Abstract
Purpose
To report the clinical features organisms and treatment outcomes in patients with endophthalmitis after penetrating keratoplasty (PK)
Methods
Retrospective noncomparative case series.
Results
Eleven eyes of 11 patients with culture positive endophthalmitis after PK were included. The time to diagnosis of endophthalmitis from last PK was less than 1 week in 3/11 (27%), between 1 and 4 weeks in 3/11 (27%), and greater than one month in 5/11 (46%) (range 2–924 days). The distribution of isolates included gram positive (GP) 9/11 (82%), gram negative (GN) 1/11 (9%), and fungal 1/11 (9%) species, respectively. Of GP bacteria tested, 9/9 (100%) were sensitive to Vancomycin. Of fungal isolates tested, none (0/1) were sensitive to Amphoteracin, Fluconazole, and/or Voriconazole. Among patients with rim culture data available, 1/7 (14%) donor rims were culture positive for Candida glabrata and 6/7 (86%) were culture negative. Patients were treated with primary tap and inject in 10/11 (91%) and primary vitrectomy in 1/11 (9%). VA of ≥5/200 was present in 2/11 (18%) at time of endophthalmitis diagnosis, and was recorded in 6/11 (55%) at last follow-up.
Conclusions and Importance
Patients with endophthalmitis after PK presented at variable time points after surgery. Gram positive organisms were the most common isolate. VA outcomes after treatment were generally poor.
Keywords: Penetrating keratoplasty, Endophthalmitis
Abbreviations: BPEI, Bascom Palmer Eye Institute; CE, cataract extraction; DMEK, Descemet membrane endothelial keratoplasty; DSAEK, descemet stripping automated endothelial keratoplasty; GN, gram negative; GP, Gram positive; HM, Hand motion; IOL, intraocular lens implant; LP, Light perception; NLP, No light perception; PK, penetrating keratoplasty; PPV, vitrectomy; TAP, tap and inject
1. Introduction
Endophthalmitis after penetrating keratoplasty (PK) is a rare but devastating post-operative complication with poor visual prognosis. The incidence of post PK endophthalmitis has been reported 0.1–2.47%,1, 2, 3, 4 and pooled estimate from 1972 to 2002 in 90549 PKs was reported as 0.382%.5 Etiologies for post PK endophthalmitis include donor contamination, intraoperative seeding, post-operative suture related complication, wound dehiscence, and post-operative keratitis. The association between infectious keratitis and post PK endophthalmitis has been well described, with an incidence of 0.05% culture positive endophthalmitis after 9934 corneal cultures for suspected infectious keratitis from 1995 to 2009 at Bascom Palmer Eye Institute, Miami, FL (BPEI).6 Reported risk factors for endophthalmitis include long term use of topical steroids, fungal keratitis, trauma, corneal thinning, and keratitis adjacent to a prior surgical wound. The purpose of this study was to identify the clinical features, organisms, and treatment outcomes in patients with endophthalmitis after penetrating keratoplasty (PK).
2. Methods
The study was approved by the University of Miami, Miller School of Medicine Medical Sciences Subcommittee Institutional Review Board. Data for this retrospective, noncomparative, consecutive case series was obtained from medical records of all patients with endophthalmitis and history of PK in the same eye who were treated at Bascom Palmer Eye Institute (BPEI) between January 1, 2006 through December 31, 2016. Not all patients had their PK performed at BPEI and only patients with culture positive endophthalmitis were included. Positive cultures from this institution's microbiology lab were reverse screened for diagnoses of endophthalmitis and keratoplasty, yielding 35 cases. From 35 cases, 5 were excluded because they had undergone Descemet stripping automated endothelial keratoplasty (DSAEK) but not PK, 10 were excluded due to PK procedure following (not preceding) endophthalmitis, 8 were excluded due to keratitis-associated endophthalmitis, and one patient was excluded given positive anterior chamber culture but negative vitreous culture. Thus 11 cases remained to be analyzed. Patients with a history of non PK surgical procedure or keratitis within 1 month of the diagnosis of endophthalmitis were excluded, so as not to re-report patients with keratitis-related or corneal suture-related endophthalmitis previously described at this institution.6,7 Clinical features before, at the time of, and after the diagnosis of endophthalmitis were noted. Culture results and treatments outcomes were reviewed.
