Abstract
Purpose
To report regression of neovascularization and reperfusion of ischemic areas of the retina on Wide-field Digital Fluorescein Angiography following anti-vascular endothelial growth factor injections in a patient with active Proliferative Diabetic Retinopathy.
Observations
Case report of sixty-one-year-old male patient with proliferative diabetic retinopathy and diabetic macular edema documented on wide field digital fluorescein angiography. The patient was treated with three intravitreal injections of ranibizumab given at monthly intervals. Repeat angiography after third intravitreal injection revealed complete regression of new vessels. Moreover, there was evident improvement in perfusion in the previously noted ischemic areas of the retina.
Conclusion and importance
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections are a valuable treatment option for reversing neovascularization in eyes with proliferative diabetic retinopathy with fewer side effects when compared to standard pan-retinal photocoagulation. Additionally, we also illustrate restoration of retinal perfusion post anti-VEGF therapy indicative of pre-existingsalvageableischemic retina tissue.
Keywords: Anti-VEGF, Proliferative diabetic retinopathy, Retinal reperfusion, Ranibizumab, Protocol-S
1. Introduction
Proliferative diabetic retinopathy(PDR) is a leading cause of vision loss in patients with diabetes mellitus1 and approximately 1.5% of adults with diabetes have PDR.2 The Diabetic Retinopathy Study Research group found that almost 50% of untreated PDR eyes have severe vision loss (i.e., visual acuity of <20/800 for at least 4 months).3 Various features of PDR include retinal neovascularization, diabetic macular edema and fibrovascular proliferation at the vitreo-retinal interface leading to vision threatening sequelae like tractional retinal detachment.4 Panretinal Photocoagulation (PRP) was established the gold standard treatment for PDR after Diabetic Retinopathy Study Research group demonstrated its benefits and has continued to remain so for the last 40 years.5 Even though over the years PRP has been proven beneficial, it has its own set of complications. The multiple concerns include loss of peripheral field of vision, worsening of macular edema and traction, difficulty to perform the procedure in eyes with media opacity and also pain during laser treatment.6 These adverse effects have prompted research into alternate therapy for PDR eyes.
The elevated intra-ocular levels of pro-angiogenic factors, especially vascular endothelial growth factor (VEGF), form the basis of pathophysiology in PDR eyes.7 Building on the concept of anti-VEGF pharmacotherapy for neovascularization, the Diabetic Retinopathy Clinical Research network (DRCR.net) conducted a multicenter randomized clinical trial to evaluate non-inferiority of intravitreal ranibizumab compared with PRP in PDR eyes.8 They demonstrated better visual outcomes in eyes treated with anti-VEGF therapy. As a secondary outcome, they also reported regression of neovascularization that was comparable in both the groups. However, this was assessed based on color fundus photographs only.
We report a case of early proliferative diabetic retinopathy that was treated with multiple intravitreal injections of ranibizumab based on Protocol-S of DRCR.net. On repeat widefield angiography, we demonstrate complete regression of neovascularization after three loading doses of intravitreal ranibizumab. Furthermore, we exhibit an overall improvement in retinal perfusion on widefield fluorescein angiogram.
