Abstract
Diabetic foot ulcers (DFUs), a life-threatening complication of diabetes mellitus, have limited treatment options, often resulting in amputations. HMG-CoA reductase inhibitors such as statins are cholesterol-reducing agents that may provide a new therapeutic option. Statins target the cholesterol pathway and block the synthesis of the wound-healing inhibitors farnesyl pyrophosphate (FPP) and cortisol, ligands for the glucocorticoid receptor (GR). Here we demonstrate that the naturally occurring statin mevastatin reverses FPP's effects and promotes healing by using in vitro wound healing assays, human ex vivo and porcine in vivo wound models, and DFU tissue. Moreover, we measured cortisol levels by ELISA and found that mevastatin inhibited cortisol synthesis in keratinocytes and biopsies from patients with DFU. Of note, topical mevastatin stimulated epithelialization and angiogenesis in vivo. Mevastatin also reversed FPP-mediated induction of the GR target, the transcription factor c-Myc (a biomarker of non-healing wounds), in porcine and human wound models. Importantly, mevastatin reversed c-Myc overexpression in DFUs. It induced expression of the long noncoding RNA Gas5 that blocks c-Myc expression, which was confirmed by overexpression studies. We conclude that topical mevastatin accelerates wound closure by promoting epithelialization via multiple mechanisms: modulation of GR ligands and induction of the long noncoding RNA Gas5, leading to c-Myc inhibition. In light of these findings, we propose that repurposing statin drugs for topical treatment of DFUs may offer another option for managing this serious condition.
Keywords: glucocorticoid receptor; long noncoding RNA (long ncRNA, lncRNA); Myc (c-Myc); skin; wound healing; diabetic foot ulcers; statin; Gas5
Introduction
Chronic wounds affect more than 6.5 million people each year in the United States and represent a major healthcare burden for patients and healthcare professionals (1, 2). Diabetic foot ulcers (DFUs)3 are one of the most debilitating complications of diabetes mellitus (DM) and a frequent cause of lower limb amputations with a 5-year mortality rate of more than 50% (1, 2). Up to 25% of patients with DM develop DFUs (2). In addition, the cost of care for patients with chronic wounds is estimated to be over $25 billion in the United States and expected to increase because of a rise in the incidence of DM and obesity and our aging population (2). There are very few treatment options that received Food and Drug Administration approval for efficacy in randomized clinical trials (3). Therefore, new therapy approaches are needed.
Statins, HMG-CoA reductase-competitive inhibitors, are used frequently to lower cholesterol plasma levels. Multiple studies suggest that statins exert cholesterol-independent pleiotropic effects, including anti-inflammatory and antibacterial properties, decreased oxidative stress, improved endothelial function, and improved healing outcomes (4–6) and that they regulate the expression of non-coding RNAs involved in the regulation of proliferation and inflammation (7, 8). All of these processes are deregulated in patients with chronic refractory wounds, making topical statins an attractive treatment modality. Use of systemic statins in patients with wounds improves healing in a variety of chronic wounds (5, 9, 10). In addition, statins are currently recommended as a standard of care therapy for patients with DM to prevent cardiovascular disease, and their use is expected to increase (11, 12). Despite this, statins taken by some populations of chronic wound patients showed a positive impact in randomized control trials (13). However, the therapeutic potential and mechanisms of topically applied statins on the epithelialization process have not been investigated.
Skin synthesizes cholesterol, the precursor to all steroid hormones (14, 15). We have shown previously that the epidermis uses cholesterol to synthesize cortisol, a glucocorticoid (GC) that inhibits keratinocyte migration and wound healing (16). Cortisol, acting through the glucocorticoid receptor (GR), targets genes involved in wound healing, including c-Myc (17–19). In addition, we have shown that an intermediate in the cholesterol biosynthesis pathway, farnesyl pyrophosphate (FPP), acts as a ligand for the GR and inhibits keratinocyte migration through repression of the keratin 6 (K6) gene, tubulins, and insulin-like growth factor signaling (20, 21). FPP-mediated inhibition of epithelialization occurs through a complex molecular mechanism that involves GR, arginine methyltransferase (CARM1), β-catenin, and GRIP1 as co-repressors in the context of the K6 promoter (21). Moreover, GR activation by both GC and FPP results in nuclear translocation of β-catenin, which leads to induction of c-myc, molecular events that represent hallmarks of chronic non-healing wounds (22–24). Activation of c-Myc in chronic wounds leads to depletion of epidermal stem cells, resulting in non-migratory and hyperproliferative epidermis, thus contributing to the inability of the tissue to respond to injury (23, 25, 26). Therefore, inhibiting the cholesterol biosynthesis pathway in the skin using statins may result in suppression of GR activation through reduction of its ligands, FPP and cortisol, and inhibition of c-myc to restore healing.
Long non-coding RNAs (lnc-RNAs) are defined as non-coding transcripts that are more than 200 nucleotides in length (27). Several functions of lnc-RNAs have been characterized that include binding and regulating protein function and regulating gene expression, whereas aberrant expression of lnc-RNAs has been implicated in having a role in diseases (27). The lnc-RNA Gas5 has been shown to play a role in regulating cell proliferation and survival (28). Gas5 can function as a glucocorticoid response element decoy to inhibit GR-mediated gene expression (28).
Because of the ability of statins to inhibit HMG-CoA reductase and subsequently reduce isoprenoid intermediates, including production of FPP, we delivered mevastatin topically in vivo using a porcine wound model and found that mevastatin reduced GR phosphorylation and improved epithelialization while simultaneously enhancing angiogenesis. We show that mevastatin treatment reversed FPP-mediated activation of c-Myc in porcine wounds in vivo and human wounds ex vivo and suppressed c-Myc in tissue specimens obtained from the patients' non-healing DFU edge. In addition, mevastatin inhibited cortisol synthesis in primary human epidermal keratinocytes (HEKs) and in DFU samples obtained from patients. Furthermore, we identified a novel mechanism by which mevastatin inhibits c-Myc through induction of expression of the lnc-RNA Gas5. Our data suggest that topical statins may be beneficial to facilitate therapeutic reprogramming and reverse the non-healing phenotype in patients with chronic wounds unresponsive to standard treatment modalities.
Results
Topical mevastatin promotes wound closure in vivo and promotes keratinocyte-driven angiogenesis
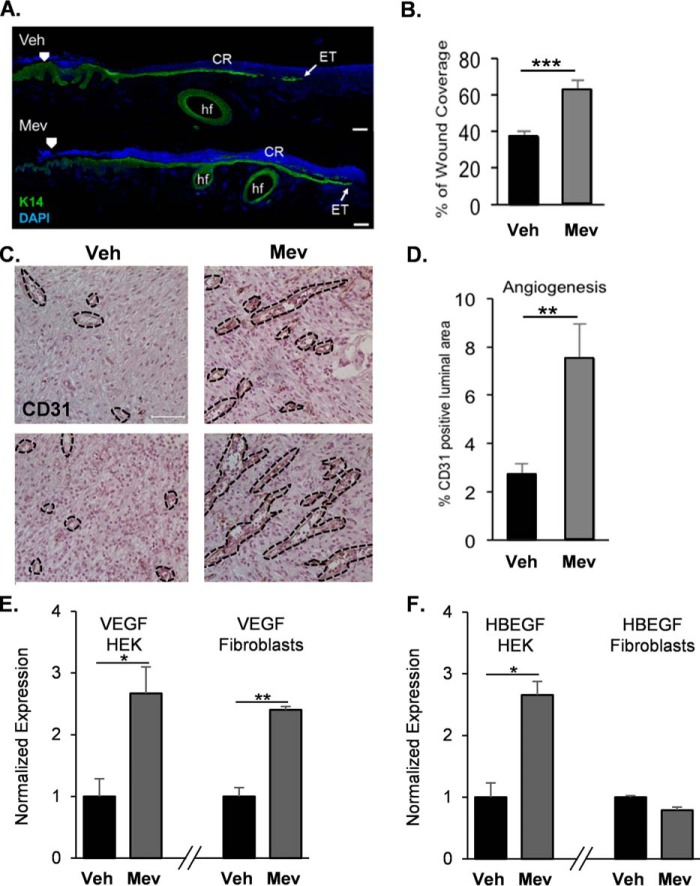
We examined the effects of topically applied mevastatin on wound healing in vivo by using an established porcine partial thickness wound model (16, 29). Wounds were topically treated daily with vehicle (0.3% ethanol) or mevastatin (250 μm). The wound samples that were evaluated at the earlier assessment time (day 2) after wounding for epithelialization by histomorphometric analyses and keratin 14 (K14) immunostaining showed that mevastatin treatment significantly promoted epithelialization and wound closure compared with less epithelialization observed in the untreated control (Fig. 1, A and B).