3. Results
Endophthalmitis after PK (excluding those associated with acute keratitis) was diagnosed in 11 eyes of 11 patients. The clinical features of these patients are summarized in Table 1. The median age was 66 years (range 42–80). Mean follow-up after infection was 36 months (range 0.3–135). The indications for PK were corneal edema or bullous keratopathy in 7/11 (64%), corneal ectasia in 2/11 (18%), graft failure in 1/11 (9%), and keratitis in 1/11 (9%). At time of PK, concurrent procedures included 2/11 (18%) cataract surgery with intraocular lens implant (IOL), and 3/11 (27%) IOL exchange with anterior vitrectomy. The time to endophthalmitis from most recent PK was less than 1 week in 3/11 (27%), between 1 and 4 weeks in 3/11 (27%), and greater than one month in 5/11 (46%) (range 2–924 days). Among patients with history of prior anterior vitrectomy and open posterior capsule at time of PKP, 6/6 (100%) presented with endophthalmitis within 1 month after PKP. At time of endophthalmitis diagnosis, the lens status of patients included 9/11 (82%) pseudophakic, 1/11 (9%) phakic, and 1/11 (9%) aphakic.
Table 1.
Demographics, past medical and ocular history of patients with endophthalmitis after PK.
| Case | Gender | Age (Years) | Ocular history | Indication for PK | PK surgery |
|---|---|---|---|---|---|
| 1 | Male | 66 | Keratoconus | Keratoconus | PK/Phaco/IOL |
| 2 | Female | 74 | PXF Glaucoma, GDI, DSAEK x2, Phaco/Ant vit/Sulcus IOL, IOL exchange for ACIOL, CME s/p IVA/IVT, PBK | PBK, Failed DSAEK x2 | PK/ACIOL explant/Ant Vit/Scleral fixated IOL |
| 3 | Female | 73 | SB/PPV/MP/EL/FAX/C3F8, PPV/PPL/Sulcus IOL/GDI, Glaucoma | PBK | PK |
| 4 | Male | 73 | Fuchs, Phaco/IOL, PBK, prior PK aborted due to dull trephine | Fuchs, PBK | PK |
| 5 | Female | 62 | Hexagonal refractive syndrome, Yag capsulotomy | Hexagonal refractive syndrome | PK/Phaco/IOL |
| 6 | Female | 62 | Fuchs | Fuchs | PK/ECCE/Ant Vit/ACIOL |
| 7 | Female | 80 | Complicated Phaco/IOL | PBK | PK |
| 8 | Female | 74 | Phaco/IOL, PBK, PK/IOL exchange, Inferior trab, Failed PK x2, Glaucoma, CME | Failed PK | PK |
| 9 | Male | 65 | Pt died of sepsis from SBO/Splenitis, Phaco/IOL, PBK, IOL dislocation | PBK | PK/Ant Vit/IOL explant/Sutured IOL |
| 10 | Male | 42 | Congenital Glaucoma, PPV/PPL/IOL/MP/EL for RD, IOL removal, ABK, GDI x2 | ABK | PK |
| 11 | Male | 57 | Pseudomonas keratitis | Keratitis | PK |
No patients were immunocompromised.
ABK = aphakic bullous keratopathy; ACIOL = anterior chamber intraocular lens; Ant vit = anterior vitrectomy; C3F8 = perfluoropropane tamponade; CME = cystoid macular edema; DSAEK = descemet stripping automated endothelial keratoplasty; FAX = fluid air exchange; ECCE = extracapsular cataract extraction; EL = Endolaser; Fuchs = Fuchs dystrophy; GDI = glaucoma drainage implant; IOL = intraocular lens; IVA = intravitreal bevacizumab; IVT = intravitreal preservative-free triamcinolone acetate; MP = membrane peel; PBK = pseudophakic bullous keratopathy; Phaco = phacoemulsification; PK = penetrating keratoplasty; PPL = pars plana lensectomy; PPV = pars plana vitrectomy; PXF = pseudoexfoliation; RD = retinal detachment; SB = scleral buckle; SBO = small bowel obstruction; Trab = trabeculectomy.
