Key Points
Silica nanoparticles act as an effector for human native and sickle cell hemoglobin while preserving their tetrameric structure.
Manipulating hemoglobin oxygenation using nanoparticles opens the way for the rational design of hemoglobin-based oxygen carriers.
Abstract
Recently, nanoparticles have attracted much attention as new scaffolds for hemoglobin-based oxygen carriers (HBOCs). Indeed, the development of bionanotechnology paves the way for the rational design of blood substitutes, providing that the interaction between the nanoparticles and hemoglobin at a molecular scale and its effect on the oxygenation properties of hemoglobin are finely controlled. Here, we show that human hemoglobin has a high affinity for silica nanoparticles, leading to the adsorption of hemoglobin tetramers on the surface. The adsorption process results in a remarkable retaining of the oxygenation properties of human adult hemoglobin and sickle cell hemoglobin, associated with an increase of the oxygen affinity. The cooperative oxygen binding exhibited by adsorbed hemoglobin and the comparison with the oxygenation properties of diaspirin cross-linked hemoglobin confirmed the preservation of the tetrameric structure of hemoglobin loaded on silica nanoparticles. Our results show that silica nanoparticles can act as an effector for human native and mutant hemoglobin. Manipulating hemoglobin oxygenation using nanoparticles opens the way to the design of novel HBOCs.
Visual Abstract
Introduction
Despite the great need for blood substitutes to treat hemorrhagic shocks, their development remains highly challenging, and clinical trials have not yet proven successful.1 Blood substitutes have to overcome the acute renal toxicity associated with the delivery of free hemoglobin in the circulatory system and to prevent fast clearance from the bloodstream, vasoconstriction, and severe cardiac effects.1,2 The 2 main strategies that have been used so far are hemoglobin cross-linking, polymerization, or conjugation; and hemoglobin encapsulation in liposomes, polymersomes, hydrogels, or porous microparticles.3-5 Recently, nanoparticles have attracted much attention as new scaffolds for hemoglobin-based oxygen carriers (HBOCs).6-10 Indeed, the development of bionanotechnology paves the way for the rational design of blood substitutes, providing that the interaction between the nanoparticles and hemoglobin at a molecular scale and its effect on the oxygenation properties of hemoglobin are finely controlled.
In our previous study, we showed that pig hemoglobin has a high affinity for silica nanoparticles (SNPs).11 The adsorption process leads to a modification of the protein structure associated with a remarkable retaining of its oxygen-binding properties and cooperativity. In this report, we investigated whether SNPs could enable one to tune the oxygenation properties of human native and mutant hemoglobin. Most of the HBOCs currently tested are based on the encapsulation of hemoglobin in larger structures (eg, in liposomes or vesicles), hemoglobin loading in porous particles, or integration in a polymeric or gel matrix. However, the rapid clearance from the bloodstream of the carrier, its degradation leading to the release of free hemoglobin, or the uncontrolled change of the oxygen-binding properties of hemoglobin impairing its oxygen delivery efficiency have limited their clinical application.1,5,12 In their meta-analysis of 16 clinical trials of hemoglobin-based blood substitutes, Natanson and coworkers concluded that the use of HBOCs is associated with a significant increase of the risk for myocardial infarction and death.2 The development of HemAssist was later abandoned by Baxter, and the clinical trial of Hemopure on trauma patients in the United States was halted by the US Food and Drug Adminsitration.1
More recently, HemoTech (by HemoBioTech), which is derived from bovine hemoglobin cross-linked with ATP and glutathione, was tested in clinical trials in India.13 However, other studies suggested a potential renal toxicity of polymerized Hb systems associated with oxidative stress.14,15 Despite great efforts and recent progress for the development of new blood substitutes, no HBOCs have yet been approved for clinical use in Europe or in the United States.