Figure 1.
Topical mevastatin promotes epithelialization and angiogenesis in vivo. A, immunolocalization of the epidermal marker K14 (green) at the wound edge on day 2 post-wounding. Mevastatin induced epithelialization compared with control. ET, epithelial tongue; CR, crust; hf, hair follicle; Veh, vehicle. Nuclei were visualized by DAPI. Arrowheads indicate wound edges after initial wounding. Scale bars = 50 μm. B, quantification of epithelialization demonstrating that topical mevastatin (250 μm) significantly promoted epithelialization compared with vehicle-treated wounds. Data are represented as mean ± S.D. and were analyzed by Student's t test. ***, p < 0.001. C, immunoperoxidase staining of the angiogenesis marker CD31 on day 2 post-wounding. Black dashed lines demarcate the luminal blood vessel area. Scale bar = 50 μm. D, quantification of CD31 staining demonstrating mevastatin-induced angiogenesis compared with vehicle treatment. Data are represented as mean ± S.D. and were analyzed by Student's t test. **, p < 0.01. E, qPCR of VEGFA in HEKs and primary human fibroblasts, demonstrating mevastatin-induced expression of VEGFA in both cell types 24 h after treatment compared with vehicle (n = 3). *, p < 0.05; **, p < 0.01. F, qPCR of HBEGF in HEKs and primary human fibroblasts, demonstrating mevastatin-induced expression of HBEGF in HEKs; mevastatin had no effect on HBEGF expression in fibroblasts 24 h after treatment compared with vehicle (n = 3). Data are represented as mean ± S.D. and were analyzed by Student's t test. *, p < 0.05.
In addition to enhancing wound closure, statins induce angiogenesis in murine wound models through the PI3K/Akt pathway (4, 30). To determine whether topical mevastatin induces angiogenesis in a porcine model, we immunostained blood vessels with the endothelial cell-specific marker CD31 (Fig. 1, C and D). We found that mevastatin significantly induced angiogenesis compared with the control. Keratinocytes are the major cell type present in skin and a major source of VEGFA and HBEGF, two potent inducers of angiogenesis (31–35), and we evaluated whether mevastatin-mediated induction of angiogenesis is keratinocyte-driven. To test this, we treated HEKs in the presence or absence of mevastatin and assessed VEGFA and HBEGF expression by qPCR. We found that mevastatin induced expression of both VEGFA and HBEGF in HEKs (Fig. 1, E and F). In addition, we assessed whether mevastatin induces expression of VEGFA and HBEGF in other cell types present in the skin. To test this, we treated primary human fibroblasts with mevastatin and assessed the expression of VEGFA and HBEGF by qPCR. We found that mevastatin induced VEGFA expression but had no effect on HBEGF expression. Our data suggest that induction of angiogenesis by mevastatin may be predominately keratinocyte-driven.
Mevastatin promotes keratinocyte migration by blocking activation of endogenous GR
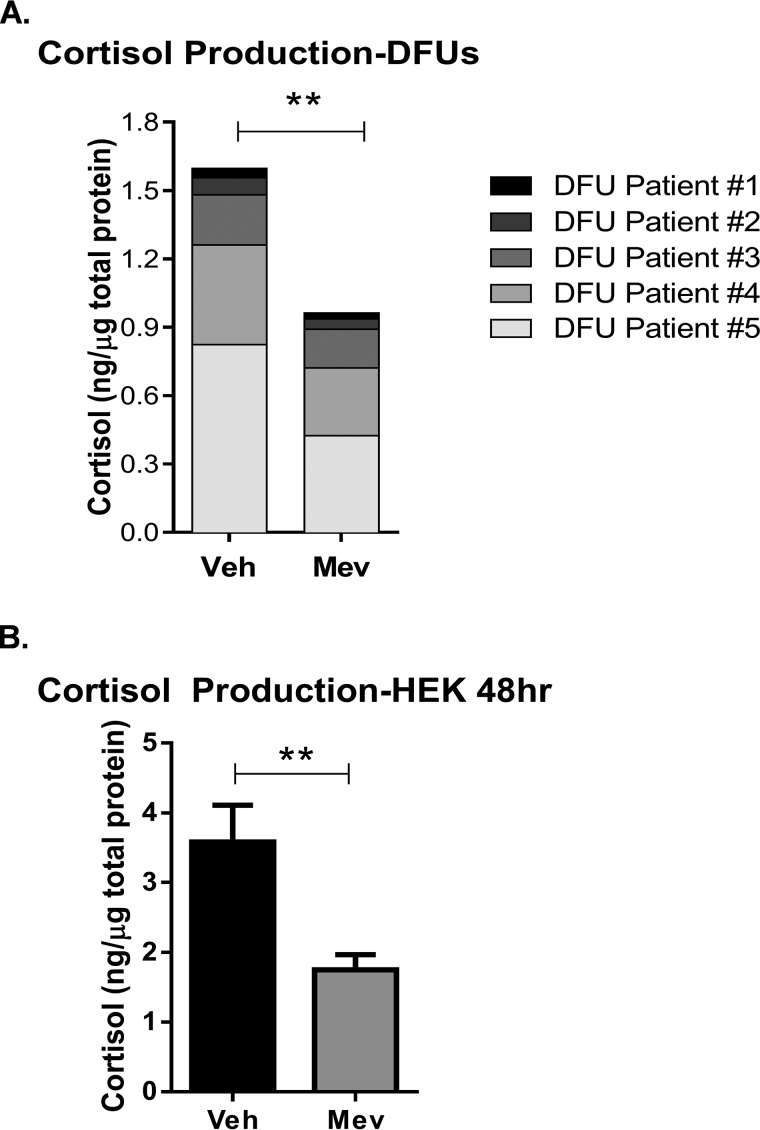
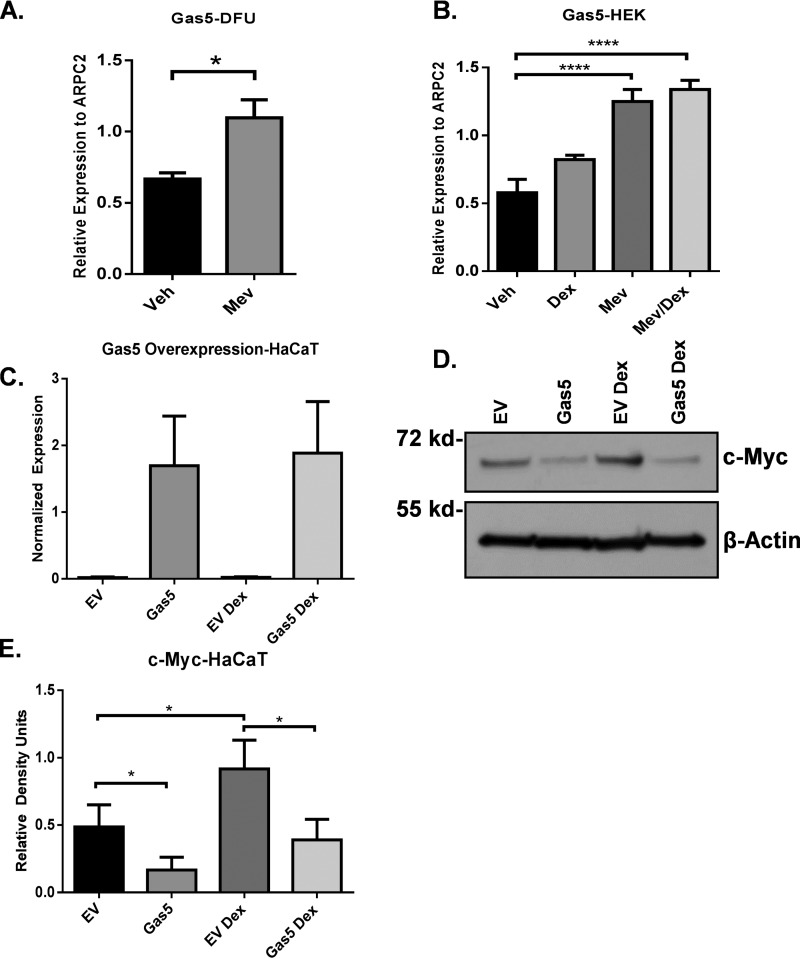
We have shown previously that the epidermis uses the cholesterol pathway to synthesize cortisol, resulting in inhibition of keratinocyte migration (16). To test whether statins inhibit synthesis of cortisol, we obtained tissue samples from the non-healing wound edge from patients with DFUs (n = 5). Freshly obtained tissue specimens were treated with mevastatin and maintained at the air–liquid interface for 48 h. Cortisol levels were measured by ELISA. We found that mevastatin inhibited cortisol synthesis in DFU tissue in comparison with the vehicle-treated control (Fig. 2A). Mevastatin-mediated inhibition of cortisol synthesis was also seen in HEKs (Fig. 2B).