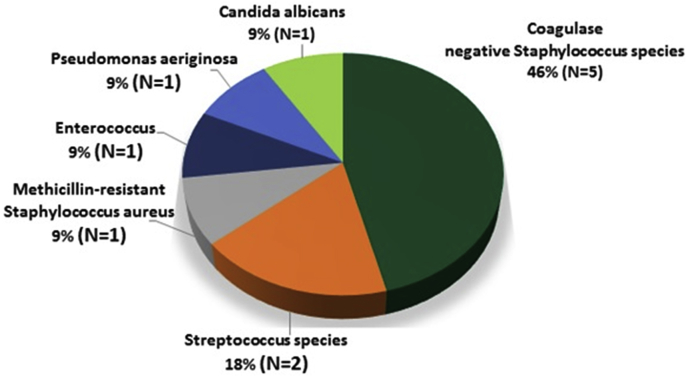
The organisms isolated from vitreous specimens (Table 2) were Gram positive (GP) 9/11 (82%), gram negative (GN) 1/11 (9%), and fungal species 1/11 (9%), respectively. The isolates included coagulase negative Staphylococcus species 5/11 (45%), Streptococcus species 2/11 (18%), Candida albicans 1/11 (9%), and miscellaneous organisms (Fig. 1). Of GP bacteria tested, 9/9 (100%) were sensitive to Vancomycin. Of fungal isolates tested, 0/1 (0%) was sensitive to Amphoteracin, Fluconazole, and/or Voriconazole. Among all patients with rim culture data available, 1/7 (14%) donor rims were culture positive for Candida glabrata and 6/7 (86%) were culture negative. The donor rim matched the vitreous isolate in 0/7 (0%) cases. 10/11 (91%) patients received primary tap and inject (TAP) and 1/11 (9%) received primary vitrectomy (PPV). All 11 patients received and intravitreal vancomycin and ceftazidime. Of those who underwent primary TAP, 2/10 (10%) had secondary TAP while 3/10 (30%) had secondary PPV for worsening infection. One of one (100%) patient with primary PPV also underwent secondary PPV for worsening infection. Other subsequent procedures included repeat PK 3/11 (27%), retinal detachment repair 2/11 (18%), glaucoma drainage implant 1/11 (9%). At time of last follow-up, VA was better than 20/400 3/11 (27%), 5/200–20/400 3/11 (27%), Hand motion (HM) – Light perception (LP) 4/11 (36%) and No light perception (NLP) 1/11 (9%) (see Fig. 2, Fig. 3, Fig. 4).
Table 2.
Endophthalmitis after PK: Causative organisms, Age of PK, Interval from Last Surgical Procedure to Diagnosis, Risk factors, and Follow-Up Data.
| Case | Duration of PK (days) | Sutures present | Lens status | Organism | Follow-up Post-Infection (months) | Last Recorded VA | Primary treatment | Further Surgical Intervention |
|---|---|---|---|---|---|---|---|---|
| 1 | 240 | Yes | IOL | Staphylococcus epidermidis | 0.3 | 20/400 | TAP V/C/D | None |
| 2 | 9 | Yes | IOL/open posterior capsule | Streptococcus oralis | 12.0 | HM | TAP V/C | Secondary PPV for worsening (PK removal/Temp Kpro/IOL removal/PPV/IV V/C/Vori/PK), late RD repair, Kpro/SOI for graft rejection |
| 3 | 2 | Yes | IOL/open posterior capsule | Streptococcus agalactiae | 1.1 | LP | PPV V/C/D | Secondary PPV for worsening |
| 4 | 262 | Yes | IOL | Staphylococcus epidermidis | 13.5 | 20/800 | TAP V/C/D | Late PPV/SB/AFX for RD |
| 5 | 216 | Yes | IOL/open posterior capsule | Staphylococcus epidermidis | 8.9 | 20/70 | TAP V/C/D | None |
| 6 | 5 | Yes | ACIOL/open posterior capsule | Staphylococcus epidermidis | 135.2 | LP | TAP V/C | Repeat TAP with IV Vanco, Late repeat PK for wound dehiscence and external drainage of HCD |
| 7 | 924 | No | IOL | Staphylococcus hominis | 64.0 | 20/200 | TAP V/C | None |
| 8 | 6 | Yes | IOL/open posterior capsule | Enterococcus faecalis | 38.7 | 20/800 | TAP V/C/D | GDI |
| 9 | 36 | Yes | IOL/open posterior capsule | Candida Albicans | 99.6 | 20/100 | TAP V/C | Secondary PPV with IV Ampho for worsening, AC washout ×2 with Vori/Ampho, late repeat PK for graft rejection |
| 10 | 17 | Yes | Aphakic/open posterior capsule | Methicillin resistant staphylococcus aureus | 13.7 | HM | TAP V/C | Secondary PPV for worsening |
| 11 | 212 | Yes | Crystalline lens | Pseudomonas aeruginosa | 6.2 | NLP | TAP V/C/D | Repeat TAP with IV Ceftaz |
AC = anterior chamber; ACIOL = anterior chamber intraocular lens; AFX = air fluid exchange; Ampho = intravitreal Amphoteracin B; C/Ceftaz = intravitreal Ceftazidime; D = intravitreal Dexamethasone HM = hand motions; GDI = glaucoma drainage implant; HCD = hemorrhagic choroidal detachment; IOL = intraocular lens; IV = intravitreal; KPro = keratoprosthesis; LP = light perception; NLP = no light perception; PK = penetrating keratoplasty; PPV = pars plana vitrectomy; RD = retinal detachment; SB = scleral buckle; SOI = silicone oil injection; TAP = vitreous paracentesis and intravitreal injection; Temp KPro = temporary keratoprosthesis; V/Vanco = intravitreal Vancomycin; Vori = intravitreal Voriconazole.