2. Case report
A sixty-one-year-old gentleman, known case of diabetes mellitus since 3 years, presented to our vitreo-retinal clinic in September 2016 with complaints of blurring of vision in right eye (OD) since 1 year. He gave history of grid laser photocoagulation done in OD 1 year ago. At presentation, his best-corrected visual acuity was 6/12, N8. On clinical examination, the anterior segment showed early cataractous changes. Dilated fundus examination revealed early proliferative diabetic retinopathy changes with clinically significant macular edema. Wide-field Digital Fluorescein Angiography(DFA) (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) confirmed the clinical diagnosis with new vessels noted along the infero-temporal arcade and nasal to disc (Fig. 1A). Angiography also revealed areas of capillary non-perfusion adjacent to the areas of neovascularization (Fig. 3A). Macular volume scan on Spectral domain optical coherence tomography (SD-OCT) (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) demonstrated serous macular detachment and an increased central macular thickness of 360 μm (Fig. 2A). In view of significant macular edema and early neovascularization, patient was advised intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections. Accordingly, three doses of Ranibizumab (0.5 mg/0.05 ml) were administered intra-vitreally at monthly intervals in OD. The patient was monitored over 3 months with serial macular scans and best-corrected visual acuity assessment (Fig. 2B and 2C). One month following the third injection, there was improvement in BCVA to 6/9, N6. SD-OCT showed complete resolution of serous macular detachment with normal central macular thickness of 247 μm (Fig. 2D). Repeat wide-field digital fluorescein angiography showed complete regression of neovascular fronds (Fig. 1B). More remarkably, there was an improvement in retinal perfusion in the areas of previously documented capillary non-perfusion (Fig. 3B). We manually calculated the peripheral ischemic index (PII), defined as ratio of the sum of all areas of capillary non-perfusion with the total area of widefield image in pixels, before and after the anti-VEGF therapy. At baseline, the PII was 6.96 indicative of substantial ischemia which improved to 0, signifying complete resolution of ischemia.
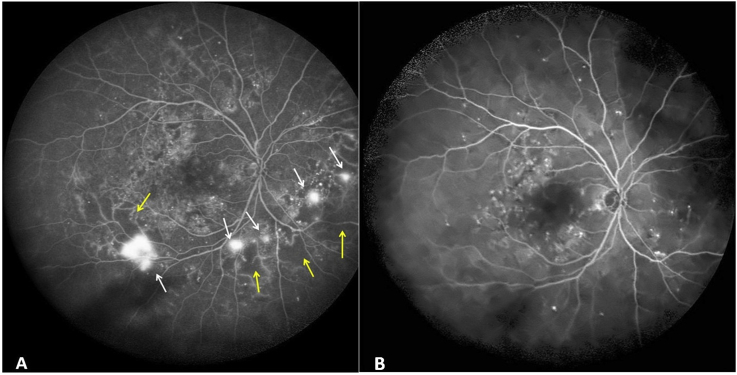
Fig. 1.
Digital Wide Field Fluorescein Angiography images before and after anti Vascular endothelial growth factor therapy. 1A: Fluorescein angiogram image at presentation showing hyperfluorescent lesions with fuzzy margins seen along the inferotemporal arcade and nasal to disc (white arrows) suggestive of neovascularization. Adjacent to new vessels, areas of hypofluorescence are seen with absent retinal capillaries (yellow arrows) suggestive of areas of capillary non perfusion. 1B: Fluorescein angiogram image post three intravitreal anti-vascular endothelial growth factor injections showing complete regression of new vessels and reperfusion of non-perfused retina. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
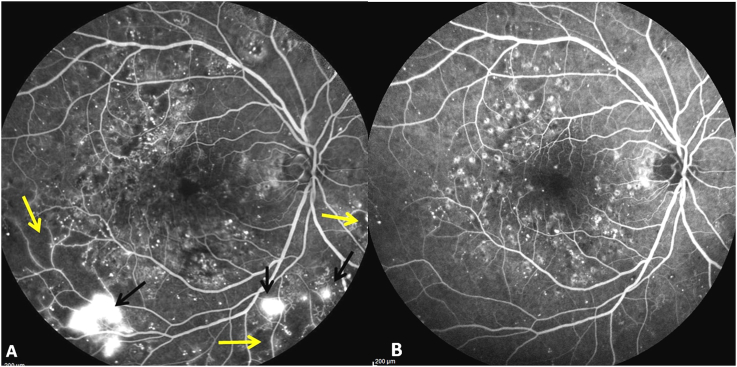
Fig. 3.