Figure 2.
Mevastatin inhibits cortisol synthesis in diabetic foot ulcers. A, cortisol ELISA from samples obtained from the non-healing edge of diabetic foot ulcers from patients (n = 5) treated with MEV for 48 h. Topical mevastatin significantly inhibited cortisol synthesis in DFU patients. Data were normalized to total protein concentration and analyzed by a ratio-paired t test. **, p < 0.01. Veh, vehicle. B, cortisol ELISA from HEKs treated with MEV for 48 h. Mevastatin significantly inhibited cortisol synthesis 48 h after treatment compared with vehicle. Data were normalized to total protein concentration and are represented as mean ± S.D. and were analyzed by Student's t test. **, p < 0.01.
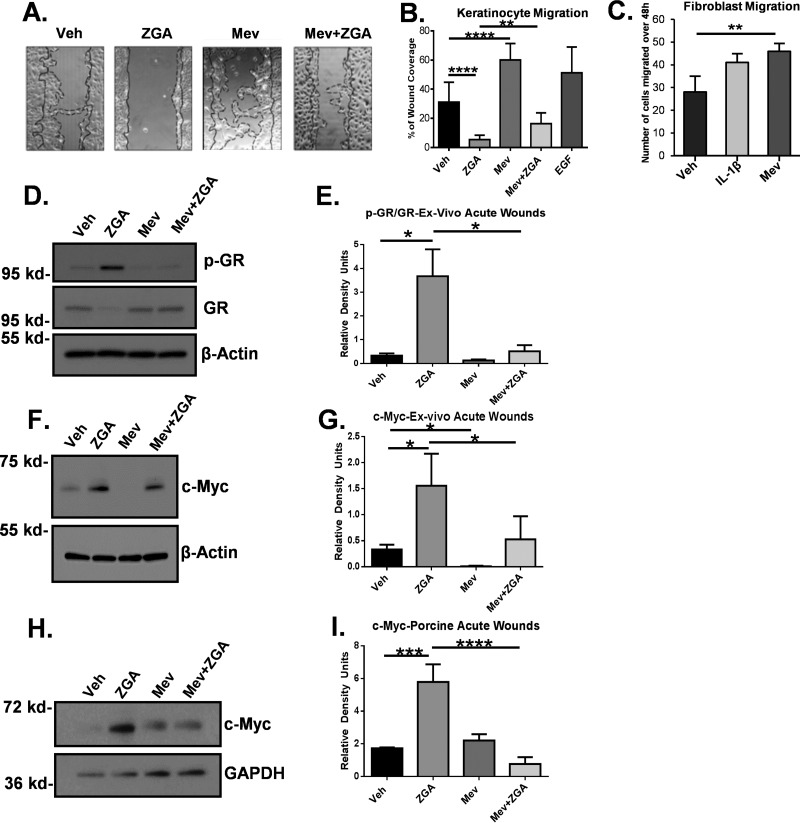
We found previously that zaragozic acid (ZGA) results in endogenous accumulation of FPP, a glucocorticoid ligand, and inhibition of wound healing (20, 36). To test whether mevastatin reverses FPP-mediated inhibition of wound healing, we performed a wound scratch assay on HEKs treated with ZGA, mevastatin, or in combination. Treatment with ZGA significantly inhibited HEK migration, whereas treatment with mevastatin induced migration to levels comparable with EGF, a potent stimulator of keratinocyte migration (Fig. 3, A and B). Interestingly, mevastatin was also able to reverse the inhibitory effect of ZGA on migration, indicating the ability of mevastatin to promote migration by decreasing cellular FPP levels (Fig. 3, A and B). In addition, mevastatin treatment increased migration of human dermal fibroblasts (Fig. 3C); thus, mevastatin can promote healing not only in keratinocytes but also in other cell types that have impaired function in DFUs.
Figure 3.
Mevastatin promotes keratinocyte migration and inhibits GR phosphorylation and c-Myc in human ex vivo and porcine partial-thickness wounds. A, HEK scratch assay (n = 3). Continuous lines represent the initial scratch, and dotted lines represent the migrating front. Veh, vehicle. B, the average coverage of scratch wound widths in percentage relative to baseline wound width 24 h after treatment. EGF was used as a positive control. ZGA inhibited keratinocyte migration, whereas mevastatin induced migration alone and reversed suppression of migration by ZGA. Data are represented as mean ± S.D. and were analyzed by one-way ANOVA followed by Bonferroni's post hoc test. **, p < 0.01; ****, p < 0.0001. C, fibroblast scratch assay (n = 3). IL-1β served as a positive control. Mevastatin induced fibroblast migration compared with the control. Data are represented as mean ± S.D. and were analyzed by Student's t test. **, p < 0.01. D, Western blot of p-GR and total GR (Ser211) from human skin ex vivo wounds topically treated with MEV in the presence or absence of ZGA for 48 h. Mevastatin abolished GR phosphorylation and reduced ZGA-mediated p-GR. E, quantification of Western blot analysis of p-GR normalized to total GR from human skin ex vivo acute wounds (n = 3). Data are represented as mean ± S.E. and were analyzed by one-way ANOVA followed by Holm-Sidak's post hoc test. *, p < 0.05. F, Western blot of c-Myc from human skin ex vivo acute wounds treated with MEV in the presence or absence of ZGA for 48 h. G, quantification of Western blot analysis of c-Myc from human skin ex vivo acute wounds. ZGA induced c-Myc expression, whereas mevastatin abolished c-Myc expression alone or in the presence of ZGA. Data were normalized to β-actin. Data are represented as mean ± S.D. and were analyzed by a paired t test. *, p < 0.05. H, representative Western blot of c-Myc from porcine partial-thickness wounds treated with MEV in the presence or absence of ZGA. I, quantification of c-Myc Western blot analysis from porcine wounds (n = 3). Mevastatin treatment suppressed c-Myc upon induction by ZGA in porcine partial-thickness wounds. Data were normalized to GAPDH. Data are represented as mean ± S.D. and were analyzed by one-way ANOVA followed by Bonferroni's post hoc test. ***, p < 0.001; ****, p < 0.0001.
To further confirm that statins reduce endogenous activity of GR by modulating its ligands, we evaluated the levels of ligand-bound phosphorylated GR (p-GR) using an established human skin ex vivo wound model. We treated human acute wounds with mevastatin in the presence or absence of ZGA. Treatment with ZGA, which leads to accumulation of endogenous FPP, resulted in induced GR phosphorylation. Topical treatment with mevastatin resulted in loss of GR phosphorylation in human ex vivo wounds. Importantly, topical treatment with mevastatin reversed ZGA-induced phosphorylation of GR (Fig. 3, D and E). We conclude that mevastatin promotes keratinocyte migration by blocking endogenous activity of GR, possibly by modulating the endogenous GR ligands FPP and cortisol.