Fig. 1.
Vitreous culture results in 11 patients.
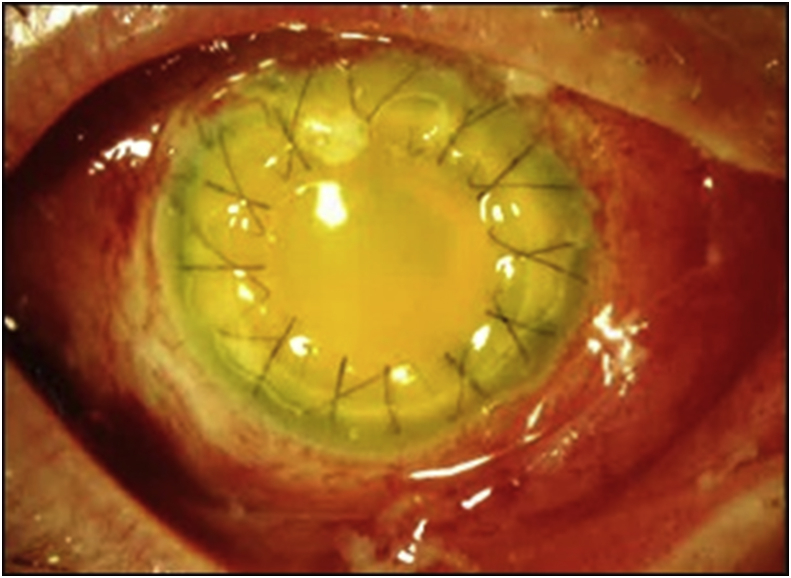
Fig. 2.
Case 3: Clinical photograph at time of endophthalmitis diagnosis, 9 days after PK. Marked conjunctival injection, intact corneal sutures, and dense fibrinous anterior chamber reaction are noted. The vitreous isolate was Streptococcus agalactiae. At last recorded visit, VA was LP due to corneal and anterior chamber opacity.
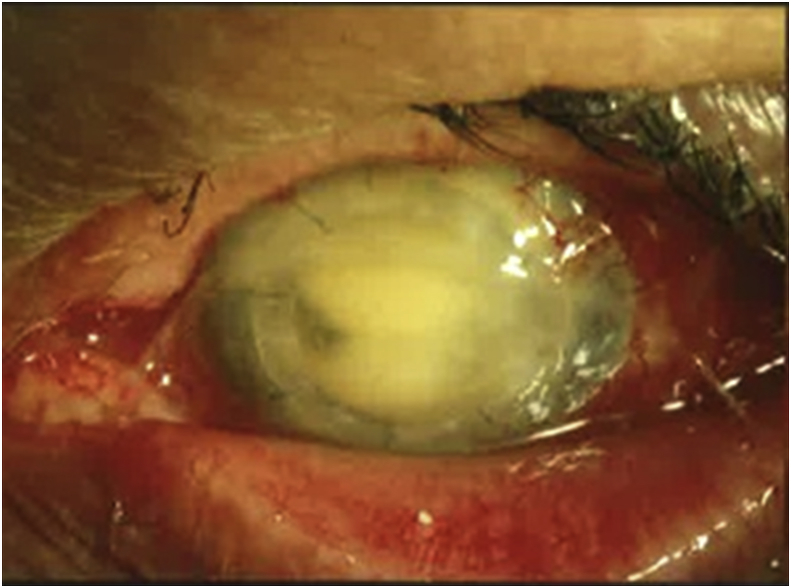
Fig. 3.
