Abstract
Background and Aims:
Routine use of pre-procedural ultrasound guided midline approach has not shown to improve success rate in administering subarachnoid block. The study hypothesis was that the routine use of pre-procedural (not real time) ultrasound-guided paramedian spinals at L5-S1 interspace could reduce the number of passes (i.e., withdrawal and redirection of spinal needle without exiting the skin) required to enter the subarachnoid space when compared to the conventional landmark-guided midline approach.
Methods:
After local ethics approval, 120 consenting patients scheduled for elective total joint replacements (Hip and Knee) were randomised into either Group C where conventional midline approach with palpated landmarks was used or Group P where pre-procedural ultrasound was used to perform subarachnoid block by paramedian approach at L5-S1 interspace (real time ultrasound guidance was not used).
Results:
There was no difference in primary outcome (difference in number of passes) between the two groups. Similarly there was no difference in the number of attempts (i.e., the number of times the spinal needle was withdrawn from the skin and reinserted). The first pass success rates (1 attempt and 1 pass) was significantly greater in Group C compared to Group P [43% vs. 22%, P = 0.02].
Conclusion:
Routine use of paramedian spinal anaesthesia at L5-S1 interspace, guided by pre-procedure ultrasound, in patients undergoing lower limb joint arthroplasties did not reduce the number of passes or attempts needed to achieve successful dural puncture.
Key words: Paramedian, spinal anaesthesia, ultrasound
INTRODUCTION
Spinal anaesthesia is conventionally performed using a landmark-guided midline approach. Various modifications have been described to reduce the morbidity related to repeated attempts and passes. These include a pre-procedure ultrasound-guided midline approach,[1] real-time ultrasound-guided approach,[2] landmark-guided paramedian approach and pre-procedure ultrasound-guided paramedian approach.[3] Ultrasound is beneficial only in patients administered a single shot spinal anaesthetic who have difficult surface landmarks or abnormal anatomy. There are insufficient data to support the routine use of ultrasound in all patients.[4]
In the previous study on patients undergoing lower limb joint replacement surgery, pre-procedural ultrasound-guided paramedian approach, performed routinely in all patients, significantly reduced the number of passes (i.e., withdrawal and redirection of spinal needle without exiting the skin) and attempts (defined as the number of times the spinal needle was withdrawn from the skin and reinserted) required for success.[3] On subgroup analysis of this study, we observed a non-significant trend towards a lower number of passes in the L5-S1 interspace compared to other intervertebral spaces, using a paramedian approach. L5–S1 had the least number of passes (mean 2 ± 1) compared to L4–5 (mean 4.27 ± 4.1) and L3–4 (mean 5.15 ± 5.01).
Anatomically, the L5-S1 interspace is the widest interlaminar space and is least affected by a patient's inability to flex.[5,6] Previous case reports on landmark-guided techniques have suggested high success rate with the paramedian approach at L5-S1 level (Taylor's approach).[7]
Hence, we hypothesised that by selective targeting of the L5-S1 interspinous space with ultrasound, we should be able to further refine the paramedian approach. The aim of the study was to compare conventional midline approach at any interspinous level to a pre-procedure ultrasound-guided L5-S1 paramedian approach.
METHODS
This was a prospective, randomised, controlled study conducted in a university teaching hospital between July 2014 and June 2015. Following approval by the clinical research ethics committee, all consented patients scheduled to undergo elective total knee or total hip arthroplasty under spinal anaesthesia during the study period were included. A written informed consent was obtained from all patients in the study. Patients with contraindications to spinal anaesthesia (allergy to local anaesthetic, coagulopathy, local infection and indeterminate neurological disease) were excluded from the study.
The patients were randomised using random number generating software (Research Randomizer Version 4.0) to undergo either conventional landmark-guided spinal anaesthesia (Group C) or pre-procedural ultrasound-guided L5-S1 paramedian spinal (Group P). Opaque sealed envelopes were used to conceal the allocation. The envelope was opened by the attending anaesthesiologist immediately before performing the procedure. Patients were not informed about their group allocation.