We also note that the oxygen affinity and the quaternary structure of hemoglobin in HBOCs vary a lot from one system to another, including the second-generation HBOCs,1,2,13 indicating that a common strategy and the best design have yet to be found. Here, we used a different strategy based on the direct adsorption of hemoglobin on the surface of the silica nanoparticles. We investigated whether this approach could allow controlling the oxygenation properties of hemoglobin to preserve its structure and to prevent the release of free hemoglobin in physiological conditions. Our results show that SNPs can act as an effector for human adult hemoglobin (HbA) and sickle cell hemoglobin (HbS) and allow manipulation of their oxygenation properties while maintaining their tetrameric structure in physiological conditions. The in vivo studies reported for nanoparticle-based systems5,6,10 suggest these new HBOCs may become a suitable blood substitute in the future.
Methods
HbA hemolysate and sickle cell hemolysate containing 87% HbS were prepared from fresh donor blood collected after ethical guidelines, and the experimental protocol abides by the Declaration of Helsinki. All donors have given their agreement for their blood to be used for experiments. Diaspirin cross-linked hemoglobin (DCLHb) was provided by Baxter Healthcare Corporation. SNPs (Sigma-Aldrich 637238) with a mean diameter of 26 ± 2 nm were extensively characterized in our previous study.16 Characterization of SNPs, hemoglobin purification, adsorption isotherms, Langmuir model, protein desorption, and oxygen binding measurements are described in the supplemental Data.
Results and Discussion
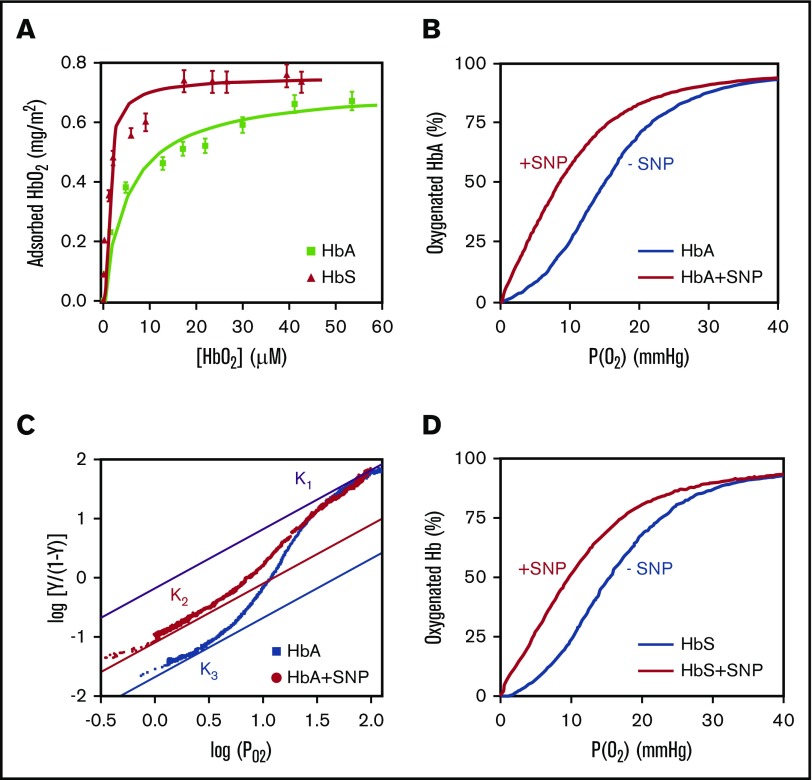
HbA has a strong affinity for SNPs and readily adsorbs on the negatively charged silica surface in phosphate buffer at pH 7.4 (Figure 1A). The nonporous SNPs are coated by the adsorbed protein. The adsorption isotherm is well fitted by the Langmuir model (supplemental Equation 1) with an affinity constant Kads = 18 ± 2 µM−1. The amount of adsorbed HbA mads = 0.67 ± 0.04 mg/m2 is twice the amount of adsorbed pig HbA on the same batch of SNPs.11 No desorption was observed at pH 6.0 and at pH 7.4. Hb adsorbed on SNPs is not covalently bound and could potentially be replaced by other blood proteins.17 However, Hb adsorption on SNPs is driven by multivalent interactions16,18 and leads to stable and irreversible binding in physiological conditions.11 Adsorption did not induce any oxidation of HbA, as indicated by spectrophotometry.11 The oxygen binding curves of HbA hemolysate with SNPs at 37°C show a significant increase of the oxygen affinity of adsorbed HbA (Figure 1B). Moreover, the lower oxygen affinity of adsorbed HbA at pH 6 (P50 = 10.7 ± 0.5 mm Hg; supplemental Figure 1) compared with adsorbed HbA at pH 7.4 (P50 = 7.2 ± 0.5 mm Hg) indicates that adsorbed HbA retains its proton exchange capacity after adsorption on SNPs, which in turn controls its affinity (Bohr effect19).
