 0.91 ± 1.42 | 0.90 |
| Caused by gram-negative bacilli (%) | 10 (35.7) | 80 (34.8) | 0.92 |
| Caused by gram-positive cocci (%) | 7 (25) | 49 (21.3) | 0.74 |
| Other infections | |||
| Patients (%) | 9 (32.1) | 59 (25.9) | 0.50 |
| Pneumonia (%) | 5 (17.9) | 20 (8.7) | 0.16 |
| Bacteriascites (%) | 2 (7.1) | 15 (6.5) | 1.00 |
| Cellulitis (%) | 2 (7.1) | 13 (5.7) | 0.67 |
Data are presented as mean ± SD or frequencies (%).
The probability of developing a bacterial infection at one-year of follow-up was 78% in the polymorphism group and 69% in the wild-type group (P = 0.54) (Figure 1A). The likelihood of infections caused by gram-negative bacilli after one year was 51% for the polymorphism group and 44% for the wild-type group (P = 0.68) (Figure 1B), for infections caused by gram-positive cocci it was 49% vs 40% (P = 0.53) (Figure 1C), and for spontaneous bacterial peritonitis it was 29% vs 34%, respectively (P = 0.99) (Figure 1D). Multivariate analysis by Cox regression showed that age (HR 1.023, 95%CI: 1.010-1.035, P ≤ 0.001), MELD score (HR 1.034, 95%CI: 1.010-1.059, P = 0.006), and previous hepatic encephalopathy (HR 1.570, 95%CI: 1.136-2.169, P = 0.006) were associated with a higher risk of infection during follow-up. The presence of TLR4 polymorphisms was not associated with the risk of infection in the univariate analysis or in the multivariate analysis.
Figure 1.
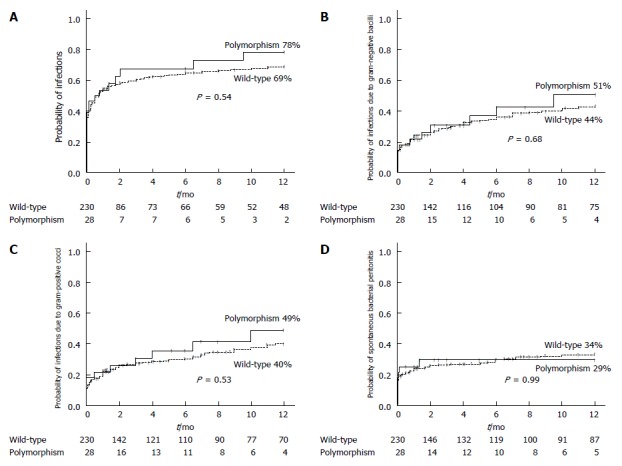
One year-probability of infections in the polymorphism group and in the wild-type group. A: All infections; B: Infections caused by gram-negative bacilli; C: Infections caused by gram-positive cocci; D: Spontaneous bacterial peritonitis.
Other complications of cirrhosis and survival
The likelihood of suffering hepatic encephalopathy after one year was 43% for the polymorphism group, and 41% for the wild-type group (P = 0.97), while the probability of developing variceal hemorrhage after one year was 17% and 12%, respectively (P = 0.35). The likelihood of developing a new hepatocellular carcinoma at two-years of follow-up was 7.1% for the polymorphism group and 6.3% for the wild-type group (P = 0.87).