Mevastatin inhibits expression of c-Myc in DFUs by inducing the long non-coding RNA Gas5
We have shown previously that activation of GR induces a wound healing inhibitor, c-myc (21, 23). To evaluate whether mevastatin inhibits GR-induced c-Myc, we evaluated the expression of c-Myc by Western blotting in human ex vivo skin wounds treated with ZGA, mevastatin, or in combination. As expected, ZGA treatment resulted in strong induction of c-Myc, whereas mevastatin treatment, which reduces the levels of FPP-activated GR, abolished c-Myc expression (Fig. 3, F and G). We then assessed c-Myc levels in wound edge tissue from a porcine wound model treated with mevastatin, ZGA, or in combination. Topical mevastatin treatment reversed ZGA-mediated induction of c-Myc in comparison with ZGA-treated porcine wounds (Fig. 3, H and I). Taken together, mevastatin reversed FPP-mediated effects in a manner that promotes wound closure in vitro and in vivo by stimulating cell migration through inhibition of GR phosphorylation and c-Myc expression.
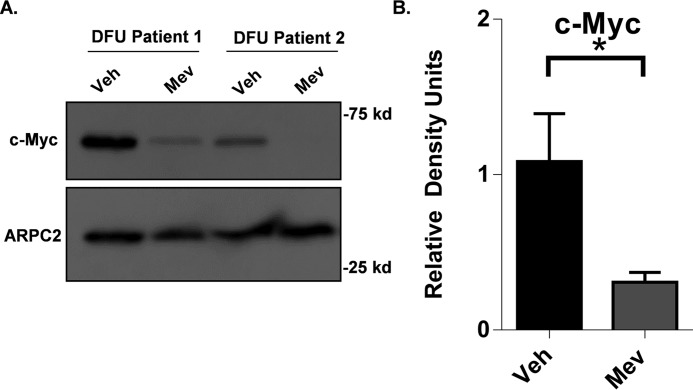
To test whether mevastatin inhibits c-Myc under pathological conditions present in DFUs, we evaluated tissue samples from patients with DFUs (n = 8). Tissue samples were treated with mevastatin and maintained at the air–liquid interface for 48 h. The expression of c-Myc was assessed by Western blotting. As expected, vehicle treatment showed strong induction of c-Myc. We found that treatment with mevastatin effectively inhibited c-Myc expression in DFUs compared with the control (Fig. 4, A and B), demonstrating the efficacy of topically applied mevastatin under pathological conditions of DFUs.
Figure 4.
Mevastatin suppresses c-Myc expression in diabetic foot ulcers. A, Western blot of c-Myc from samples obtained from the non-healing edge of DFU patients (n = 8) treated with MEV for 48 h. Western blots were performed separately. Veh, vehicle. B, topical mevastatin significantly suppressed c-Myc in samples obtained from the non-healing edge of DFU patients (n = 8). Data are represented as mean ± S.E., and a paired t test was performed. *, p < 0.05 between the indicated conditions.
Statins have been shown to regulate the expression of non-coding RNAs (7, 8). It has been shown that the lnc-RNA Gas5 can block c-Myc (37). Therefore, we postulate that statins may also block c-Myc by activation of the lnc-RNA Gas5. To test whether mevastatin induces expression of the lnc-RNA Gas5 in chronic wounds, we treated tissue samples obtained from the non-healing edge of DFU patients with mevastatin at the air–liquid interface for 48 h. We found that mevastatin induced expression of Gas5 in DFUs by qPCR (Fig. 5A). Next we assessed Gas5 expression by qPCR in mevastatin-treated HEKs and confirmed Gas5 induction by mevastatin in vitro (Fig. 5B), suggesting that inhibition of c-Myc may occur through mevastatin-induced expression of Gas5. To gain further insight into this mechanism, we overexpressed Gas5 in the HaCaT human keratinocytes cell line (Fig. 5C) and tested the level of c-Myc expression in the presence or absence of dexamethasone, a GR ligand. We found that overexpression of Gas5 inhibited c-Myc expression even in the presence of dexamethasone compared with the empty vector control (Fig. 5, D and E), demonstrating a novel mechanism by which mevastatin inhibits c-Myc to promote wound healing in keratinocytes.
Figure 5.
Mevastatin inhibits c-Myc through inducing expression of the long non-coding RNA Gas5. A, DFUs (n = 4) were treated with mevastatin at the air–liquid interface for 48 h. Shown is qPCR demonstrating mevastatin-induced expression of Gas5 in DFUs. Data are represented as mean ± S.E., and a paired t test was used. *, p < 0.05. Veh, vehicle. B, qPCR demonstrating mevastatin-induced expression of Gas5 in HEKs. Data are represented as mean ± S.D., and one-way ANOVA followed by Bonferroni's post hoc test was used. ****, p < 0.0001. C, qPCR confirming overexpression of Gas5 in HaCaT cells (n = 3). EV, empty vector. D, Western blot of c-Myc in HaCaT cells overexpressing Gas5 treated with or without dexamethasone compared with empty vector control. Gas5 inhibited c-Myc even in the presence of dexamethasone. E, Gas5 overexpression significantly inhibited c-Myc in HaCaT cells overexpressing Gas5 (n = 3). Data are represented as mean ± S.D., and one-way ANOVA followed by Holm-Sidak's post hoc test was used. *, p < 0.05.
Discussion
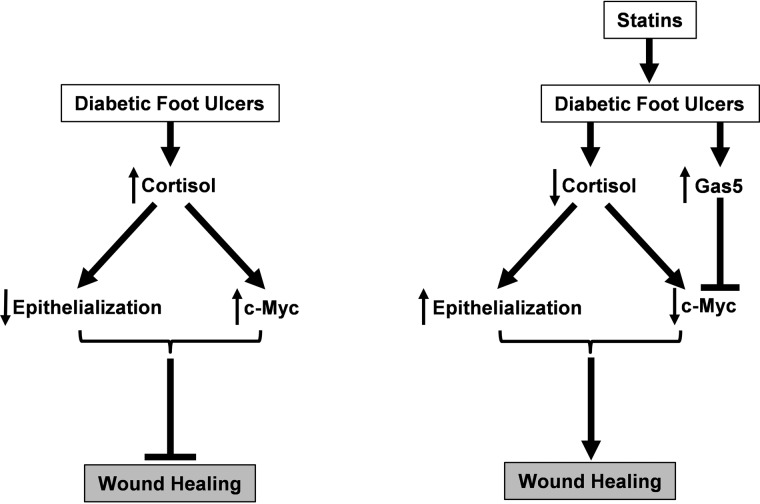
In this study, we show that statins impact epidermal biology in a manner that accelerates epithelialization in vivo through a novel mechanism by inducing expression of the lnc-RNA Gas5. In addition, topical statins facilitated therapeutic reprogramming by inhibiting activation of GR and reversing negative effects on wound healing through inhibition of GR ligands, i.e. FPP, and cortisol synthesis. Moreover, statins stimulated keratinocyte migration while simultaneously promoting angiogenesis. Topical mevastatin reversed FPP-induced expression of c-Myc, a hallmark of chronic wounds, in vivo and ex vivo as well as under pathological conditions of DFUs. Inhibition of c-Myc occurs via a novel mechanism whereby statins induce expression of Gas5 (Fig. 6). These findings demonstrate that topical statins exert beneficial diverse effects on wound healing, highlighting the therapeutic potential of repurposing these already Food and Drug Administration–approved drugs for topical treatment for DFUs. This approach may enable rapid clinical translation from bench to bedside.
Figure 6.
Model demonstrating that statins inhibit cortisol synthesis and induce expression of the long non-coding RNA Gas5 to inhibit c-Myc in DFUs to promote wound healing.