In both groups, spinal anaesthesia was performed by one of three consultant anaesthetists (FL, PL, GI), each having performed more than 100 neuraxial ultrasound scans before the study. After application of standard monitoring (non-invasive blood pressure, pulse oximetry and three-lead electrocardiogram) and obtaining intravenous access, the patients were positioned sitting on a level trolley with feet resting on a footrest. An assistant supported the patient to aid positioning, and the patients were asked to maintain an arched back position during scanning and performance of spinal anaesthesia.
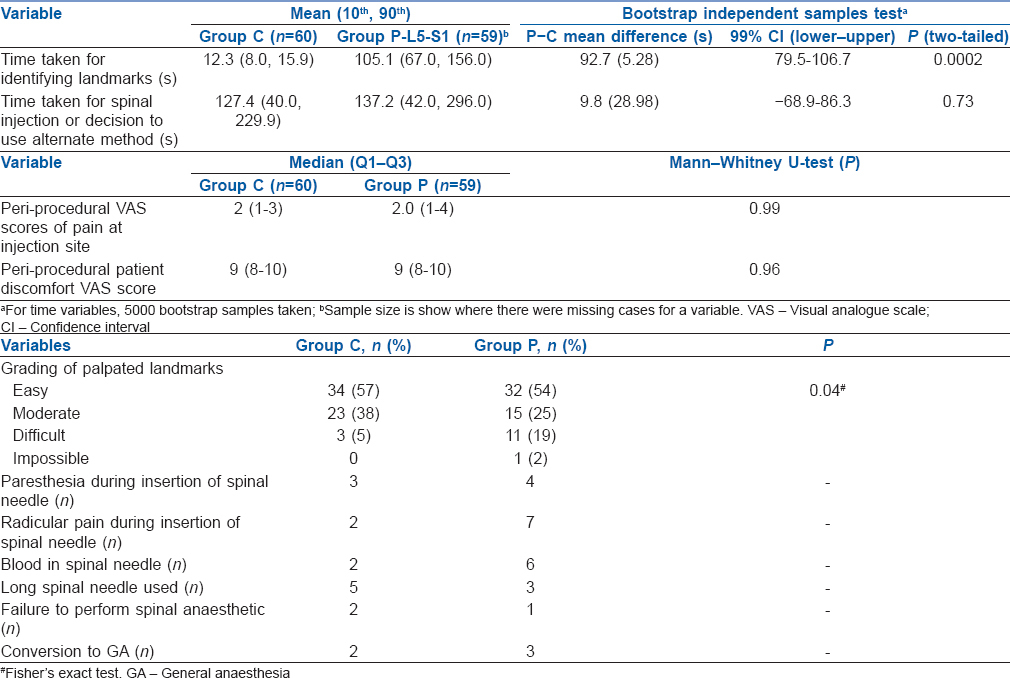
In Group C, the anaesthesiologist selected the preferred interspace and graded the ease of palpation after positioning on a 4-point scale (easy, moderate, difficult or impossible) as described in previous studies.[1] There was no restriction on the interspace selected for this group. Asepsis was maintained, and the anaesthesiologist scrubbed before the procedure, wearing mask and sterile gloves. The skin was prepared with 0.5% chlorhexidine spray (CareFusion Corporation, San Diego, CA 92130, USA) following which 1% lidocaine (2–5 ml) was used for skin infiltration. A 25G Whitacre spinal needle (Becton, Dickinson and Company, Franklin Lakes, New Jersey, 07417-1880, USA) was used initially in all patients. The procedural anaesthesiologist chose the length (90 mm or 119 mm length). Patients in each group received 3.5 ml of 0.5% hyperbaric bupivacaine for spinal anaesthesia. After completion of spinal anaesthetic injection, the patient was placed in lateral decubitus (with operating side in dependent position). Ultrasound scan was then done to identify the level at which dural tap was performed.