The mortality during follow-up was 46.4% (13/28) in the polymorphism group and 46.5% (107/230) in the wild-type group (P = 1.00). The causes of mortality were ACLF or liver insufficiency in 46.15% (6/13) in the polymorphism group and 51.4% (55/107) in the wild-type group (P = 0.77); infection in 15.4% (2/13) and 20.6 % (22/107) (P = 1.00), variceal bleeding in 7.7% (1/13) and 8.4% (9/107) (P = 1.00), and hepatocellular carcinoma in 7.7% (1/13) and 5.6 % (6/107) (P = 0.56). In the polymorphism group, another three patients (23%) died from other causes unrelated to the liver disease: two from coronary heart disease and one from advanced oropharyngeal cancer. In the wild-type group, another fourteen patients (14%) (P = 0.41 with respect to polymorphism group) died from other causes unrelated to the liver disease: five from coronary heart disease and nine from advanced neoplasia (two lung neoplasm, one oropharyngeal cancer, one colon neoplasm, two non-identified advanced neoplasias, one breast cancer, one lymphoma, and one brain cancer). The likelihood of transplant-free survival at one year was 55% for the polymorphism group and 66% for the wild-type group (P = 0.15) (Figure 2), while the figures at two-years of follow up were 48% and 57%, respectively (P = 0.16).
Figure 2.
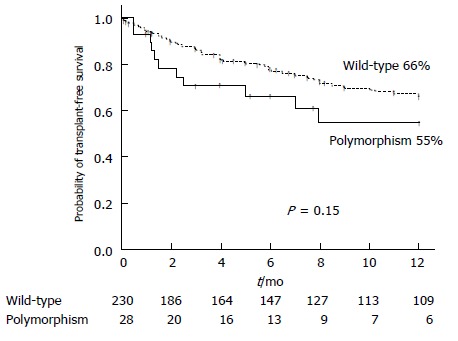
One year-probability of transplant-free survival in the polymorphism group and in the wild-type group.
We performed a multivariate analysis to analyse the contribution of baseline characteristics and the presence of TLR4 polymorphisms on the risk of death. In the univariate analysis we found age, HCV (hepatitis C virus) infection, active alcohol intake, Child-Pugh score, MELD score, previous decompensation, previous ascites, previous hepatic encephalopathy, hepatic encephalopathy at admission, hepatocellular carcinoma, treatment with beta-blockers or diuretics, serum creatinine, and serum urea were associated with a higher risk of death during follow-up (Table 4). Multivariate analysis by Cox regression confirmed that age, Child-Pugh score, active alcohol intake, previous hepatic encephalopathy, hepatocellular carcinoma and serum creatinine were associated with a higher risk of death during follow-up. The presence of TLR4 polymorphisms was not associated with mortality in the univariate analysis or in the multivariate analysis.
Table 4.
Univariate and multivariate analysis of baseline characteristics regarding the risk of death during follow-up in all patients
| Variables |
Univariate |
Multivariate |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.042 (1.026-1.056) | < 0.001 | 1.035 (1.016-1.054) | < 0.001 |
| HCV etiology | 1.99 (1.385-2.865) | < 0.001 | ||
| Diabetes mellitus | 1.583 (1.097-2.283) | 0.010 | ||
| Active alcohol intake | 0.353 (0.230-0.542) | < 0.001 | 0.568 (0.345-0.935) | 0.020 |
| Child-Pugh score | 1.170 (1.041-1.317) | 0.008 | 1.263 (1.113-1.432) | < 0.001 |
| MELD score | 1.036 (1.007-1.066) | 0.010 | ||
| Previous decompensation | 2.33 (1.473-3.690) | < 0.001 | ||
| Previous ascites | 2.50 (1.620-3.860) | < 0.001 | ||
| Previous encepalophathy | 2.794 (1.908-4.092) | < 0.001 | 2.216 (1.493-3.290) | < 0.001 |
| Hepatocellular carcinoma | 2.872 (1.097-4.817) | < 0.001 | 2.381 (1.411-4.018) | < 0.001 |
| Previous β-blockers | 1.727 (1.194-2.498) | 0.004 | ||
| Previous diuretics | 1.957 (1.334-2.869) | 0.001 | ||
| Serum creatinine (μmol/L) | 1.006 (1.004-1.008) | < 0.001 | 1.005 (1.002-1.007) | < 0.001 |
| Serum urea (mmol/L) | 1.026 (1.013-1.040) | < 0.001 | ||
| Platelet count (× 109/L) | 0.997 (0.995-1.00) | 0.032 | ||
| TLR4 polymorphisms | 1.372 (0.770-2.445) | 0.280 | ||
HCV: Hepatitis C virus; MELD: Model for end-stage liver disease.