Topical application of statins offers several advantages over systemic delivery that include directly targeting skin with avoidance of first-pass metabolism and systemic adverse effects. In addition, patients with DFUs often have compromised macro- and microvasculature, which leads to reduced blood perfusion and renal failure as a result of DM complications (1, 24, 38), which may interfere with drug distribution, making systemic delivery of statins less effective. Therefore, topical application of statins may be advantageous for cutaneous wound healing disorders in which epithelialization ensures positive healing outcome (1, 39). To investigate the outcome of topical mevastatin on wound closure, we utilized a well-established porcine skin wound model (29, 40), and we found accelerated epithelialization by mevastatin. Although incisional polyvinyl alcohol sponge murine and rabbit wound models have been used previously to assess the therapeutic potential of either systemic (32, 41) or topical applications (4), the porcine model offers benefits that include a similar physiology and healing by re-epithelialization like human skin in addition to a large surface area, which allows creation of multiple wounds on a single animal (29, 42). Porcine skin is structurally similar to human skin, including parameters such as epidermal thickness and dermal–epidermal thickness ratios (29, 40, 43), patterns of hair follicles, and blood vessels. Also, porcine wounds, unlike murine and rabbit wounds, which heal primarily by contraction, heal by epithelialization in a manner similar to human wounds and therefore represent a validated preclinical wound model (29). Interestingly, our data from the porcine wound model revealed additional and novel molecular mechanisms and cellular responses, confirming the pleiotropic effects of statins.
Our data suggest that mevastatin may promote keratinocyte-driven angiogenesis during wound healing by inducing expression of VEGFA and HBEGF. Although our study did not functionally confirm the extent of the role of keratinocytes in promoting angiogenesis in response to statins, keratinocytes are the major cell type present in skin and are a major source for growth factors such as VEGFA and HBEGF (31, 44), two potent stimulators of angiogenesis we found to be induced by mevastatin in keratinocytes. Although we found that mevastatin induced VEGFA expression in primary human fibroblasts, we did not observe induction of HBEGF expression, further suggesting that pro-angiogenic effects of topical statins may be predominately keratinocyte-driven through a synergistic effect of both VEGFA and HBEGF by keratinocytes. Decreased local angiogenesis is a major contributor to the development of DFUs (1), making this process a primary target for therapeutic approaches (33). Vasculature abnormalities observed in patients with DM are attributed to impaired recruitment of endothelial cell progenitors from the bone marrow as well as impaired growth factor responses, all of which lead to decreased peripheral blood flow and suppressed local angiogenesis, which contributes to the lack of healing (24). Topical mevastatin-mediated induction of two potent stimulators of angiogenesis and keratinocyte migration, VEGFA and HBEGF (31, 33–35), reveals an important mechanism of action. Keratinocytes have been established as a major source of growth factors and cytokines that coordinate wound healing in a spatiotemporal fashion, which is why they are used as effective therapy for chronic wounds (31, 44, 45). Furthermore, keratinocytes are major site for cortisol synthesis, and we have shown previously that selective inhibition of endogenous GR activity promoted wound healing, suggesting that endogenous activity of GR plays a significant role in this mechanism (15, 16, 20). We have previously generated transcriptional profiles from keratinocytes treated with the GR ligands cortisol and FPP and identified several processes profoundly regulated by GR that negatively impact keratinocyte migration wound closure (17, 21, 46). Moreover, keratinocytes utilize the cholesterol pathway to endogenously synthesize both ligands (16, 21). Thus, statins, by targeting the rate-limiting step of the cholesterol pathway and decreasing GR ligands (FPP and cortisol) reverse their effects on keratinocyte migration and facilitate closure. Taken together, these data suggest that, using topical mevastatin, one can use the endogenous therapeutic effects of keratinocytes and stimulate patient cells into therapeutic reprogramming, stimulating a non-healing cell phenotype to become pro-healing.
Another unique aspect of topical mevastatin treatment is reversal of FPP-mediated activation of c-Myc in human skin ex vivo, porcine wounds in vivo, and specimens derived from the non-healing edge of DFUs obtained from patients. Activation of c-Myc affects epidermal biology and inhibits keratinocyte migration in murine models (26). Transgenic mouse models overexpressing c-Myc develop a spontaneous chronic wound phenotype (26, 47), further supporting the notion that, although it triggers hyperproliferation, overexpression of c-Myc inhibits healing. Although c-Myc is required for transition from G1 to S phase of the cell cycle and promotes proliferation of transit-amplifying cells, deregulation of c-Myc depletes epidermal stem cells in chronic wounds (25), thus contributing to the inability of the tissue to respond to injury. Furthermore, transcriptional profiling patterns from non-healing ulcers confirmed induction of c-Myc in non-healing tissues from chronic wounds (46). We have also demonstrated that skin derived from the non-healing edge of chronic wounds exhibits distinctive histopathology; the epidermis becomes hyperproliferative and hyper- and parakeratotic, with an accumulation of mitotically active cells in the suprabasal layers and activation of epidermal stem cells, all consistent with c-Myc overexpression (23, 25, 46, 48). Importantly, our findings demonstrate the efficacy of topically applied statins to reverse c-Myc overexpression in DFU tissue, shifting the cell phenotype into healing. In addition, statins have been shown previously to exert antiproliferative effects on epithelial cancer cell lines (49), which can play a crucial role in their therapeutic potential for wound healing disorders characterized by a hyperproliferative non-migratory epidermis driven by c-myc overexpression (23). This further suggests that statins may shift chronic wounds from a hyperproliferative to a migratory phenotype to promote healing. Our data show that inhibition of c-Myc occurs by inducing expression of Gas5 (in both HEK and DFU patient samples). Gas5 has been shown to bind the mRNA of c-Myc to block its translation (37). In addition, it has been shown to function as a glucocorticoid response element decoy to inhibit the activity of GR (28), a potent stimulator of c-Myc. Although there may be multiple ways in which statins can inhibit c-Myc, we found that statins significantly induced Gas5 expression in HEKs and DFUs, and overexpression of Gas5 in HaCaT cells inhibited c-Myc even in the presence of its stimulator, GC, suggesting that mevastatin inhibits c-Myc by inducing Gas5 expression. Although one cannot fully exclude non-physiological consequences of Gas5 overexpression, the data presented in this manuscript highlight a role of statin-induced Gas5 in inhibiting c-Myc even in the presence of its epidermal inducer, dexamethasone. These findings have significant implications in which statins can modulate the glucocorticoid response during wound healing. Not only can statins inhibit GC synthesis by targeting the cholesterol pathway, but statins can also inhibit hormone-bound and activated GR by inducing expression of Gas5 to promote healing and reverse overexpression of c-Myc in patients with DFUs.
Statins are a widely used class of drugs and it is expected that the use of statins will increase in the future (12). Our findings demonstrate a complex mechanism of action whereby topical statins not only stimulate endogenous cells to enter a healing mode but also directly act on molecular inhibitors of healing, such as c-Myc, both ex vivo and in vivo. Together, our data suggest that topical statins may have considerable therapeutic potential for patients suffering from chronic wounds that do not respond to standard treatment modalities. Additional benefits of topical statins include a positive safety profile, low cost, and avoidance of adverse systemic effects (50, 51), making them a very attractive treatment approach.
Experimental procedures
Cell culture and wound scratch assay
Primary HEKs were maintained as described previously (17) in serum-free keratinocyte medium supplemented with epidermal growth factor and bovine pituitary extract (Keratinocyte-SFM, LifeSci, 10724–011). Prior to treatments, cells were incubated for 24 h in a basal serum-free medium custom-made without hydrocortisone (LifeSci, ME14041L1) and treated as follows: 1 μm dexamethasone (Sigma, D8893), 50 μm ZGA (Sigma-Aldrich, Z2626), or 5 μm mevastatin (Sigma-Aldrich, M2537). Prior to the wound scratch assay, the cells were treated with 8 μg/ml mitomycin C (Sigma, M4287) for 1 h and washed with basal medium. Scratches and migration quantifications were performed as described previously (20, 23). Primary human fibroblasts were maintained as published previously (52) in DMEM, 10% FBS, and 1× penicillin/streptomycin. Fibroblasts were treated with 5 μm mevastatin 3 times daily for 24 h. Samples were harvested for RNA analysis by qPCR as described below.