Digital fluorescein angiogram image (55°) showing areas of capillary non-perfusion (yellow arrows) and neovascularization (black arrows) at presentation (3A). At the end of therapy same areas show reperfusion with complete regression of neovascularization (3B). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
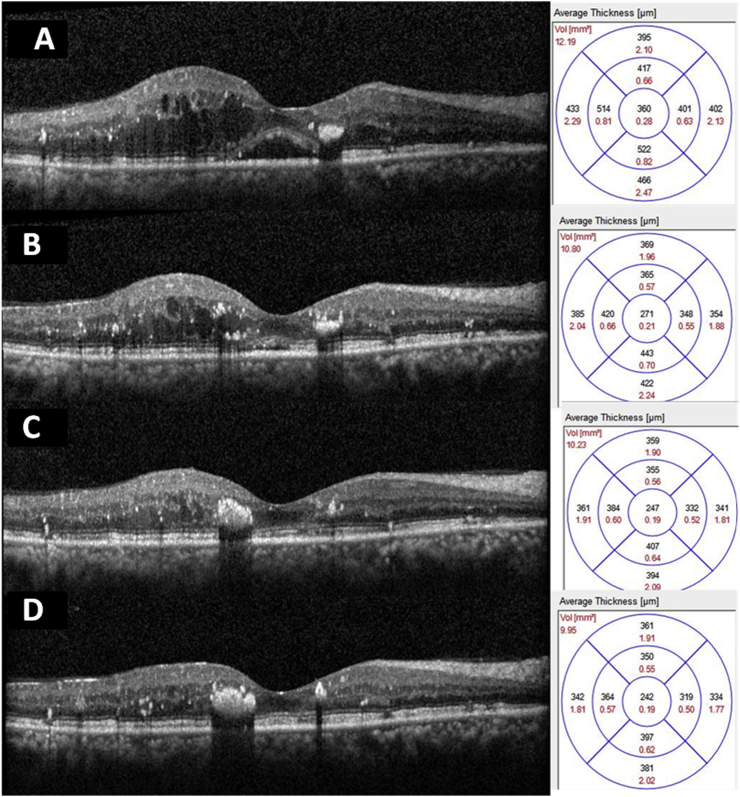
Fig. 2.
Sequential spectral domain optical coherence tomography (SD-OCT) images through the fovea of right eye showing gradual reduction in macular edema. 2A: Scan through fovea at presentation showing intraretinal cystic changes with serous macular detachment (SMD). Central macular thickness of 360 μm. 2B: Scan through fovea showing central macular thickness of 271 μm. 2C: Scan through fovea showing central macular thickness of 247 μm 2D: Scan through fovea at the end of three injections of ranibizumab showing complete resolution of intraretinal edema and SMD. Central macular thickness 247 μm.
3. Discussion
Panretinal photocoagulation has stood the test of time and is the only evidence-based treatment available for PDR. Studies have shown almost 50–60% reduction in risk of sever visual loss with regression of neovascularization post laser treatment.5 It is postulated that PRP decreases the retinal demand for oxygen by destroying the active outer retinal cells that are metabolically extremely active and improves the oxygenation from choroidal circulation. Several investigators have attempted to modify PRP laser techniques to decrease laser-related side-effects, including decreased visual acuity, peripheral field loss and macular oedema.9 Even so, many patients eventually need multiple sittings of supplemental laser treatment throughout the course of disease, and nearly 4.5% show disease progression that ultimately requires pars plana vitrectomy (PPV), even when PRP was considered ‘adequate’.10 This has impelled the efforts to provide alternate treatment options that may delay or obviate the need for PRP. With the advent of anti-vascular endothelial growth factor (VEGF) agents, studies have focused on their role in the management of PDR.11
Hypoxia-driven angiogenesis is the key pathway responsible for the development and progression of severity of proliferative diabetic retinopathy. Among the pro-angiogenic cytokines involved, Vascular Endothelial Growth Factor (VEGF) plays a key role. High levels of VEGF in vitreous humor and intraocular fibrovascular tissues reported in eyes with PDR have confirmed its pivotal role in angiogenesis12 and it has been demonstrated that VEGF mediates its physiological and pathological effects through two tyrosine-kinase receptors VEGFR-1 and VEGFR-2. Activation of these receptors stimulates the angiogenesis and vascular permeability leading to neovascularization and macular edema respectively in PDR eyes.13 Multiple studies have demonstrated the effectiveness of anti-VEGF therapy in transiently regressing PDR.15,14