DISCUSSION
The main finding in the present prospective study was that we failed to show significant differences in the incidence and number of infections, complications of cirrhosis and prognosis between cirrhotic patients with ascites who had D299G and/or T399I TLR4 polymorphisms and patients who were not carriers of these polymorphisms.
Infections in patients with cirrhosis are common and a major cause of morbidity and mortality[4]. Attempts to prevent bacterial infections are therefore reasonable, and successful strategies have been developed in recent years, particularly those using antibiotic prophylaxis[3,4]. Antibiotic prophylaxis, however, mainly in long-term treatments, is not devoid of side effects, especially the development of bacterial resistances[1,3]. This is a serious world-wide problem that decreases the efficacy of prophylactic antibiotics and increases morbidity and mortality, not only in the general population but also in patients with cirrhosis[3]. To minimize bacterial resistance it is important to identify the risk factors for infection in order to develop comprehensive prevention strategies that restrict antibiotic prophylaxis to high-risk groups[3]. Many clinical factors have been associated with an increased risk of infection in cirrhosis, such as high degree of hepatic insufficiency, variceal bleeding, low levels of protein in ascites, prior spontaneous bacterial peritonitis, and hospital admission in the last 3 mo[15].
In addition to clinical factors, the relationship between genetic variants that can modify the immune response and the incidence of infections in cirrhotic patients has gained increasing interest in recent years[11,14,16-18]. Recent studies in patients with cirrhosis have shown that some genetic variants of NOD2 (nucleotide-binding oligomerization domain-containing 2), TLR2 and NDP52 (nuclear dot protein 52 kDa) are involved in the predisposition to spontaneous bacterial peritonitis, probably through alterations at the intestinal barrier and the immune response[16-18].
The presence of the genetic polymorphisms D299G and/or T399I of TLR4 is also thought to modify the immune response to LPS from gram-negative bacilli, and therefore increase susceptibility to infection in patients with cirrhosis[11-14]. In a preliminary retrospective study, it was found that cirrhotic patients with D299G TLR4 polymorphism had more previous infections than wild-type patients[14]. Therefore, the present study was designed to prospectively evaluate whether patients diagnosed with cirrhosis and ascites and D299G and/or T399I TLR4 polymorphisms had a higher risk of bacterial infections during follow-up than wild-type patients. However, we observed a non-significant trend to a higher predisposition to bacterial infections, infections caused either by gram-negative bacilli or by gram-positive cocci in the polymorphism group. Regarding the types of infection, there was a trend to a higher incidence of pneumonia in the polymorphism group, but the incidence of other infections more characteristic of cirrhosis such as SBP, urinary infection or bacteremia was similar in the two groups. The findings of the present study are contradictory with those from our previous preliminary retrospective study. However, we consider the results of this study more reliable because it was prospective and included a higher number of patients.
These negative results may be due to the low prevalence of the polymorphisms evaluated, 10.8% of all patients - a prevalence similar to that in the general population[9,10], an insufficient number of patients studied, and a short follow-up, particularly in the polymorphism group. It should be noted, however, that we prospectively evaluated a relatively high number of patients with decompensated cirrhosis over a 6-year period and with a mean overall follow-up of 26.6 ± 31.7 mo. Moreover, we corrected for the difference in the length of follow-up between the two groups by calculating Kaplan-Meier curves. We consider that, if we failed to show statistically significant differences in the development of infections under these conditions, the effect, if any, of the studied polymorphisms has little clinical relevance, and their determination should not be included in the design of new preventive strategies.