Case 7: Clinical photograph at time of endophthalmitis diagnosis, 924 days after PK. Marked conjunctival injection, intact corneal sutures, and dense fibrinous anterior chamber reaction are noted. The vitreous isolate was Staphylococcus hominis. At last recorded visit, VA was 20/200 due to vitreous opacities and corneal edema.
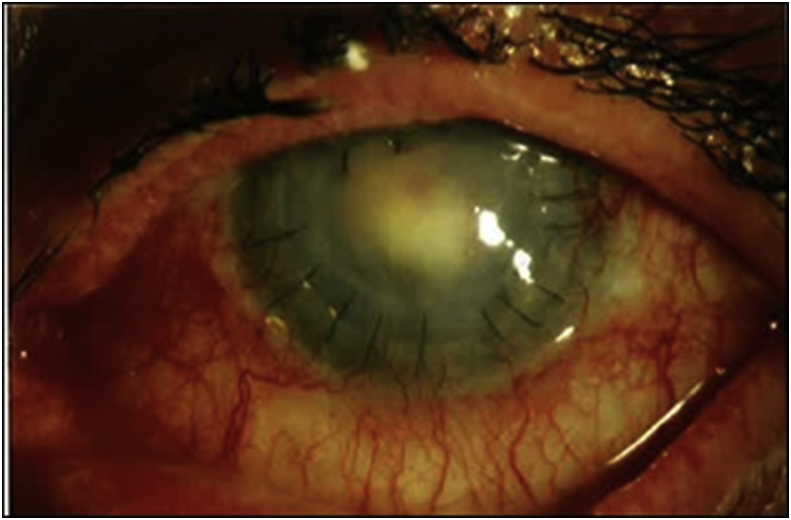
Fig. 4.
Clinical photograph at time of endophthalmitis diagnosis, 214 days after PK. Marked conjunctival injection, intact corneal sutures, and dense fibrinous anterior chamber reaction are noted. The vitreous culture was negative, thus this patient was not included in the current series. The anterior chamber isolate was Candida albicans. At last recorded visit, VA was 20/200 due to corneal edema after multiple repeat PKs and removal of pupillary membrane.
4. Discussion
Variations in post PK rates reported lie in varying study methodologies, inclusion criteria, and study definition of endophthalmitis (Table 3). Additionally, outside of the United States, septisemia is not always a contraindication to tissue donation, different sterilization technique at time of retrieval, and long death to preservation time may also contribute to a higher reported international estimate 0.38% endophthalmitis after PK.5 In the UK annual incidence of post PK endophthalmitis was reported as high as 0.67% in 11 320 first PKs from 1999-2006.8 Incidence was not calculated in this study given several cases were referred from outside institutions, thus the total number of PK procedures performed was not known.
Table 3.
Comparison of post PK endophthalmitis studies.
| Leveille1 | Guss2 | Pardos3 | Kloess4 | Taban5 | Henry6 | Chen7 | Kunimoto10 | Antonios11 | Cameron12 | Alharbi13 | Cameron14 | Keyhani15 | Edelstein17 | Hassan18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 1983 | 1983 | 1982 | 1993 | 2005 | 2013 | 2015 | 2004 | 1991 | 1991 | 2014 | 1998 | 2005 | 2016 | 2005 |
| Country | USA | USA | USA | USA | ◊ | USA | UK | USA | Saudi Arabia | Saudi Arabia | Saudi Arabia | Saudi Arabia | USA | USA | USA |
| Number of PKs | 1876 | 445 | 1880 | 1010 | 90549◊◊ | – | 11320 | – | 2210 | 3000 | 6752 | 16 | 2466 | 195859 | – |
| Number of reported endophthalmitis cases | 4 | 11 | 2 | 4 | – | 5 | 76 | 14 | 9 | 10 | 55° | 4 | 3 | 37 | 234 |
| Reported incidence of endophthalmitis | 0.21% | 2.47% | 0.1% | 0.40% | 0.38%◊◊ | – | 0.67%● | – | 0.41% | 0.33% | 0.68% | – | 0.12% | 0.018% | – |
| Number of culture positive endophthalmitis cases | 4/4 | 10/11 | 1/2 | 4/4 | – | 5/5 | 13/24●● | 13/14 | 9/9 | 6/10♠ | 49/55° | 4/4 | 3/3 | 77/99¥ | 176/234 |
| Etiology | |||||||||||||||
| Suture related complication | – | 0/11 | 0/2 | – | – | 5/5 | – | – | – | 0/6 | 19/55° | 0/4 | 0/3 | – | – |
| Wound leak/dehiscence | – | 0/11 | 0/2 | 4/4 | – | 0/5 | – | – | – | 0/6 | 4/55° | 0/4 | 0/3 | – | – |
| Keratitis | – | 6/11 | 0/2 | 1/4 | – | 5/5 | – | – | – | 1/6 | 47/55° | 0/4 | 0/3 | – | – |