Of the many landmark trials evaluating the efficacy of anti-VEGF therapy in diabetic retinopathy eyes, the RISE and RIDE trials are worth mentioning.16 They evaluated the efficacy of ranibizumab in diabetic macular edema. Results revealed significant improvements in macular edema, but more importantly they also showed that retinopathy was less likely to worsen and more likely to improve in ranibizumab-treated eyes. The DRCR network recently published the two-year results of Protocol S8 that compared panretinal photocoagulation and intravitreal ranibizumab (Lucentis, Genentech) for patients with high-risk proliferative diabetic retinopathy. Protocol S was designed as a noninferiority study to determine if intravitreal ranibizumab was non-inferior to PRP for treatment of high-risk PDR. Mean visual acuity letter improvement was +2.8 in ranibizumab group versus +0.2 in the PRP group with p < .001 for non-inferiority. As secondary outcome measure they also studied the regression of neovascularization on digital fundus photographs. Eyes without active or regressed neovascularization at the end of 2 years were not significantly different in both the groups (35% in ranibizumab group versus 30% in PRP group). These results reinforce the therapeutic efficacy of anti VEGF in eyes with active PDR.
Avery et al.17 reported complete resolution of angiographic leakage of neovascularization of the disc due to PDR in 19 of 26 eyes (73%) that were treated with intravitreal bevacizumab. Gonzalez et al.18 in their open-label exploratory study randomized 20 subjects with active PDR to treatment with intravitreal pegaptanib versus PRP. By week 12, all pegaptanib-treated eyes demonstrated complete regression of neovascularization, which was maintained through the final study visit at week 36.
Our case is analogous to the aforementioned reports in that there was complete regression of active neovascularization post three doses of intravitreal ranibizumab. Furthermore, our case is exclusive as we illustrate these findings on widefield digital fluorescein angiography system, unlike the previous reports that were based on normal 55° fundus based camera system and DRCR.net Protocol-S which was based on digital color photographs.
Retinal reperfusion in diabetic retinopathy eyes after anti-VEGF therapy with ranibizumab and bevacizumab has been documented by Levin et al.19 on ultrawide field fluorescein angiography. Our case is complementary to this reported series since it illustrates retinal reperfusion after anti-VEGF therapy. Additionally, we also demonstrate complete resolution of neovascular tissue. Moreover, we performed a quantitative assessment of retinal perfusion by analyzing the peripheral ischemic index (PII) which showed significant improvement from 6.96 to 0, post three doses of ranibizumab. The mechanism by which the anti-VEGF agents stabilizes retinal vasculature and causes reperfusion remains unexplored. The proposed hypotheses include decrease in hyperpermeability, pericyte restoration, and normalization of the basement membrane.20 This normalized basement membrane provides a scaffolding for regrowth of retinal microvasculature. This hypothesis has been utilized to a great extent while treating cancers, in which a combination treatment of anti-VEGF agent with chemotherapy is utilized for greater efficacy. Anti-VEGF causes stabilization of tumor vessels which augments efficacy of the chemotherapeutic agent.21 However, as demonstrated by Levin et al., not all patients demonstrated retinal reperfusion. This may be hypothesized to occur because of either significant ischemia and infarction of the retina or the possible need of higher doses of anti-VEGF agent for reperfusion to occur.