Our results are in agreement with those of Lee et al[19] in patients who underwent a liver transplant. They observed no association connecting D299G and T399I TLR4 polymorphisms with a risk of developing infection or liver disease. Recently, Piñero et al[10] also failed to find a relationship between D299G TLR4 polymorphism and the development of infections in patients with cirrhosis and ascites. These findings are probably due to poor functional impact of these polymorphisms and/or the multifactorial and complex nature of the immune response[11].
Patients with polymorphisms of TLR4, the receptor to LPS of gram-negative bacilli, would be expected to present a greater predisposition to infection caused by these bacteria. It is therefore surprising that such patients also had a greater, although not statistically significant, predisposition to infections caused by gram-positive cocci and pneumonia, an infection usually caused by gram-positive cocci, than wild-type patients. Possible explanations could again be the complexity of the immune response, and the association between polymorphisms of TLR4 and other polymorphisms of other PRRs (pattern recognition receptors), such as TLR2 (involved in ligand recognition of gram-positive cocci) or NOD2, which were not evaluated in this study[11].
Inflammation is one of the factors that is increasingly recognized to favor the occurrence of hepatic encephalopathy[20,21]. In a previous study, we reported a greater occurrence of previous hepatic encephalopathy in cirrhotic patients carrying the D299G TLR4 polymorphism than in wild-type group patients[14]. As described by Nieto et al[12], cirrhotic patients with D299G and/or T399I TLR4 polymorphisms have less spontaneous production of IL-6 and IL-10 by peripheral monocytes, but a similar production after receptor stimulation compared to wild-type patients. This distinct cytokine production pattern may favor the development of hepatic encephalopathy in cirrhotic patients who are carriers of any of these polymorphisms[12]. In the present study, although previous episodes of hepatic encephalopathy were more frequent in patients with TLR4 polymorphisms than in patients in the wild-type group in agreement with previous data[12,14], this predisposition was not confirmed in the prospective follow-up.
A different inflammatory response could influence the evolution of cirrhosis and survival in patients with TLR4 polymorphisms[22]. In the present study a non-significant trend to higher mortality was observed during the follow-up period in patients with TLR4 polymorphisms than in patients from the wild-type group. Nevertheless, the presence of TLR4 polymorphisms neither in the univariate nor in the multivariate analysis was a predictive factor of mortality. Moreover, we did not observe differences in the cause of mortality between patients with TLR4 polymorphisms and wild-type patients. Most of the independent predictive factors of mortality in the multivariate analysis, such as age, Child-Pugh score, previous hepatic encephalopathy, hepatocellular carcinoma and serum creatinine, coincided with previous studies[23]. Regarding active alcoholism, this was an independent factor of survival probably due to the fact that more than half of patients actively drinking at inclusion in the study remained abstinent during follow-up. This percentage of abstainers was similar to that in previous studies showing that alcohol abstinence improves survival in patients with alcoholic cirrhosis[24]. In contrast, at the time the present study was performed, patients with decompensated cirrhosis due to HCV infection were not usually treated with antivirals.
Hepatocellular carcinoma is associated with inflammation. TLR4 stimulation can induce hepatocarcinogenesis[25] and increase invasiveness of hepatocellular carcinoma[26]. Therefore, a different inflammatory response as a consequence of the presence of TLR4 polymorphisms could influence the development of hepatocellular carcinoma in cirrhotic patients. In the present study, a similar likelihood of developing a new hepatocellular carcinoma was observed in both patients with TLR4 polymorphisms and in wild-type patients, though the follow-up period was too short to accurately evaluate this outcome.
We conclude that the presence of D299G and/or T399I TLR4 polymorphisms in cirrhotic patients with ascites is not a relevant risk factor for the development of bacterial infections and does not seem to significantly modify the evolution of the disease. It would be interesting to study the potential role of other genetic polymorphisms in the susceptibility to infections and the evolution of patients with cirrhosis.
ARTICLE HIGHLIGHTS
Research background
Toll-like receptor (TLR) 4 genetic polymorphisms, particularly D299G, have been previously associated with an increased predisposition to infection in several populations. However, few data regarding the role of these polymorphisms in patients with cirrhosis are available.