Human ex vivo wound models
Healthy skin samples were used to generate acute wounds as described previously (53, 54). Wounds were treated daily with 50 μm ZGA, 10 μm MEV, or both ZGA and MEV. Vehicle (0.3% ethanol)-treated wounds served as controls. Discarded unidentified DFU samples were considered non-human subject research by the University of Miami Intuitional Review Board. Specimens from each patient were maintained at the air–liquid interface in DMEM with 10% charcoal-stripped FBS and treated with either vehicle (ethanol) or 5 μm MEV three times daily for 48 h. Samples were harvested for protein assessment (Western blotting) and cortisol synthesis (ELISA).
Western blotting
Extracts for immunoblotting were prepared from a subconfluent normal HEK or wound tissue as described previously (20). Cell extracts were separated on 4–20% Criterion Tris-Glycine eXtended precast gels (Bio-Rad, 5671094) and transferred onto PVDF membranes (Bio-Rad, 1620177). Membranes were blocked with I-Block (Applied Biosystems, T2015) in phosphate-buffered saline containing 0.1% Tween 20 and then incubated with anti-Ser(P)211 GR antibody (1:1000, Cell Signaling Technology, 4161S), anti-total GR antibody (1:1000, Cell Signaling Technology, 3660S), or anti c-Myc (1:1000, EMD Millipore, 06-340). As a loading control, we used anti β-actin antibody (1:10,000, Sigma-Aldrich, A5441), anti GAPDH (1:1000, Santa Cruz Biotechnology, sc-25788), or anti-ARPC2 (1:5000, Abcam, ab133315).
Cortisol ELISA
Primary human epidermal keratinocytes and discarded DFU samples were treated and harvested as described above. Cortisol levels from cell and tissue lysates were analyzed using the Cortisol Parameter Assay Kit (R&D Systems, KGE008) according to the manufacturer's instructions with a Spark 10 M spectrophotometer (Tecan U.S., Inc.). Cortisol levels were normalized to total protein concentration.
RNA isolation and RT-PCR
Total RNA was isolated from HEK and primary human fibroblasts treated with 5 μm MEV three times daily for 24 h and vehicle-treated cells using the miRNeasy Mini Kit (Qiagen, 217004) according to the manufacturer's protocol. cDNA was synthesized using 100 ng of total RNA using the qScript cDNA Synthesis Kit (Quantabio, 95047-100). Real-time PCR was performed in triplicates with 1 ng of cDNA per reaction using the CFX96 qPCR thermal cycler and detection system (Bio-Rad) and PerfeCTa SYBR Green Supermix (Quantabio, 95054-500). Relative expression was normalized for levels of actin-related protein 2/3 complex, subunit 2 (ARPC2). The primer sequences used were as follows: ARPC2, 5′-TCCGGGACTACCTGCACTAC-3′ (forward) and 5′-GGTTCAGCACCTTGAGGAAG-3′ (reverse); Gas5, 5′-CTTCTGGGCTCAAGTGATCCT-3′ (forward) and 5′-TTGTGCCATGAGACTCCATCAG-3′ (reverse); VEGFA, 5′-AGGGAAAGGGGCAAAAACGA-3′ (forward) and 5′-CCTCGGCTTGTCACATCTGC-3′ (reverse); and HBEGF, 5′-CTTGTGCTCAAGGAATCGGC-3′ (forward) and 5′-CAACTGGGGACGAAGGAGTC-3′ (reverse).
Plasmids and transient transfection
Gas5 was PCR-amplified from cDNA isolated from primary human keratinocytes. The primer sequences used were as follows: Gas5, 5′-ATAGGGCTAGCTTTCGAGGTAGGAGTCGACT-3′ (forward) and 5′-ATAGGCGGCCGCGGATTGCAAAAATTTATTAA-3′ (reverse). PCR products were directionally cloned into the pEGFP-N3 plasmid using Nhe1 and Not1 restriction enzymes and the Quick Ligation Kit (New England Biolabs, M2200S). Plasmids were confirmed by sequencing. HaCaT cells were plated on 12-well plates at 300,000 cells/well. HaCaT cells were transfected the next day using Attractene transfection reagent (Qiagen, 301005). 2 μl of Attractene was combined with 1 μg of plasmid DNA per well in Opti-MEM I reduced serum medium (Gibco, 11058-021), and the complex was allowed to form at room temperature for 15 min. HaCaT cells were switched to fresh medium with DMEM, 10% FBS, and 1× penicillin/streptomycin/glutamine, and 60 μl of the complex was added per well. The next day, HaCaT cells were switched to DMEM, 0.1% FBS, and 1× penicillin/streptomycin/glutamine for 24 h, followed by treatment with or without 1 μm dexamethasone for 24 h, and harvested for protein, and c-myc was assessed by Western blotting as described above.
Experimental animals, wounding, and treatments
Five young, female, specific pathogen-free pigs (Ken-O-Kaw Farms, Windsor, IL) weighing between 25 and 35 kg were used. Animals were fed a non-antibiotic chow ad libitum, fasted overnight before the procedures, and housed individually with controlled temperature and controlled light and dark cycles. The experimental animal protocols were approved by the University of Miami Institutional Animal Care and Use Committee (protocol 13-140). The methods for animal preparation and wounding were explained in detail in our previous study (40). Briefly, the flank and the back of experimental animals were prepped on the day of the experiment. Animals were anesthetized, and partial-thickness wounds were made on the paravertebral area using a modified electrokeratome set at 0.5 deep × 10 × 7 mm. The wounds were separated from one another by ∼50 mm areas of unwounded skin. Wounds were treated within 20 min (or when hemostasis has been achieved) after creation with either vehicle (ethanol in PBS), 250 μm MEV, 100 μm ZGA, or a combination of MEV and ZGA. At least six wounds from each group per animal were used for histological evaluation and RNA and protein isolation at days 2 and 4 post-wounding.
Evaluation of wound epithelialization
To evaluate wound healing rates, a transverse section ∼3 mm thick was cut through the central part of the wound, including 5 mm of adjacent uninjured skin at day 2 post-wounding. The tissues were fixed and processed for paraffin embedding. 7-μm sections were cut and stained with hematoxylin and eosin. Staining was analyzed using a Nikon Eclipse E800 microscope, and the digital images were collected using the SPOT camera advanced program. The wounds were quantified by planimetry as described previously (16, 53). Paraffin sections were also used for staining with anti-K14 antibody (1:50, Abcam, ab9220) as described previously (40). K14 staining was visualized with Alexa Fluor 488–conjugated goat anti-mouse antibody (Invitrogen, A11001), and mounted with Prolong DAPI Gold antifade reagent (Invitrogen, P36931) to visualize cell nuclei. Specimens were analyzed using a Nikon Eclipse E800 microscope, and digital images were collected using the NIS Elements program.
Evaluation of wound angiogenesis
Wedge biopsies from the 10-mm full-thickness wounds were collected at day 8 post-wounding for the evaluation of angiogenesis. The 5-μm-thick sections were deparaffinized and quenched for endogenous peroxidase. Antigen retrieval was performed with Target Retrieval Solution (Dako Corp., S1699). The sections were blocked for 10 min with Background Sniper (BioCare Medical, BS966JJ) and incubated overnight with anti-CD31 antibody (1:100, Thermo Fisher Scientific, PA1-36181). Diaminobenzidine (DAB) chromagen solution (BioCare Medical, BDB2004L) was applied for 5 min, and the slides were counterstained with hematoxylin. Staining was analyzed using a Nikon Eclipse E400 microscope, and digital images were collected with the QImaging camera and NIS Elements BR 3.1 software. For each slide, six different images of the granulation tissue at the wound bed were captured. NIS Elements BR 3.1 software was used to quantify the luminal vessels that were CD31-positive. Percent vascularization was determined as the ratio of the positively stained endothelial cells in blood vessels to the total wound area for each image.
