Abstract
Purpose
To report a case of juvenile xanthogranuloma involving the iris and skin that clincally was diagnosed with an obvious cutaneous lesion.
Observations
A four month-old girl with hyphema and increased intraocular pressure of the left eye persisting for 2 weeks. A suspicious yellow-brown mass with nodular surface and traversed by irregular vascularization was noted on the inferior iris surface. Ultrasound biomicroscopy (UBM; 35 MHz) of the mass revealed multiple nodular irregular hyperreflective lesions in the peripheral iris. Using a biopsy of an obvious cutaneous abdominal skin lesion a diagnosis was made based on histopathological analyses. The biopsy showed dense dermal infiltrate consisting of foamy histiocytes. Additional stains revealed CD68 positivity and CD1a and S100 negativity. This mass revealed histopathologic features identical to juvenile xanthogranuloma and was concurrent with the iris lesion. Next-generation sequencing using Ion AmpliSeqTM Cancer Hotspot Panel revealed a missense mutation of FGFR3 (p.F386L).
Conclusion and importance
The diagnosis of a xanthogranuloma of the iris with hyphema can be made easier in patients with obvious cutaneous lesions as described in our case. The significance of FGFR3 mutation in association with JXG is unknown and should be further investigated.
Keywords: Juvenile xanthoganuloma, Iris, Ultrasound biomicroscopy, Cutaneous lesion, FGFR3 mutation
1. Introduction
Juvenile xanthogranuloma (JXG) is a rare benign hystiocytic proliferation most common in infants and young children. Typically it manifests primarily as a self-limited dermatologic disorder and consists of solitary or multifocal yellow-pink cutaneous nodules.1 JXG can rarely be associated with extracutaneous sites including mostly the eye. Ocular JXG typically manifests as a circumscript iris nodule, occasionally with hyphema and elevated intraocular pressure.2 We describe a case of nodular iris JXG with associated skin lesion of which an additional molecular-pathological examination was performed.
2. Case report
2.1. Clinical history
A four month-old girl was referred to our eye clinic for cloudiness and redness of the left eye which had persisted for 2 weeks. The medical history including pregnancy and birth was unremarkable. The parents denied any history of trauma or signs for allergy. The child presented with an increased intraocular pressure (IOP) of the left eye of 40 mm Hg (right eye 16 mm Hg), mild edema of the cornea and primary hyphema up to the pupillary edge which later developed into a “pseudohypopyon”. Slight anisocoria was observed. To reduce the IOP, treatment was started with local acetazolamide and β-blocker (twice a day) and topical steroid (three times a day). Ten days later, left eye, IOP had decreased to 16 mm Hg and hyphema regressed. A suspicious yellow-brown mass with irregular vascularisation was noted on the inferior iris surface. In addition, heterochromia with green left iris and blue right iris was obvious.