| Contaminated donor | 3/4 | 0/11 | 1/2 | 3/4 | – | 0/5 | – | – | 5/9 | 6/6 | – | 4/4 | 3/3 | – | – |
| Other | 1/4 | 5/11 | 0/2 | – | – | 0/5 | – | – | – | 0/6 | – | 0/4 | 1/3 | – | – |
| Isolate | |||||||||||||||
| Gram positive | 3/4 | 8/10 | 0/1 | 2/4 | – | 5/5 | 9/13●●● | 10/13 | 6/9 | 2/6 | 68/80°° | 3/4 | 0/3 | 27/82¥¥ | 130/176ǁ |
| Gram negative | 1/4 | 1/10 | 1/1 | 0/4 | – | – | 9/13●●● | 3/13 | 0/9 | 1/6 | 10/80°° | 0/4 | 0/3 | 2/82¥¥ | 130/176ǁ |
| Fungal | 0/4 | 1/10 | 0/1 | 2/4 | – | – | 4/13 | 0/13 | 3/0 | 3/6 | 2/80°° | 1/4 | 3/3 | 53/82¥¥ | 46/176 |
| Primary management | |||||||||||||||
| Intravitreal antimicrobials | 1/4 | 4/11 | 0/2 | 1/4 | – | 4/5 | – | – | ? | 6/6 | 38/53°°° | 2/4ǂ | – | – | – |
| PPV | 2/4 | 7/11 | 2/2 | 3/4 | – | 2/5 | – | – | ? | 0/6 | 13/53°°° | 0/4 | – | – | – |
| Evisceration | 1/4 | 0/11 | 0/2 | 0/4 | – | – | – | 0/6 | 15/53°°° | 0/4 | – | – | – | ||
| Last VA | |||||||||||||||
| < 5/200 | 2/4 | 9/11 | 2/2 | 4/4 | – | 3/5 | – | – | ? | 3/6 | 48/55° | 2/4 | – | – | – |
| ≥ 5/200 (better) | 2/4 | 2/11 | 0/2 | 0/4 | – | 2/5 | – | – | ? | 3/6 | 7/55° | 2/4 | – | – | – |
In contrast to other categories of post-operative endophthalmitis, which tend to have acute onset,9,10 there was a wide distribution of time between PK to endophthalmitis diagnosis among patients in the current study. This time distribution is comparable to a similar prior report.11 The results of vitreous isolate data in this studies showed predominance of Staphylococcus and Streptococcus species, and is consistent with prior post PK and generalized post-operative endophthalmitis data.2,9,10,12, 13, 14 While there has been suggestion of increased incidence of fungal endophthalmitis after PK in recent years (0.1%12,15,16-0.22%.2,17−19), only 1/11 cultures were positive for fungal species in the current series. This lower incidence of post PK fungal endophthalmitis may result from of different microbial biomes, small study number, or differing study inclusion/exclusion criteria.
While concordance of donor rim or preservation medium with positive vitreous culture have been reported higher in fungal than bacterial endophthalmitis,19 this was not present in this series. One of 7 rim cultures were positive for Candida glabrate, 1/11 vitreous cultures was positive for Candida albicans, and neither positive culture matched its counterpart. There is no clear consensus of predictive and prognostic value of donor rim culture in the literature.20,21 In one study, bacterial contamination of donor rim was common (12–39%), but transmission rate was low.22 In another study, endophthalmitis was 12 times more likely with positive donor rim culture (95% CI 5–29%) more likely in positive rim. Based on these data, the routine use of antifungal agents in preservation media or the use of rim culture data in guiding management is not well defined.