Statistics
Statistical analyses were performed using one-way ANOVA followed by Bonferroni's or Holm-Sidak's post hoc test, Student's t test, or ratio-paired t test was used where indicated. A p value of 0.05 or less was considered significant.
Author contributions
M. T. C., A. P. S., I. P., O. S., and S. C. D. designed the research. A. P. S., I. P., O. S., S. L., and J. G. performed the research. S. C. D. and R. S. K. contributed reagents. A. P. S., I. P., O. S., and M. T. C. analyzed data. A. P. S., I. P., O. S., and M. T. C. wrote the paper.
Acknowledgments
We thank S. Patel, N. Yin, and J. Valdes for support and technical assistance and Dr. A. Barrientos, Dr. R. W. Keane, Dr. J. P. Vaccari, and Dr. S. Elliot for generously sharing laboratory resources and equipment. We also thank our laboratory members for continuous support and constructive criticism.
This work was supported by National Institutes of Health Grants AR060562, NR015649, and NR013881 (to M. T. C.) and University of Miami SAC Award SAC 2013-19. M. T. C. is listed as the inventor of Patent PCT/US2010/062361, “Composition and Methods for Promoting Epithelialization and Wound Closure,” issued to New York University based on the data presented, in part, in this study and stands to potentially gain royalties from future commercialization.
- DFU
- diabetic foot ulcers
- DM
- diabetes mellitus
- GC
- glucocorticoid
- GR
- glucocorticoid receptor
- FPP
- farnesyl pyrophosphate
- lnc-RNA
- long non-coding RNA
- HEK
- human epidermal keratinocyte
- qPCR
- quantitative PCR
- ZGA
- zaragozic acid
- MEV
- mevastatin
- ANOVA
- analysis of variance
- HBEGF
- heparin-binding epidermal growth factor.
References
- 1. Eming S. A., Martin P., and Tomic-Canic M. (2014) Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sen C. K., Gordillo G. M., Roy S., Kirsner R., Lambert L., Hunt T. K., Gottrup F., Gurtner G. C., and Longaker M. T. (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 17, 763–771 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gould L., Abadir P., Brem H., Carter M., Conner-Kerr T., Davidson J., DiPietro L., Falanga V., Fife C., Gardner S., Grice E., Harmon J., Hazzard W. R., High K. P., Houghton P., et al. (2015) Chronic wound repair and healing in older adults: current status and future research. J. Am. Geriatrics Society 63, 427–438 10.1111/jgs.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asai J., Takenaka H., Hirakawa S., Sakabe J., Hagura A., Kishimoto S., Maruyama K., Kajiya K., Kinoshita S., Tokura Y., and Katoh N. (2012) Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am. J. Pathol. 181, 2217–2224 10.1016/j.ajpath.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 5. Evangelista M. T., Casintahan M. F., and Villafuerte L. L. (2014) Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br. J. Dermatol. 170, 1151–1157 10.1111/bjd.12883 [DOI] [PubMed] [Google Scholar]
- 6. Stojadinovic O., Lebrun E., Pastar I., Kirsner R., Davis S. C., and Tomic-Canic M. (2010) Statins as potential therapeutic agents for healing disorders. Expert Rev. Dermatol. 5, 689–698 10.1586/edm.10.60 [DOI] [Google Scholar]
- 7. Satoh M., Tabuchi T., Minami Y., Takahashi Y., Itoh T., and Nakamura M. (2012) Expression of let-7i is associated with Toll-like receptor 4 signal in coronary artery disease: effect of statins on let-7i and Toll-like receptor 4 signal. Immunobiology 217, 533–539 10.1016/j.imbio.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 8. Takwi A. A., Li Y., Becker Buscaglia L. E., Zhang J., Choudhury S., Park A. K., Liu M., Young K. H., Park W. Y., Martin R. C., and Li Y. (2012) A statin-regulated microRNA represses human c-Myc expression and function. EMBO Mol. Med. 4, 896–909 10.1002/emmm.201101045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox J. D., Baquerizo-Nole K. L., Macquhae F., Herskovitz I., Freedman J. B., Vileikyte L., Margolis D. J., and Kirsner R. S. (2016) Statins may be associated with six-week diabetic foot ulcer healing. Wound Repair Regen. 24, 454–457 [DOI] [PubMed] [Google Scholar]
- 10. Johansen O. E., Birkeland K. I., Jørgensen A. P., Orvik E., Sørgård B., Torjussen B. R., Ueland T., Aukrust P., and Gullestad L. (2009) Diabetic foot ulcer burden may be modified by high-dose atorvastatin: a 6-month randomized controlled pilot trial. J. Diabetes 1, 182–187 10.1111/j.1753-0407.2009.00031.x [DOI] [PubMed] [Google Scholar]
- 11. Corrao G., Ibrahim B., Nicotra F., Soranna D., Merlino L., Catapano A. L., Tragni E., Casula M., Grassi G., and Mancia G. (2014) Statins and the risk of diabetes: evidence from a large population-based cohort study. Diabetes Care 37, 2225–2232 10.2337/dc13-2215 [DOI] [PubMed] [Google Scholar]
- 12. Pencina M. J., Navar-Boggan A. M., D'Agostino R. B. Sr., Williams K., Neely B., Sniderman A. D., and Peterson E. D. (2014) Application of new cholesterol guidelines to a population-based sample. N. Engl. J. Med. 370, 1422–1431 10.1056/NEJMoa1315665 [DOI] [PubMed] [Google Scholar]
- 13. Herskovitz I., MacQuhae F. E., Dickerson J. E. Jr., Cargill D. I., Slade H. B., Margolis D. J., and Kirsner R. S. (2017) Opioids' effect on healing of venous leg ulcers. J. Invest. Dermatol. 137, 2646–2649 [DOI] [PubMed] [Google Scholar]
- 14. Menon G. K., Feingold K. R., Moser A. H., Brown B. E., and Elias P. M. (1985) De novo sterologenesis in the skin: II: regulation by cutaneous barrier requirements. J. Lipid Res. 26, 418–427 [PubMed] [Google Scholar]
- 15. Jozic I., Stojadinovic O., Kirsner R. S., and Tomic-Canic M. (2014) Stressing the steroids in skin: paradox or fine-tuning? J. Invest. Dermatol. 134, 2869–2872 10.1038/jid.2014.363 [DOI] [PubMed] [Google Scholar]
- 16. Vukelic S., Stojadinovic O., Pastar I., Rabach M., Krzyzanowska A., Lebrun E., Davis S. C., Resnik S., Brem H., and Tomic-Canic M. (2011) Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J. Biol. Chem. 286, 10265–10275 10.1074/jbc.M110.188268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stojadinovic O., Lee B., Vouthounis C., Vukelic S., Pastar I., Blumenberg M., Brem H., and Tomic-Canic M. (2007) Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-γ pathway, and wound healing along with promotion of terminal differentiation. J. Biol. Chem. 282, 4021–4034 [DOI] [PubMed] [Google Scholar]
- 18. Jozic I., Vukelic S., Stojadinovic O., Liang L., Ramirez H. A., Pastar I., and Tomic Canic M. (2017) Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3β/β-catenin pathway to inhibit wound closure. J. Invest. Dermatol. 137, 1144–1154 10.1016/j.jid.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z., Frederick J., and Garabedian M. J. (2002) Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J. Biol. Chem. 277, 26573–26580 10.1074/jbc.M110530200 [DOI] [PubMed] [Google Scholar]
- 20. Vukelic S., Stojadinovic O., Pastar I., Vouthounis C., Krzyzanowska A., Das S., Samuels H. H., and Tomic-Canic M. (2010) Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. J. Biol. Chem. 285, 1980–1988 10.1074/jbc.M109.016741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pastar I., Stojadinovic O., Sawaya A. P., Stone R. C., Lindley L. E., Ojeh N., Vukelic S., Samuels H. H., and Tomic-Canic M. (2016) Skin metabolite, farnesyl pyrophosphate, regulates epidermal response to inflammation, oxidative stress and migration. J. Cell. Physiol. 231, 2452–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindley L. E., Stojadinovic O., Pastar I., and Tomic-Canic M. (2016) Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 138, 18S–28S 10.1097/PRS.0000000000002682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stojadinovic O., Brem H., Vouthounis C., Lee B., Fallon J., Stallcup M., Merchant A., Galiano R. D., and Tomic-Canic M. (2005) Molecular pathogenesis of chronic wounds: the role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 167, 59–69 10.1016/S0002-9440(10)62953-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brem H., and Tomic-Canic M. (2007) Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 117, 1219–1222 10.1172/JCI32169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stojadinovic O., Pastar I., Nusbaum A. G., Vukelic S., Krzyzanowska A., and Tomic-Canic M. (2014) Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen. 22, 220–227 10.1111/wrr.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waikel R. L., Kawachi Y., Waikel P. A., Wang X. J., and Roop D. R. (2001) Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet. 28, 165–168 10.1038/88889 [DOI] [PubMed] [Google Scholar]