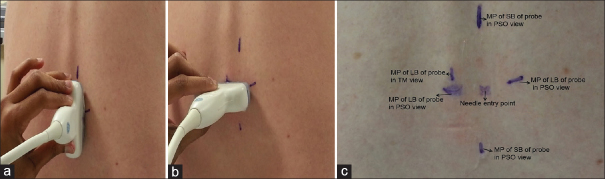
In Group P, a 2–5 MHz curvilinear probe (SonixTablet, Peabody, MA, USA) was used for initial pre-procedural marking. The sacrum was identified first in parasagittal oblique view following which the interlaminar space between L5 and S1 was noted. This space was selected for all patients. At this interspace, and with the probe positioned to obtain the clearest ultrasound image with the interspace in the middle of the screen, a skin marker was used to mark the midpoint of the long and short borders of the probe. The medial angulation of the probe was also noted to guide the insertion of the spinal needle. At the same horizontal level as the midpoint of the long border of the probe, the midpoint of the line drawn between the two short border midpoints of the probe was used as paramedian insertion point for the spinal needle. A transverse median view at the same level was also obtained and the midline was marked. This marking was used to aid the medial angulation of the spinal needle [Figure 1]. Following skin marking, the injection site was cleared of any residual ultrasound gel prior to needle insertion. The spinal anaesthesia was performed as described for the control group. In Group P, the anaesthesiologist palpated and graded the landmarks immediately after the administration of spinal anaesthetic in sitting position. This was done to minimise bias if palpation were to occur before scanning.
Figure 1.
(a) Skin markings with probe positioned to get the best possible parasagittal oblique view of neuraxis (b) midpoint of long border of probe marked in transverse median view. (c) Paramedian skin entry point shown after skin markings. It is marked at the intersection of the lines joining midpoint of long border of probe and midpoint of short border of the probe marked during parasagittal oblique view. The midpoint of long border of probe in transverse median view was used to aid the medial angulation of the needle in addition to probe angle in parasagittal oblique view. MP – Midpoint; LB – Long border; SB – Short border
In both groups, after three unsuccessful attempts, the anaesthesiologists were allowed to use alternative methods when felt necessary. For patients in Group C, another interspinous space could be used or ultrasound employed. For patients in Group P, a midline approach or a conventional landmark palpation technique could be used.
Outcomes were measured by two observers (KK, AML) for all patients. Due to the nature of the study, these observers were not blinded to the groups. Time for identifying landmarks in Group C was defined as time from which the anaesthesiologist started palpating to identify the landmarks to completion of the process as declared by the anaesthesiologist. In Group P, it was defined as time from which the ultrasound probe was placed on the skin to the anaesthesiologist declaring that the skin markings were completed.
Time taken to perform spinal anaesthesia was defined as the time from insertion of introducer needle to completion of injection. The number of passes, defined as the number of forward advancements of the spinal needle in a given interspinous space (i.e., withdrawal and redirection of spinal needle without exiting the skin) and the number of needle insertion attempts (defined as the number of times the spinal needle was withdrawn from the skin and reinserted) were noted. The number of passes and attempts were recorded either until the completion of spinal anaesthetic or until the anaesthesiologist converted to an alternate technique.
The incidence of radicular pain, paraesthesia and blood in the spinal needle hub was also noted. All patients who experienced paraesthesia or radicular pain were followed over the next 24 h, and patients with persistent symptoms were managed as per local department protocol.
In both groups following administration of spinal anaesthesia, patients were positioned on either left or right lateral position depending on the site of surgery. After positioning and before administration of sedation, patients were asked for their peri-procedural pain scores measured using an 11-point verbal rating scale (0 = no pain, 10 = most pain imaginable) and peri-procedural discomfort scores measured using an 11-point verbal rating measured (0 = no discomfort and 10 = most discomfort imaginable). Level of block (loss of cold sensation to ethyl chloride spray) was noted 30 min after the local anaesthetic injection. Type and dose of sedation (Midazolam ± Propofol infusion) was left to the discretion of the anaesthesiologist.