Regarding risk factors, prior studies suggest risk factors for post PK endophthalmitis include prolonged used of steroids, keratitis, suture related complication, and wound dehiscence.6 The following trends were recorded in the current study: combined CE/IOL at time of PK 5/11 (45%), anterior vitrectomy at time of PK 3/11 (27%), sutures present at time of endophthalmitis 10/11 (91%), recent suture removal 0/11 (0%), and wound leak 0/11 (0%). Nine of 11 patients in this study were pseudophakic, 1 phakic, and 1 aphakic. Patients with open posterior capsule at time of PK had earlier presentation with endophthalmitis (within 1 month of PK) likely due to greater ease of passage of pathogens from anterior to posterior chamber. It should also be noted that a majority of patients in this series received primary PK for corneal edema or bullous keratopathy, many of whom were referred from outside institutions. At this institution since 2006, it is uncommon practice to pursue a primary PK for corneal edema unless the patient has already failed prior Descemet stripping automated endothelial keratoplasty (DSAEK) or Descemet membrane endothelial keratoplasty (DMEK) procedures.
The predominance of TAP compared to PPV as initial treatment in this study reflects the lack of a defined protocol and management selection by individual surgeons. It is likely that the opacity of the PK graft in the setting of acute onset endophthalmitis precluded a satisfactory view for safe PPV. Due to the small number of patients, correlation between treatment and final visual acuity could not be performed; however, the wide variation in procedures including repeat TAP, secondary PPV, repeat PK, among others reflects the individuality of each patient presentation and lack of defined treatment protocol on which patients benefit most from surgical versus medical intervention. Data from study isolates support the continued use of vancomycin and ceftazidime as first line empiric intravitreal antibiotics, and the use of intravitreal antifungal agents when identified by culture results from the patient or the donor rim.
Limitations of this study include retrospective design, small number of patients, and lack of standardized prospective protocol. In cases where the infection clears, vision is often limited by other complications including secondary glaucoma, cystoid macular edema, graft failure and/or rejection, hypotony, cataract, retinal detachment. Cases of refractory or recurrent post PK endophthalmitis are also reported and management in these cases can be especially challenging.17 Routine culture of donor rims and preservative media must be continued for patient safety. The role and timing of vitrectomy for post PK endophthalmitis with persistent corneal opacity and vitreous haze requires further study.
5. Conclusion
Endophthalmitis after PK is uncommon. The current study suggests that patients at highest risk are those with a concurrent procedure at time of PK, intraoperative complication (anterior vitrectomy), and those with open posterior capsule. Patients may present at variable time points after PK. Gram positive organisms were the most common isolate. VA outcomes were generally poor.
Patient consent
This report does not contain any personal information that could lead to the identification of the patient. A Waiver of HIPAA Authorization was approved by the University of Miami, Miller School of Medicine Medical Sciences Subcommittee Institutional Review Board for this study in accordance with IRB requirements.
Funding
This study is supported in part by NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Conflicts of interest
The following authors have no financial disclosures: KDT, NAY, NS, NAP, DM, GA, AMB, HWF.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Leveille A.S., McMullan F.D., Cavanagh H.D. Endophthalmitis following penetrating keratoplasty. Ophthalmology. 1983;90(1):38–39. doi: 10.1016/s0161-6420(83)34601-7. [DOI] [PubMed] [Google Scholar]
- 2.Guss R.B., Koenig S., De La Pena W., Marx M., Kaufman H.E. Endophthalmitis after penetrating keratoplasty. Am J Ophthalmol. 1983;95(5):651–658. doi: 10.1016/0002-9394(83)90385-9. [DOI] [PubMed] [Google Scholar]
- 3.Pardos G.J., Gallagher M.A. Microbial contamination of donor eyes. A retrospective study. Arch Ophthalmol. 1982;100(10):1611–1613. doi: 10.1001/archopht.1982.01030040589006. [DOI] [PubMed] [Google Scholar]