- 27. Batista P. J., and Chang H. Y. (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307 10.1016/j.cell.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kino T., Hurt D. E., Ichijo T., Nader N., and Chrousos G. P. (2010) Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3, ra8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan T. P., Eaglstein W. H., Davis S. C., and Mertz P. (2001) The pig as a model for human wound healing. Wound Repair Regen. 9, 66–76 10.1046/j.1524-475x.2001.00066.x [DOI] [PubMed] [Google Scholar]
- 30. Dimmeler S., Aicher A., Vasa M., Mildner-Rihm C., Adler K., Tiemann M., Rütten H., Fichtlscherer S., Martin H., and Zeiher A. M. (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Invest. 108, 391–397 10.1172/JCI200113152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrientos S., Stojadinovic O., Golinko M. S., Brem H., and Tomic-Canic M. (2008) Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601 10.1111/j.1524-475X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- 32. Bitto A., Minutoli L., Altavilla D., Polito F., Fiumara T., Marini H., Galeano M., Calo M., Lo Cascio P., Bonaiuto M., Migliorato A., Caputi A. P., and Squadrito F. (2008) Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol. Res. 57, 159–169 10.1016/j.phrs.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 33. Brem H., Kodra A., Golinko M. S., Entero H., Stojadinovic O., Wang V. M., Sheahan C. M., Weinberg A. D., Woo S. L., Ehrlich H. P., and Tomic-Canic M. (2009) Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J. Invest. Dermatol. 129, 2275–2287 10.1038/jid.2009.26 [DOI] [PubMed] [Google Scholar]
- 34. Mehta V. B., and Besner G. E. (2007) HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 25, 253–263 10.1080/08977190701773070 [DOI] [PubMed] [Google Scholar]
- 35. Mehta V. B., Zhou Y., Radulescu A., and Besner G. E. (2008) HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors 26, 301–315 10.1080/08977190802393596 [DOI] [PubMed] [Google Scholar]
- 36. Das S., Schapira M., Tomic-Canic M., Goyanka R., Cardozo T., and Samuels H. H. (2007) Farnesyl pyrophosphate is a novel transcriptional activator for a subset of nuclear hormone receptors. Mol. Endocrinol. 21, 2672–2686 10.1210/me.2007-0080 [DOI] [PubMed] [Google Scholar]
- 37. Hu G., Lou Z., and Gupta M. (2014) The long non-coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c-Myc translation. PLoS ONE 9, e107016, 10.1371/journal.pone.0107016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramirez H. A., Liang L., Pastar I., Rosa A. M., Stojadinovic O., Zwick T. G., Kirsner R. S., Maione A. G., Garlick J. A., and Tomic-Canic M. (2015) Comparative genomic, microRNA, and tissue analyses reveal subtle differences between non-diabetic and diabetic foot skin. PLoS ONE 10, e0137133 10.1371/journal.pone.0137133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pastar I., Stojadinovic O., Yin N. C., Ramirez H., Nusbaum A. G., Sawaya A., Patel S. B., Khalid L., Isseroff R. R., and Tomic-Canic M. (2014) Epithelialization in wound healing: a comprehensive review. Adv. Wound Care 3, 445–464 10.1089/wound.2013.0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pastar I., Nusbaum A. G., Gil J., Patel S. B., Chen J., Valdes J., Stojadinovic O., Plano L. R., Tomic-Canic M., and Davis S. C. (2013) Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE 8, e56846 10.1371/journal.pone.0056846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laing T., Hanson R., Chan F., and Bouchier-Hayes D. (2010) Effect of pravastatin on experimental diabetic wound healing. J. Surg. Res. 161, 336–340 10.1016/j.jss.2009.01.024 [DOI] [PubMed] [Google Scholar]
- 42. Haag W. G., Abril-Hörpel O., Becquerelle S. D., Mertz P. M., and Davis S. C. (2011) Statistical approach for avoiding pseudoreplication and increasing power in wound-healing studies. Wound Repair Regen. 19, 442–448 10.1111/j.1524-475X.2011.00693.x [DOI] [PubMed] [Google Scholar]
- 43. Liu Y., Chen J. Y., Shang H. T., Liu C. E., Wang Y., Niu R., Wu J., and Wei H. (2010) Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp. Med. 60, 142–148 [PMC free article] [PubMed] [Google Scholar]
- 44. Barrientos S., Brem H., Stojadinovic O., and Tomic-Canic M. (2014) Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 22, 569–578 10.1111/wrr.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stone R. C., Stojadinovic O., Rosa A. M., Ramirez H. A., Badiavas E., Blumenberg M., and Tomic-Canic M. (2017) A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci. Transl. Med. 9, eaaf8611 10.1126/scitranslmed.aaf8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stojadinovic O., Pastar I., Vukelic S., Mahoney M. G., Brennan D., Krzyzanowska A., Golinko M., Brem H., and Tomic-Canic M. (2008) Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J. Cell. Mol. Med. 12, 2675–2690 10.1111/j.1582-4934.2008.00321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arnold I., and Watt F. M. (2001) c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11, 558–568 10.1016/S0960-9822(01)00154-3 [DOI] [PubMed] [Google Scholar]
- 48. Stojadinovic O., Landon J. N., Gordon K. A., Pastar I., Escandon J., Vivas A., Maderal A. D., Margolis D. J., Kirsner R. S., and Tomic-Canic M. (2013) Quality assessment of tissue specimens for studies of diabetic foot ulcers. Exp. Dermatol. 22, 216–218 10.1111/exd.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sivaprasad U., Abbas T., and Dutta A. (2006) Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol. Cancer Ther. 5, 2310–2316 10.1158/1535-7163.MCT-06-0175 [DOI] [PubMed] [Google Scholar]
- 50. Banach M., Rizzo M., Toth P. P., Farnier M., Davidson M. H., Al-Rasadi K., Aronow W. S., Athyros V., Djuric D. M., Ezhov M. V., Greenfield R. S., Hovingh G. K., Kostner K., Serban C., Lighezan D., et al. (2015) Statin intolerance: an attempt at a unified definition: position paper from an International Lipid Expert Panel. Expert Opin. Drug Saf. 14, 935–955 10.1517/14740338.2015.1039980 [DOI] [PubMed] [Google Scholar]
- 51. Apostolopoulou M., Corsini A., and Roden M. (2015) The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Invest. 45, 745–754 [DOI] [PubMed] [Google Scholar]
- 52. Liang L., Stone R. C., Stojadinovic O., Ramirez H., Pastar I., Maione A. G., Smith A., Yanez V., Veves A., Kirsner R. S., Garlick J. A., and Tomic-Canic M. (2016) Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 24, 943–953 10.1111/wrr.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pastar I., Khan A. A., Stojadinovic O., Lebrun E. A., Medina M. C., Brem H., Kirsner R. S., Jimenez J. J., Leslie C., and Tomic-Canic M. (2012) Induction of specific microRNAs inhibits cutaneous wound healing. J. Biol. Chem. 287, 29324–29335 10.1074/jbc.M112.382135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stojadinovic O., and Tomic-Canic M. (2013) Human ex vivo wound healing model. Methods Mol. Biol. 1037, 255–264 10.1007/978-1-62703-505-7_14 [DOI] [PubMed] [Google Scholar]