The primary outcome was the number of passes in the two groups. Secondary outcomes included the number of spinal needle insertion attempts, first pass success rates (1 attempt and 1 pass), time for identifying landmarks, time taken to administer spinal anaesthetic, level of block at 30 min, incidence of radicular pain, paraesthesia and blood in the spinal needle, peri-procedural pain and peri-procedural discomfort.
In a pilot observational study done in our department, the average number of passes for conventional midline approach, per spinal anaesthetic for an experienced anaesthesiologist was noted to be 6.4 ± 8.6 (mean ± standard deviation). We hypothesised that using pre-procedural paramedian spinal at L5-S1 level, the number of passes could be reduced to two. A minimum of sixty patients in each group would therefore be needed to achieve 80% power to detect a difference with a less than 0.05 chance of type 1 error. We randomised sixty patients to each group. All data were analysed on an intention-to-treat basis.
Data were visually inspected for normality and Shapiro–Wilks test was done to check for normal distribution. Categorical data were analysed using the Chi-square test or Fisher's exact test as appropriate. Normally distributed parametric data were analysed using two-tailed Student's t-test. Non-parametric data were analysed using the Mann–Whitney U-test. Zero-truncated negative binomial regression was used for count data (passes and attempts). P < 0.05 was considered statistically significant. For primary outcome variables, 95% confidence interval (CI) was reported and for other variables 99% CI was reported. SPSS version 20 (Property of IBM © Copyright IBM Corporation 2000, 2013, Armonk, NY) and STATA (1996–2016 Statacorp LP, College station, Texas) were used during statistical analysis.
RESULTS
A total of 120 patients consented to take part in the study and sixty patients were randomised to each group [Figure 2]. In one patient in Group P, spinal anaesthetic was not attempted due to poor visualisation of anatomy in ultrasound and palpated landmarks were impossible to locate. This patient received a general anaesthetic. Sixty patients in Group C and 59 patients in Group P were included in the final analysis. No dropouts or incomplete data acquisition was noted. No patients were lost for follow-up [Figure 2]. The distribution of demographic data [Table 1] was similar between the groups.
Figure 2.
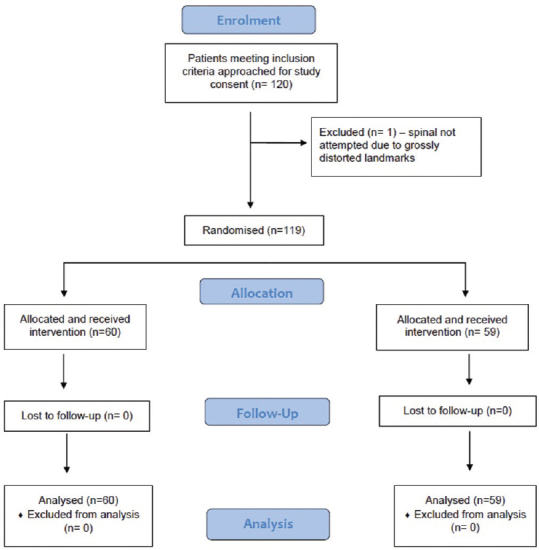
Consort flow sheet
Table 1.
Patient characteristics in Group C and Group P-L5S1
The average number of passes and attempts were similar between the groups [Table 2]. The distribution of the number of passes and number of attempts was highly skewed, and all values exceeded 1 [Supplemental Figures 1 (66KB, tif) and 2 (62.9KB, tif) ]; therefore, the zero-truncated negative binomial (STATA) was used to compare the two groups. A patient in the paramedian group L5-S1 had an expected number of passes equal to 1.195 times (95% CI: 0.57–2.47) that of a patient in the conventional group (P = 0.63) i.e., similar number of passes were expected in both groups. A patient in the paramedian group L5-S1 had an expected number of attempts equal to 1.079 times (99% CI: 0.41–2.8) that of a patient in the conventional group (P = 0.84) i.e., a similar number of attempts were expected in both groups. The first pass success rates (1 attempt and 1 pass) was significantly greater in Group C compared to Group P [43% vs. 22%, P = 0.02, Table 3].