In literature, especially the recent RIDE and RISE trials16 have demonstrated reduction in rate of retinal non-perfusion in patients treated with intravitreal ranibizumab. Although these trials were not powered to measure reperfusion, slowing down of rate of non-perfusion is indicative of affirmative action of anti-VEGF agents in stabilization of retinal microvasculature. In these trials, retinal non-perfusion was measured in terms of disc areas (DA), due to which there are chances of missing subtle areas of retinal non-perfusion or reperfusion. In contrast, in our report, we performed a detailed quantitative assessment of retinal ischemia by manually delineating all areas of capillary non-perfusion at baseline and post three injections. This gave us an accurate quantitative estimation of amount of retinal reperfusion, in which the peripheral ischemic index (PII) recovered from 6.96 to 0. Also, in the RIDE and RISE trials, perfusion analysis was performed based on the7-standard fields fluorescein angiography (7-SF FA) images, while in our study, wide-field FA images were utilized for the perfusion assessment. This is advantageous as it reduces chances of missing areas of reperfusion outside the 7SF-FA, which would have probably occurred in the RIDE and RISE trials.
Our report thus illustrates the efficacy of ranibizumab in regression of neovascular tissue with notable improvement in retinal perfusion, both qualitatively and quantitatively on widefield imaging. These significant outcomes may provide probable explanation for previous studies which have documented halting or reversal of diabetic retinopathy secondary to anti-VEGF therapy.
4. Conclusion
Laser pan-retinal photocoagulation, which is currently the gold standard for management of PDR, has potential adverse effects on visual function secondary to retinal tissue damage. Anti-VEGF treatment on the other hand, is effective in regressing active neovascularization and simultaneously prevents laser-associated vision loss by precluding the need for PRP. Our case is singular as it emphasizes the additional advantage of anti-VEGF therapy which is establishment of retinal reperfusion both qualitatively and quantitatively.
Patient consent
Obtained in writing.
Acknowledgments and disclosures
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: JS, AG, SC, MG.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., Saaddine J.B., Chou C.F. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetic Retinopathy Study Research Group Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 4.Fong D.S., Aiello L.P., Ferris F.L., 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–2553. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 6.Jardeleza M.S., Miller J.W. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- 7.Witmer A.N., Vrensen G.F., Van Noorden C.J., Schlingemann R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 8.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross J.G., Glassman A.R., Jampol L.M. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–2146. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brucker A.J., Qin H., Antoszyk A.N. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Diabetic Retinopathy Clinical Research Network. Arch Ophthalmol. 2009;127:132–140. doi: 10.1001/archophthalmol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn H.W., Jr., Chew E.Y., Simons B.D., Barton F.B., Remaley N.A., Ferris F.L., 3rd Pars plana vitrectomy in the early treatment diabetic retinopathy study. ETDRS report number 17. The early treatment diabetic retinopathy study research group. Ophthalmology. 1992;99:1351–1357. doi: 10.1016/s0161-6420(92)31779-8. [DOI] [PubMed] [Google Scholar]
- 11.Adamis A.P., Miller J.W., Bernal M.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 12.Chung E.J., Kang S.J., Koo J.S., Choi Y.J., Grossniklaus H.E., Koh H.J. Effect of intravitreal bevacizumab on vascular endothelial growth factor expression in patients with proliferative diabetic retinopathy. Yonsei Med J. 2011;52:151–157. doi: 10.3349/ymj.2011.52.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 14.Mason J.O., 3rd, Nixon P.A., White M.F. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142(4):685–688. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Hussain G., Ghanekar Y., Kaur I. The future implications and indications of anti-vascular endothelial growth factor therapy in ophthalmic practice. Indian J Ophthalmol. 2007;55:445–450. doi: 10.4103/0301-4738.36480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen Q.D. RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Avery R.L., Pearlman J., Pieramici D.J. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10) doi: 10.1016/j.ophtha.2006.05.064. 1695 e1–15. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez V.H., Giuliari G.P., Banda R.M., Guel D.A. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93(11):1474–1478. doi: 10.1136/bjo.2008.155663. [DOI] [PubMed] [Google Scholar]
- 19.Levin A.M., Rusu I., Orlin A. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol (Auckl, NZ) 2017;11:193–200. doi: 10.2147/OPTH.S118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetsuichiro I., Mancuso M., Hashizume H. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165(1) doi: 10.1016/S0002-9440(10)63273-7. 35–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giantonio B.J., Catalano P.J., Meropol N.J. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]