Figure 1.
Functional analysis of human HbA and HbS adsorbed on SNPs. (A) Adsorption isotherms of human HbA and HbS on SNPs. (B) Oxygen binding curve of HbA hemolysate at 37°C with and without SNPs. (C) Hill plot of the oxygen binding curves of purified HbA with and without SNPs at 37°C. The upper and lower asymptotes represent the limits for the high-affinity and low-affinity oxygen binding. K1 is the oxygen association equilibrium constant for the final oxygen binding step and is identical for free and adsorbed HbA. K2 and K3 are the association equilibrium constants for the initial oxygen binding of adsorbed HbA and free HbA, respectively. (D) Oxygen binding curves of HbS hemolysate at 37°C with and without SNPs. All the experiments were performed in 0.1 M phosphate buffer at pH 7.4. In these conditions, hemoglobin is totally adsorbed on SNPs.
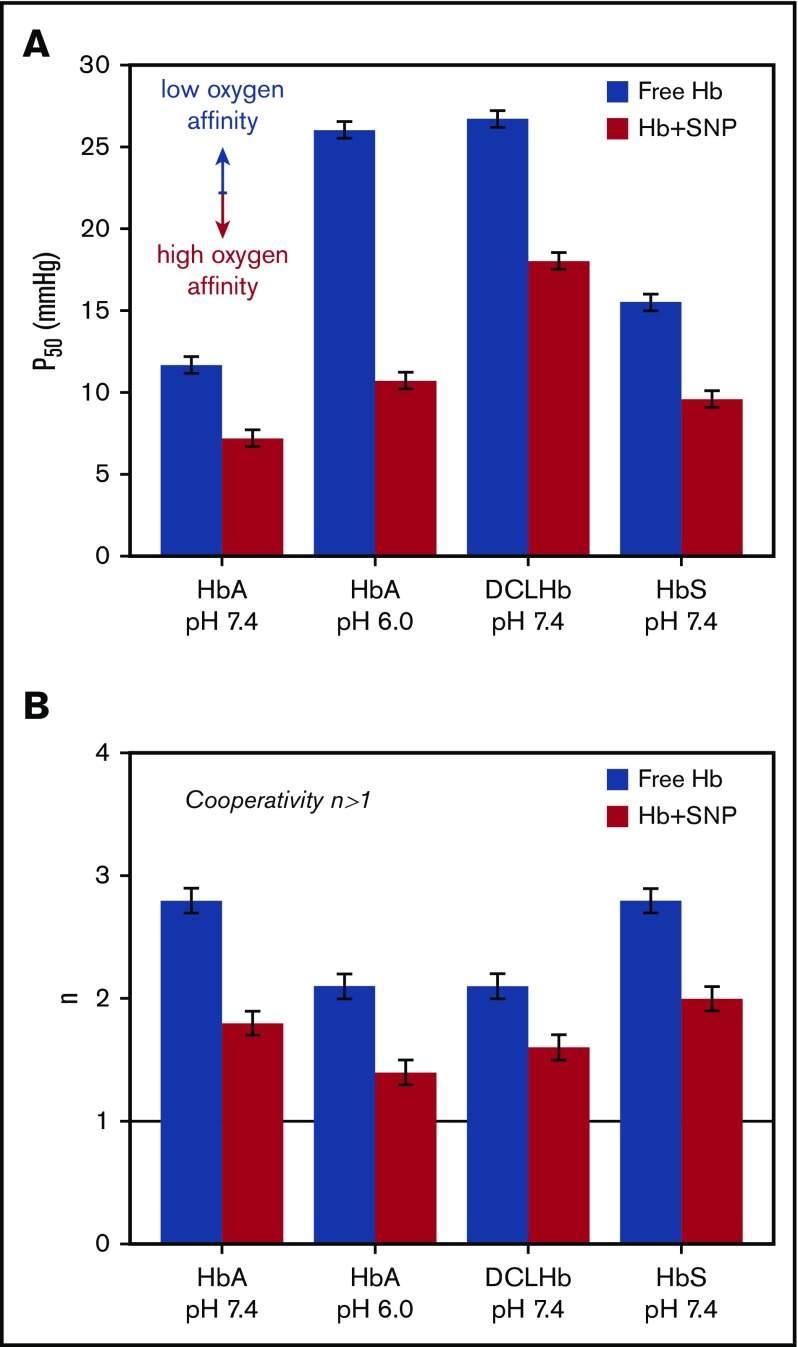
Adsorbed HbA maintains cooperativity despite a significant decrease, as indicated by the Hill coefficient (n = 1.8 ± 0.1 compared with n = 2.8 ± 0.1 for free HbA; Figure 2B). The cooperative binding of oxygen (n > 1) is clear evidence that adsorbed HbA retains its tetrameric structure after adsorption on SNPs.20 A similar effect is observed after the adsorption of DCLHb on SNPs (Figure 2; supplemental Figure 2). As DCLHb cannot dissociate into dimers (n = 2.1 ± 0.1),21 this result further confirms that the modification of the oxygenation properties of adsorbed HbA is not a result of the breakdown of the tetramer but, rather, a result of specific changes of hemoglobin structure and dynamics on the silica surface.11,18 Interestingly, the Hill plot shows that the high-affinity constant K1 = 0.68 mm Hg−1 is the same for free and adsorbed HbA, whereas the low-affinity constant is higher for adsorbed HbA (K2 = 0.08 mm Hg−1) compared with free HbA (K3 = 0.02 mm Hg−1) (Figure 1C).22 This means that the change in the oxygenation properties is the result of a difference in the initial oxygen binding step (K2, K3) between free HbA and adsorbed HbA, whereas the final binding step (K1) is identical. The increase of the association equilibrium constant for the initial oxygen binding could therefore account for the decrease in cooperativity of adsorbed HbA.23