- 4.Kloess P.M., Stulting R.D., Waring G.O., Wilson L.A. Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J Ophthalmol. 1993;115(3):309–316. doi: 10.1016/s0002-9394(14)73580-9. [DOI] [PubMed] [Google Scholar]
- 5.Taban M., Behrens A., Newcomb R.L., Nobe M.Y., McDonnell P.J. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005;123(5):605–609. doi: 10.1001/archopht.123.5.605. [DOI] [PubMed] [Google Scholar]
- 6.Henry C.R., Flynn H.W., Miller D., Forster R.K., Alfonso E.C. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012;119(12):2443–2449. doi: 10.1016/j.ophtha.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry C.R., Flynn H.W., Miller D., Schefler A.C., Forster R.K., Alfonso E.C. Delayed-onset endophthalmitis associated with corneal suture infections. J Ophthalmic Inflamm Infect. 2013;3(1):51. doi: 10.1186/1869-5760-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J.Y., Jones M.N., Srinivasan S. Endophthalmitis after penetrating keratoplasty. Ophthalmology. 2015;122(1):25–30. doi: 10.1016/j.ophtha.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Eifrig C.W., Flynn H.W., Scott I.U., Newton J. Acute-onset postoperative endophthalmitis: review of incidence and visual outcomes (1995-2001) Ophthalmic Surg Laser. 2002;33(5):373–378. [PubMed] [Google Scholar]
- 10.Eifrig C.W., Scott I.U., Flynn H.W., Smiddy W.E., Newton J. Endophthalmitis after pars plana vitrectomy: incidence, causative organisms, and visual acuity outcomes. Am J Ophthalmol. 2004;138(5):799–802. doi: 10.1016/j.ajo.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Kunimoto D.Y., Tasman W., Rapuano C. Endophthalmitis after penetrating keratoplasty: microbiologic spectrum and susceptibility of isolates. Am J Ophthalmol. 2004;137(2):343–345. doi: 10.1016/S0002-9394(03)00874-2. [DOI] [PubMed] [Google Scholar]
- 12.Antonios S.R., Cameron J.A., Badr I.A., Habash N.R., Cotter J.B. Contamination of donor cornea: postpenetrating keratoplasty endophthalmitis. Cornea. 1991;10(3):217–220. doi: 10.1097/00003226-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Cameron J.A., Antonios S.R., Cotter J.B., Habash N.R. Endophthalmitis from contaminated donor corneas following penetrating keratoplasty. Arch Ophthalmol. 1991;109(1):54–59. doi: 10.1001/archopht.1991.01080010056032. [DOI] [PubMed] [Google Scholar]
- 14.Alharbi S.S., Alrajhi A., Alkahtani E. Endophthalmitis following keratoplasty: incidence, microbial profile, visual and structural outcomes. Ocul Immunol Inflamm. 2014;22(3):218–223. doi: 10.3109/09273948.2013.841956. [DOI] [PubMed] [Google Scholar]
- 15.Cameron J.A., Badr I.A., Miguel Risco J., Abboud E., Gonnah el-S. Endophthalmitis cluster from contaminated donor corneas following penetrating keratoplasty. Can J Ophthalmol. 1998;33(1):8–13. [PubMed] [Google Scholar]
- 16.Keyhani K., Seedor J.A., Shah M.K., Terraciano A.J., Ritterband D.C. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea. 2005;24(3):288–291. doi: 10.1097/01.ico..0000138832.3486.70. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell M.C., Perfect J.R., Carlson A.N., Proia A.D. Candida glabrata endophthalmitis following penetrating keratoplasty. J Cataract Refract Surg. 2009;35(3):598–602. doi: 10.1016/j.jcrs.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 18.Edelstein S.L., DeMatteo J., Stoeger C.G., Macsai M.S., Wang C.H. Report of the eye bank association of America medical review subcommittee on adverse reactions reported from 2007 to 2014. Cornea. 2016;35(7):917–926. doi: 10.1097/ICO.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 19.Hassan S.S., Wilhelmus K.R. America MRSotEBAo. Eye-banking risk factors for fungal endophthalmitis compared with bacterial endophthalmitis after corneal transplantation. Am J Ophthalmol. 2005;139(4):685–690. doi: 10.1016/j.ajo.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Wiffen S.J., Weston B.C., Maguire L.J., Bourne W.M. The value of routine donor corneal rim cultures in penetrating keratoplasty. Arch Ophthalmol. 1997;115(6):719–724. doi: 10.1001/archopht.1997.01100150721003. [DOI] [PubMed] [Google Scholar]
- 21.Everts R.J., Fowler W.C., Chang D.H., Reller L.B. Corneoscleral rim cultures: lack of utility and implications for clinical decision-making and infection prevention in the care of patients undergoing corneal transplantation. Cornea. 2001;20(6):586–589. doi: 10.1097/00003226-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelmus K.R., Hassan S.S. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology. 2007;114(3):440–445. doi: 10.1016/j.ophtha.2006.09.006. [DOI] [PubMed] [Google Scholar]