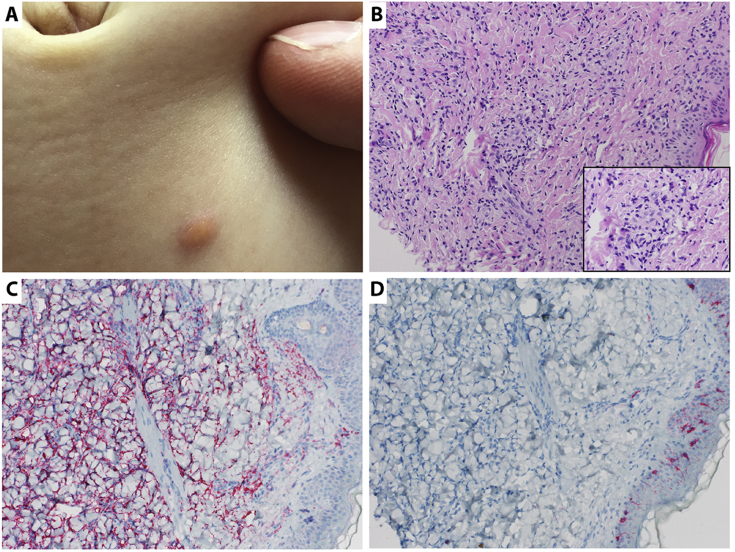
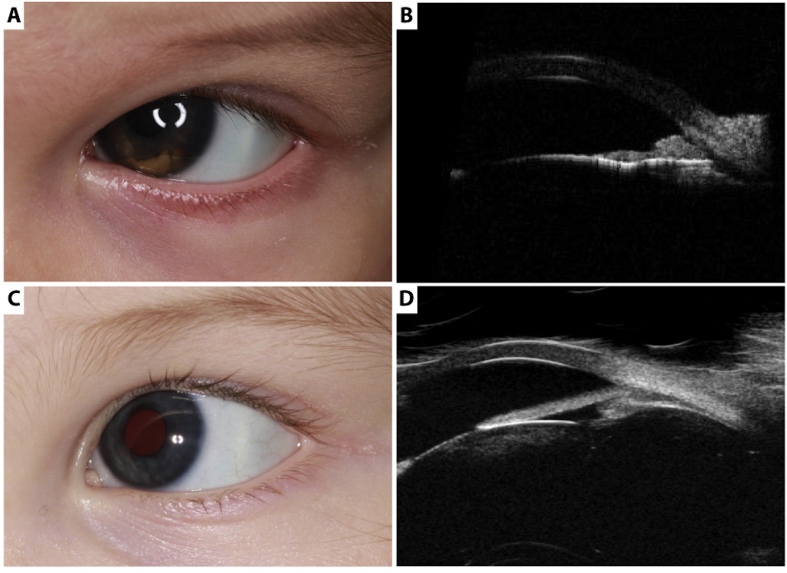
During further physical examination we found one yellowish-brown papule on the abdomen of the child (Fig. 2A). Under general anaesthesia, ultrasound biomicroscopy of the yellow brown mass on the iris surface (UBM; 35 MHz) revealed multiple nodular irregular hyperreflective lesions in the peripheral iris between 2 and 7 o'clock, growing into the anterior chamber angle and towards the corneal endothelium. The pigmented epithelium of the iris was intact, the ciliary body was not involved (Fig. 1A and B). Fluorescence angiography of the iris showed slight staining in the late phase. Retinal examination of the posterior pole of both eyes and B-scan echography showed no lesion in the vitreous, retina/choroidea or optic nerve thus a retinoblastoma was ruled out. Under the same general anaesthesia the papule on the abdomen was totally excised and submitted for histological examination.
Fig. 2.
Histopathological analyses of the skin lesion. A. Abdominal skin lesion used for histopathological evaluation. B. Hematoxylin and eosin staining (20x) showing dense dermal infiltrate consisting of histiocytes, some lymphocytes and plasma cells. Touton giant cells were not detected. A 60× magnification of the respective area is shown in the lower right corner. C. Positive CD68 staining (20x) for confirmation of the histiocytic phenotype. D. Negative CD1a and S100 staining (20x) ruling out Langerhans cell histiocytosis.
Fig. 1.
Clinical evaluation of the iris lesion. A. A Yellow-brown mass with irregular vascularization at the inferior part of the iris surface; became visible after ten days of treatment (dorzolamid/timolol and topical steroid). B. Ultrasound biomicroscopy of the anterior segment revealing multiple nodular hyperreflective heterogenous mass in the peripheral iris between 2 and 7 o'clock, growing into the anterior chamber angle and to the corneal endothelium (section at 5 o'clock). C. After 2 month of high-dose corticosteroid treatment iris lesion decreased completely without recurrence of hyphae or iritis. D. Ultrasound biomicroscopy confirming decrease of iris lesion (section at 5 o'clock).