Figure 2.
Oxygenation properties of human HbA, HbS, and DCLHb after adsorption on SNPs. Oxygen partial pressure at half saturation P50 (A) and Hill coefficient n (B) of HbA, DCLHb, HbS in 0.1 M phosphate buffer at pH 7.4, and HbA in 0.1 M BisTris-HCl buffer at pH 6.0, with and without SNPs. The black line represents the limit of cooperative oxygen binding (n > 1).
We investigated whether SNPs could also change the oxygenation properties of mutant HbS (β6: Glu→Val) responsible for sickle cell anemia. The adsorption isotherm of HbS reveals some differences compared with native HbA (Figure 1A). The higher amount of adsorbed HbS (mads = 0.74 ± 0.04 mg/m2) shows that the hemoglobin payload on SNPs could be further optimized. The adsorption isotherm does not follow a Langmuir model, which could be because of the irreversible adsorption, multiple binding sites, or lateral interactions between proteins.24,25 HbS polymerization is very unlikely at this low protein concentration. Moreover, the amount of adsorbed HbS does not exceed 1 monolayer of tetramers.26 However, the effect of SNPs on the polymerization of HbS at high protein concentration has yet to be determined. The oxygen binding curve of adsorbed HbS shows that SNPs have a similar effect on HbS and HbA (Figure 1D) associated with an increase of the oxygen affinity (P50 = 9.6 ± 0.5 mm Hg) and a decrease of the cooperativity (n = 2.0 ± 0.1) (Figure 2B). Despite differences in the adsorption of pig HbA, human HbA, and mutant HbS, the effect of SNPs on the oxygenation properties of hemoglobin are virtually identical.11 This could indicate that local structural modifications may not be the key to explain the effect of SNPs on the oxygenation properties of hemoglobin but, rather, large structural or dynamic changes that globally affect adsorbed hemoglobin. Using incoherent neutron scattering experiments, we observed in a previous study that myoglobin adsorption on SNPs results in a large decrease of the protein dynamics.18 According to Yonetani’s model,27 a similar decrease in hemoglobin dynamics could result in an increase of the oxygen affinity. This hypothesis could explain the change of the oxygenation properties of various forms of hemoglobins after adsorption on SNPs.