Research motivation
Few data regarding the role of TLR4 genetic polymorphisms in patients with cirrhosis are available
Research objectives
The aim of this study was to prospectively assess the relationship between the presence of D299G and/or T399I TLR4 polymorphisms and the incidence of bacterial infections in cirrhotic patients with ascites.
Research methods
The present study was designed to confirm the previous retrospective data and to further explore the relationship between the presence of TLR4 polymorphisms and bacterial infections in cirrhotic patients with ascites. The authors included consecutive patients with cirrhosis and ascites hospitalized during a 6-year period. The presence of D299G and/or T399I TLR4 polymorphisms was determined by sequencing and related to the incidence of infections during follow-up.
Research results
The authors included 258 patients: 28 (10.8%) were carriers of D299G and/or T399I TLR4 polymorphisms (polymorphism group) and 230 patients were not (wild-type group). The probability of developing any bacterial infection at one-year follow-up was 78% in the polymorphism group and 69% in the wild-type group (P = 0.54). The one-year probability of presenting infections caused by gram-negative bacilli (51% vs 44%, P = 0.68), infections caused by gram-positive cocci (49% vs 40%, P = 0.53), and spontaneous bacterial peritonitis (29% vs 34%, respectively, P = 0.99) did not differ between the two groups. The one-year probability of transplant-free survival was 55% in the polymorphism group and 66% in the wild-type group (P = 0.15).
Research conclusions
The presence of the genetic polymorphisms D299G and/or T399I of TLR4 does not seem to play a relevant role in the predisposition of cirrhotic patients with ascites to develop bacterial infections.
Research perspectives
To study the potential role of other genetic polymorphisms in the susceptibility to infections and the evolution of patients with cirrhosis.
ACKNOWLEDGMENTS
We thank Carolyn Newey and David Bridgewater for English language revision.
Footnotes
Supported by (partially) from the Instituto de Salud Carlos III, Madrid, Spain, No. PI0900357; and cofinanced by Fondos FEDER (Fondo Europeo de Desarrollo Regional), “Una manera de hacer Europa”, European Union, and CERCA Programme, Generalitat de Catalunya; Silvia Vidal was supported by Fondo de Investigaciones Sanitarias (FIS) and is a participant in the Program for Stabilization of Investigators of the Direcció d’Estrategia i Coordinació del Departament de Salut, Generalitat de Catalunya; Edilmar Alvarado-Tapias is a recipient of a “Río Hortega” fellowship grant from the Instituto de Salud Carlos III, No. CM16/00133.
Institutional review board statement: This study was approved by the Ethics Committee of Hospital de la Santa Creu i Sant Pau. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Manuscript source: Unsolicited manuscript
Peer-review started: November 17, 2017
First decision: December 1, 2017
Article in press: December 29, 2017
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Garbuzenko DV, Grgurevic I, Kai K, Kapoor S, Kaya M, Qi X, Quarleri J, Shimizu Y, Yoshioka K S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
Contributor Information
Edilmar Alvarado-Tapias, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
Carlos Guarner-Argente, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Elida Oblitas, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Elisabet Sánchez, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
Silvia Vidal, Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Department of Immunology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Eva Román, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain; Escola Universitària d’Infermeria EUI-Sant Pau, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Mar Concepción, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Maria Poca, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
Cristina Gely, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain.