2.2. Dermal pathology
Histology of the cutaneous abdominal skin lesion showed a dense dermal infiltrate consisting of histiocytes, some lymphocytes and plasma cells without touton giant cells. The overlying epidermis was focally attenuated. The histiocytes were mostly mononuclear with a narrow cytoplasmic rim and cytoplasmic vacuoles, reaching focally up to the epidermis. Immunohistochemically, the histiocytic phenotype was confirmed with a positive staining for CD68. Langerhans cell histiocytosis was ruled out with a negative reaction for CD1a and S100. A malignant lymphoma could be excluded (Fig. 2).
2.3. Molecular pathology
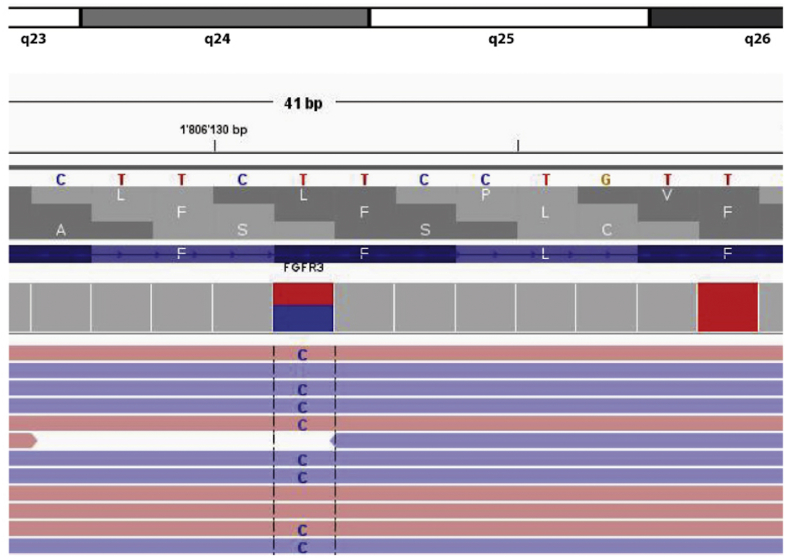
DNA was extracted from paraffin tissue. Next-generation sequencing from the tumor tissue was performed using an Ion AmpliSeqTM Cancer Hotspot Panel v2: A BRAF mutation as described for Langerhans cell histiocytosis3 could be excluded. Instead, FGFR3 missense mutation (p.F386L) was found, so far undescribed in Juvenile Xanthogranuloma (Fig. 3).
Fig. 3.
Molecularpathological analyses of the skin lesion. Next-generation sequencing using Ion AmpliSeqTM Cancer Hotspot Panel v2 revealing FGFR3 (p.F386L) missense mutation.
Treatment was initiated with topical high-dose corticosteroid (every 2 hours during waking hours and ointment at bedtime) with a slow taper. During the next month the iris lesion decreased completely without recurrence of hyphae or iritis and after three weeks the therapy with corticosteroids was stopped (Fig. 1C and D). However a secondary esotropia with deep amblyopia of the left eye was observed. Visual acuity (VA) was measured using Teller Acuity Cards. VA was 4.8 cy/cm at 38 cm (corresponding to VA 20/180) in the right eye and 0.86 cy/cm at 38 cm (corresponding to VA 20/1000) in left eye. After correction of the hyperopia with sph +4.0 glasses for both eyes as well as an occlusion therapy with patching of the non-amblyopic eye for 2.5 hours/day were started. After 5 months the visual outcome increased in the left eye to 2.4 cy/cm (corresponding to VA 20/360; lower limit of normal value).
2.4. Diagnosis
Diagnosis of juvenile xanthogranuloma (JXG) was made based on the histology obtained from a cutaneous lesion. Genetically, a missense mutation of the FGFR3 gene (p.F386L) with unknown significance was found.