Our study shows that SNPs control the oxygenation properties of native and mutant hemoglobins while retaining their tetrameric structure in physiological conditions. Adsorbed hemoglobin-based oxygen carriers represent a promising approach for the rational design of blood substitutes, using nanoparticles to manipulate the structure and activity of hemoglobin. Moreover, the grafting of active molecules could be used to prevent opsonization, fast clearance from the blood stream, and adverse effects.4,6,7,17,28 Another strategy consists of encapsulating nanoparticles inside liposomes, as shown by Liu and coworkers.8 The use of biocompatible nanoparticles prevents the release of free Hb in the bloodstream, which is associated with acute renal toxicity. Biodistribution studies showed that silica nanoparticles of similar size (20-25 nm) are excreted after a period of 2 weeks in mice without inducing any toxicity.29 Studies of nonporous SNPs30,31 and mesoporous SNPs32,33 showed a size-, shape-, and chemistry-dependent biodistribution. In the future, the assessment of the degradation,34 clearance,30 and toxicity35,36 of Hb-loaded SNPs will be an important step for the development of adsorbed hemoglobin-based oxygen carriers.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This study was supported by the Programme Transversal de Toxicologie du CEA.
Authorship
Contribution: S.D., J.P.R., and S.P. designed research; S.D. performed research and analyzed data; L.K., F.G., V.B.-C., and M.M. recruited patients with sickle cell anemia disease and purified mutant hemoglobins; and S.D., J.P.R., and S.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphanie Devineau, Paris Diderot University, Unit of Functional and Adaptive Biology, CNRS UMR 8251, 5 rue Thomas Mann, 75205 Paris, France; e-mail: stephanie.devineau@univ-paris-diderot.fr.
References
- 1.Chen J-Y, Scerbo M, Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (São Paulo). 2009;64(8):803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piras AM, Dessy A, Chiellini F, et al. Polymeric nanoparticles for hemoglobin-based oxygen carriers. Biochim Biophys Acta. 2008;1784(10):1454-1461. [DOI] [PubMed] [Google Scholar]
- 4.Baudin-Creuza V, Chauvierre C, Domingues E, et al. Octamers and nanoparticles as hemoglobin based blood substitutes. Biochim Biophys Acta. 2008;1784(10):1448-1453. [DOI] [PubMed] [Google Scholar]
- 5.Jia Y, Duan L, Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv Mater. 2016;28(6):1312-1318. [DOI] [PubMed] [Google Scholar]
- 6.Chauvierre C, Marden MC, Vauthier C, Labarre D, Couvreur P, Leclerc L. Heparin coated poly(alkylcyanoacrylate) nanoparticles coupled to hemoglobin: a new oxygen carrier. Biomaterials. 2004;25(15):3081-3086. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Yuan Y, Shan X, et al. Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int J Pharm. 2009;377(1-2):199-206. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Gan L, Chen L, et al. A novel liposome-encapsulated hemoglobin/silica nanoparticle as an oxygen carrier. Int J Pharm. 2012;427(2):354-357. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Zhao C, Wang Q, et al. Preparation, characterization and in vivo investigation of blood-compatible hemoglobin-loaded nanoparticles as oxygen carriers. Colloids Surf B Biointerfaces. 2016;139:171-179. [DOI] [PubMed] [Google Scholar]
- 10.Sen Gupta A. Bio-inspired nanomedicine strategies for artificial blood components. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(6):1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devineau S, Zargarian L, Renault JP, Pin S. Structure and function of adsorbed hemoglobin on silica nanoparticles: relationship between the adsorption process and the oxygen binding properties. Langmuir. 2017;33(13):3241-3252. [DOI] [PubMed] [Google Scholar]
- 12.Moradi S, Jahanian-Najafabadi A, Roudkenar MH. Artificial blood substitutes: First steps on the long route to clinical utility. Clin Med Insights Blood Disord. 2016;9:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simoni J, Simoni G, Moeller JF, Feola M, Wesson DE. Artificial oxygen carrier with pharmacologic actions of adenosine-5′-triphosphate, adenosine, and reduced glutathione formulated to treat an array of medical conditions. Artif Organs. 2014;38(8):684-690. [DOI] [PubMed] [Google Scholar]