Oana Pavel, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Juan Camilo Nieto, Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Department of Immunology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Cándido Juárez, Department of Immunology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Carlos Guarner, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
Germán Soriano, Department of Gastroenterology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Instituto de Salud Carlos III, Institut de Recerca IIB-Sant Pau, Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès) 08193, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
References
- 1.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 2.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 3.Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2016;63:2019–2031. doi: 10.1002/hep.28330. [DOI] [PubMed] [Google Scholar]
- 4.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27–31. doi: 10.1097/00042737-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Skevaki C, Pararas M, Kostelidou K, Tsakris A, Routsias JG. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseases. Clin Exp Immunol. 2015;180:165–177. doi: 10.1111/cei.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen A, Obaro S, Bojang K, Awomoyi AA, Greenwood BM, Whittle H, Sirugo G, Newport MJ. Variation in Toll-like receptor 4 and susceptibility to group A meningococcal meningitis in Gambian children. Pediatr Infect Dis J. 2003;22:1018–1019. doi: 10.1097/01.inf.0000095431.15606.68. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 9.Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SE, Lowry SF. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 10.Piñero P, Juanola O, Caparrós E, Zapater P, Giménez P, González-Navajas JM, Such J, Francés R. Toll-like receptor polymorphisms compromise the inflammatory response against bacterial antigen translocation in cirrhosis. Sci Rep. 2017;7:46425. doi: 10.1038/srep46425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5:156–164. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 12.Nieto JC, Sánchez E, Román E, Vidal S, Oliva L, Guarner-Argente C, Poca M, Torras X, Juárez C, Guarner C, et al. Cytokine production in patients with cirrhosis and TLR4 polymorphisms. World J Gastroenterol. 2014;20:17516–17524. doi: 10.3748/wjg.v20.i46.17516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testro AG, Gow PJ, Angus PW, Wongseelashote S, Skinner N, Markovska V, Visvanathan K. Effects of antibiotics on expression and function of Toll-like receptors 2 and 4 on mononuclear cells in patients with advanced cirrhosis. J Hepatol. 2010;52:199–205. doi: 10.1016/j.jhep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Guarner-Argente C, Sánchez E, Vidal S, Román E, Concepción M, Poca M, Sánchez D, Juárez C, Soriano G, Guarner C. Toll-like receptor 4 D299G polymorphism and the incidence of infections in cirrhotic patients. Aliment Pharmacol Ther. 2010;31:1192–1199. doi: 10.1111/j.1365-2036.2010.04291.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 16.Appenrodt B, Grünhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F. Nucleotide-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis in liver cirrhosis. Hepatology. 2010;51:1327–1333. doi: 10.1002/hep.23440. [DOI] [PubMed] [Google Scholar]
- 17.Nischalke HD, Berger C, Aldenhoff K, Thyssen L, Gentemann M, Grünhage F, Lammert F, Nattermann J, Sauerbruch T, Spengler U, et al. Toll-like receptor (TLR) 2 promoter and intron 2 polymorphisms are associated with increased risk for spontaneous bacterial peritonitis in liver cirrhosis. J Hepatol. 2011;55:1010–1016. doi: 10.1016/j.jhep.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Lutz P, Krämer B, Kaczmarek DJ, Hübner MP, Langhans B, Appenrodt B, Lammert F, Nattermann J, Hoerauf A, Strassburg CP, et al. A variant in the nuclear dot protein 52kDa gene increases the risk for spontaneous bacterial peritonitis in patients with alcoholic liver cirrhosis. Dig Liver Dis. 2016;48:62–68. doi: 10.1016/j.dld.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Lee SO, Brown RA, Kang SH, Abdel Massih RC, Razonable RR. Toll-like receptor 4 polymorphisms and the risk of gram-negative bacterial infections after liver transplantation. Transplantation. 2011;92:690–696. doi: 10.1097/TP.0b013e31822b589f. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, et al. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. doi: 10.1038/srep26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Córdoba J, Mínguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70–80. doi: 10.1055/s-2008-1040322. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Xie YD, Feng B, Gao Y, Wei L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol Res. 2014;44:436–449. doi: 10.1111/hepr.12131. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong YQ, Lu CW, Zhang L, Yang J, Hameed W, Chen W. Toll-like receptor 4 signaling promotes invasion of hepatocellular carcinoma cells through MKK4/JNK pathway. Mol Immunol. 2015;68:671–683. doi: 10.1016/j.molimm.2015.10.015. [DOI] [PubMed] [Google Scholar]