3. Discussion
JXG is a rare benign histiocytic proliferation most common as benign cutaneous disorder in infants and young children. It is regarded as the common form of non-Langerhans cell histiocytosis.4 Extracutaneous JXG can affect the eye and ocular adnexa as well as central nervous system, lung, liver, spleen and other sites. Eye involvement is rather rare with a range of 0.3%–10% of children with cutaneous JXG.1 Ocular JXG preferentially affects the iris and can potentially lead to blinding complications such as hyphema, secondary glaucoma and amblyopia.5 In a survey of 449 iris tumors occured in children (less than 20 years old) 14 (3%) were xanthogranulomatous lesions.6 In another study of Chang MW et al. cutaneous lesions have been found in up to 40% of children with ocular JXG.1, 7
Spontaneous hyphema with or without increase of intraocular pressure – in our case with an increased IOP - is a common sign of iris JXG. The differential diagnosis of hyphema in childhood includes trauma, neoplastic conditions like retinoblastoma, medulloepithelioma and leukemia, retinopathy of prematurity and blood dyscrasias and should clinically be ruled out.5
UBM as well as anterior segment optical coherence tomography can help confirm the diagnosis, demonstrating the iris nodular lesions with intact iris pigment epithelium like in our case or demonstrating flat lesions on the iris surface in other pattern of JXG.8 In atypical presentations and lesions of poor response to steroid a fine-needle aspiration biopsy is recommended.9 The diagnosis can be easier made in patients with obvious cutaneous lesions like in our case.
Pathologically a Langerhans cell histiocytosis has been excluded with a CD 1a negative staining of the histiocytes. In early stage of JXG touton giant cells could not be detected like in our case.10 The pathogenesis of JXG is still unclear. Although well-charcterised by morphology and immunohistology the genetic profile of JXG is not yet elucidated.
Genetically a missense mutation of FGFR3 (p.F386L) was found. The FGFR3 p.F386L mutation has been reported in patients with cancer11 and aggressive fibromatosis.12 In addition FGFR3 mutations have been identified in benign acanthotic skin tumors such as in seborrheic keratoses and epidermal nevi.13, 14 However, the biological significance of this mutation in JXG is unknown.
Therapeutically there is no standard of care for the ophthalmic manifestation of JXG. Nonsurgical approaches are preferred because of less risk of intraocular bleeding. Corticosteroids are recommended as principal therapy. Topical application starting with a regimen every 2 hours a day, then slowly tapering over a time period of 3–4 month would be preferred.5 Systemic corticosteroids are generally avoided and only considered in uncontrolled lesions of the iris. The control rate with topical corticosteroids alone is 63% with a mean duration of 8 weeks.5 To avoid the danger of amblyopia the therapy should be started as fast as possible. In our case a secondary esotropia with deep amblyopia of the left eye was observed which required an adequate therapy. In two children with JXG refractory to local administration of corticosteroid the successful use of off-label intraocular bevacizumab was reported. Bevacizumab was administered leading to reduced vascularity and flattening of the JXG lesion 3 months after treatment. The authors suggest that intraocular bevacizumab has a lower risk of cataract formation and secondary glaucoma than topical corticosteroids.15
4. Conclusions
In summary we report a case of JXG based on the histology obtained from a cutaneous lesion. Genetically a missense mutation within the FGFR3 gene was found. The biological significance of this mutation in JXG is unknown. Clinically imaging of the iris with fluorescein angiography, UBM aided in the detection and the differential diagnosis of the iris lesion leading to hyphema and secondary glaucoma. With use of topical corticosteroids the JXG was successfully treated, however a secondary esotropia with deep amblyopia of the left eye was observed.
Patient consent
Informed written consent was obtained from the patient's legal guardian for publication of personal and medical record details.
Disclosures
Funding
No funding or grant support.
Conflict of interest
The following authors have no financial disclosures: MP, GE, KC, MF, BE.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
We gratefully thank the patient and her family for participation in this study. We also thank Myriam Vonlanthen for her technical help with the immunohistochemistry samples.