- 14.Rentsendorj O, Zhang X, Williams MC, et al. Transcriptional suppression of renal antioxidant enzyme systems in guinea pigs exposed to polymerized cell-free hemoglobin. Toxics. 2016;4(6):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alayash AI. Hemoglobin-based blood substitutes and the treatment of sickle cell disease: More harm than help? Biomolecules. 2017;7(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathé C, Devineau S, Aude JC, et al. Structural determinants for protein adsorption/non-adsorption to silica surface. PLoS One. 2013;8(11):e81346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devineau S, Zanotti JM, Loupiac C, et al. Myoglobin on silica: a case study of the impact of adsorption on protein structure and dynamics. Langmuir. 2013;29(44):13465-13472. [DOI] [PubMed] [Google Scholar]
- 19.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Interactions with Ligands. Frontiers of Biology. Vol 21 Amsterdam, The Netherlands: North Holland Publishing Company; 1971. [Google Scholar]
- 20.Hewitt JA, Kilmartin JV, Eyck LF, Perutz MF. Noncooperativity of the dimer in the reaction of hemoglobin with oxygen (human-dissociation-equilibrium-sulfhydryl-absorption-x-ray analysis). Proc Natl Acad Sci USA. 1972;69(1):203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee R, Welty EV, Walder RY, et al. Isolation and characterization of a new hemoglobin derivative cross-linked between the alpha chains (lysine 99α1→lysine 99α2). J Biol Chem. 1986;261(21):9929-9937. [PubMed] [Google Scholar]
- 22.Tsuneshige A, Kanaori K, Samuni U, et al. Semihemoglobins, high oxygen affinity dimeric forms of human hemoglobin respond efficiently to allosteric effectors without forming tetramers. J Biol Chem. 2004;279(47):48959-48967. [DOI] [PubMed] [Google Scholar]
- 23.Yonetani T, Kanaori K. How does hemoglobin generate such diverse functionality of physiological relevance? Biochim Biophys Acta. 2013;1834(9):1873-1884. [DOI] [PubMed] [Google Scholar]
- 24.Mura-Galelli MJ, Voegel JC, Behr S, Bres EF, Schaaf P. Adsorption/desorption of human serum albumin on hydroxyapatite: a critical analysis of the Langmuir model. Proc Natl Acad Sci USA. 1991;88(13):5557-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meissner J, Prause A, Bharti B, Findenegg GH. Characterization of protein adsorption onto silica nanoparticles: influence of pH and ionic strength. Colloid Polym Sci. 2015;293(11):3381-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington DJ, Adachi K, Royer WE Jr.. The high resolution crystal structure of deoxyhemoglobin S. J Mol Biol. 1997;272(3):398-407. [DOI] [PubMed] [Google Scholar]
- 27.Yonetani T, Laberge M. Protein dynamics explain the allosteric behaviors of hemoglobin. Biochim Biophys Acta. 2008;1784(9):1146-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez DST, Paula AJ, Fonseca LC, et al. Monitoring the hemolytic effect of mesoporous silica nanoparticles after human blood protein corona formation. Eur J Inorg Chem. 2015;2015(27):4595-4602. [Google Scholar]
- 29.Kumar R, Roy I, Ohulchanskky TY, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4(2):699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu T, Hubbard D, Ray A, Ghandehari H. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J Control Release. 2012;163(1):46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns AA, Vider J, Ow H, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009;9(1):442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souris JS, Lee CH, Cheng S-H, et al. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31(21):5564-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Li L, Liu T, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5(7):5390-5399. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332-348. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov S, Zhuravsky S, Yukina G, et al. In vivo toxicity of intravenously administered silica and silicon nanoparticles. Materials (Basel). 2012;5(12):1873-1889. [Google Scholar]
- 36.Murugadoss S, Lison D, Godderis L, et al. Toxicology of silica nanoparticles: an update. Arch Toxicol. 2017;91(9):2967-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.