References
- 1.Chang M.W. Update on juvenile xanthogranuloma: unusual cutaneous and systemic variants. Semin Cutan Med Surg. 1999;18(3):195–205. doi: 10.1016/s1085-5629(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 2.Danzig C.J., Shields C.L., Mashayekhi A., Ehya H., Manquez M.E., Shields J.A. Fluorescein angiography of iris juvenile xanthogranuloma. J Pediatr Ophthalmol Strabismus. 2008;45(2):110–112. doi: 10.3928/01913913-20080301-03. [DOI] [PubMed] [Google Scholar]
- 3.Tatsuno M., Shioda Y., Iwafuchi H. BRAF V600 mutations in Langerhans cell histiocytosis with a simple and unique assay. Diagn Pathol. 2016;11:39. doi: 10.1186/s13000-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Martin A., Baselga E., Drolet B.A., Esterly N.B. Juvenile xanthogranuloma. J Am Acad Dermatol. 1997;36(3 Pt 1):355–367. doi: 10.1016/s0190-9622(97)80207-1. quiz 368–359. [DOI] [PubMed] [Google Scholar]
- 5.Samara W.A., Khoo C.T., Say E.A. Juvenile xanthogranuloma involving the eye and ocular adnexa: tumor control, visual outcomes, and globe salvage in 30 patients. Ophthalmology. 2015;122(10):2130–2138. doi: 10.1016/j.ophtha.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Shields C.L., Kancherla S., Patel J. Clinical survey of 3680 iris tumors based on patient age at presentation. Ophthalmology. 2012;119(2):407–414. doi: 10.1016/j.ophtha.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 7.Chang M.W., Frieden I.J., Good W. The risk intraocular juvenile xanthogranuloma: survey of current practices and assessment of risk. J Am Acad Dermatol. 1996;34(3):445–449. doi: 10.1016/s0190-9622(96)90437-5. [DOI] [PubMed] [Google Scholar]
- 8.Samara W.A., Khoo C.T., Magrath G., Shields C.L. Multimodal imaging for detection of clinically inapparent diffuse iris juvenile xanthogranuloma. J Pediatr Ophthalmol Strabismus. 2015;52:e30–33. doi: 10.3928/01913913-20150506-01. Online. [DOI] [PubMed] [Google Scholar]
- 9.Karcioglu Z.A., Mullaney P.B. Diagnosis and management of iris juvenile xanthogranuloma. J Pediatr Ophthalmol Strabismus. 1997;34(1):44–51. doi: 10.3928/0191-3913-19970101-10. [DOI] [PubMed] [Google Scholar]
- 10.Janssen D., Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29(1):21–28. doi: 10.1097/01.pas.0000147395.01229.06. [DOI] [PubMed] [Google Scholar]
- 11.Feng S., Zhou L., Nice E.C., Huang C. Fibroblast growth factor receptors: multifactorial-contributors to tumor initiation and progression. Histol Histopathol. 2015;30(1):13–31. doi: 10.14670/HH-30.13. [DOI] [PubMed] [Google Scholar]
- 12.Meazza C., Belfiore A., Busico A. AKT1 and BRAF mutations in pediatric aggressive fibromatosis. Cancer Med. 2016;5(6):1204–1213. doi: 10.1002/cam4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafner C., van Oers J.M., Vogt T. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116(8):2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logie A., Dunois-Larde C., Rosty C. Activating mutations of the tyrosine kinase receptor FGFR3 are associated with benign skin tumors in mice and humans. Hum Mol Genet. 2005;14(9):1153–1160. doi: 10.1093/hmg/ddi127. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazy N., Henry C.R., Abbey A.M., McKeown C.A., Berrocal A.M., Murray T.G. Successful treatment of juvenile xanthogranuloma using bevacizumab. J AAPOS. 2014;18(3):295–297. doi: 10.1016/j.jaapos.2014.01.007. [DOI] [PubMed] [Google Scholar]