Table 2.
Analysis of number of needle passes and number of attempts
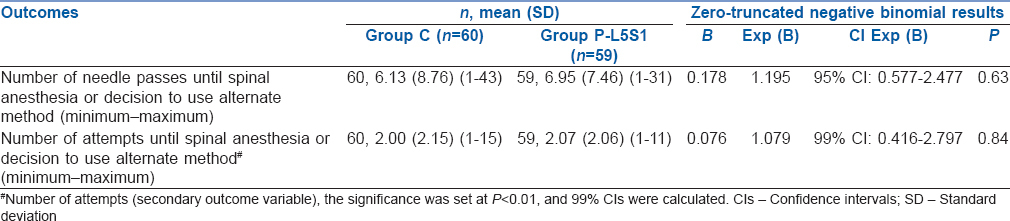
Table 3.
Successful dural puncture rates

Comparison of number of passes between the groups
Comparison of number of attempts between the groups
It took an average of 93 s longer (99% CI: 79.5, 106.7, P < 0.0002) for landmarks to be established in Group P compared to Group C [Table 4]. Other parameters were comparable between the groups [Table 4] with the exception of grading palpated landmarks.
Table 4.
Spinal anesthesia variables
Alternative techniques were employed in three patients in Group C (technique used-ultrasound-guided paramedian spinal) and five patients in Group P (technique used-midline approach by conventional palpation). Despite the use of alternative techniques, dural puncture could not be achieved in two patients in Group C, and one patient in Group P. All nine patients in the study who had radicular pain or paraesthesia during needle placement were followed up for 24 h post-surgery and no patient had persistent symptoms.
Of the two patients in Group C who required general anaesthesia (GA), spinal anaesthesia could not be performed in one patient and the second patient did not have any measurable block post-administration of spinal anaesthetic. Of the three patients in Group P who needed GA, in one patient the spinal could not be performed, and in two patients the block level was inadequate. A non-parametric Mann–Whitney U-test (U = 1890.5, P = 0.32) showed that the distributions of sensory block level at 30 min were similar (U = 1890.5, P = 0.32) in Group C and Group P, with a median of T6; (Q1 = T5; Q2 = T8) in both groups.
A significantly greater number of patients had the spinal needle inserted at or above L2-3 (n = 10) in Group C versus Group P (n = 0) with P < 0.001 (Chi-square test). Of note, none of the patients in conventional group had spinal administered at L5-S1 level.
DISCUSSION
In patients undergoing elective hip or knee joint replacements, routine use of pre-procedure ultrasound-guided paramedian spinal performed at the L5-S1 level did not reduce the number of passes or attempts required to achieve a successful spinal anaesthetic when compared to a conventional landmark-guided midline approach.
Since 2011, four randomised controlled studies, and one cohort study have been published on pre-procedural ultrasound to facilitate spinal anaesthesia in non-obstetric patients.[1,3,4,8,9] Of these, three studies looked at the routine use of ultrasound[3,4,8] and others were done in patients in whom the procedure was anticipated to be difficult. While the use of ultrasound in patients with difficult anatomy has been largely positive, the data on its routine use are conflicting.[3,4,8]
Abdelhamid et al.[8] studied 90 patients undergoing spinal anaesthesia by midline approach. The nature of surgery was not mentioned, and the study population was relatively young (mean age 34.7 years). Lim et al.[4] on the other hand, looked at 170 patients undergoing various procedures under spinal anaesthesia (paramedian approach) with an older population (mean age 62.2 years). The former study reported a significantly improved success rate and the latter showed no difference.
The study by Lim et al. was different to this study in many ways. First, the population group was different. Second, spinal anaesthesia was attempted by trainee anaesthetist with 0 to 3 years of experience whereas in this study it was done by experienced consultant anaesthetist. Third, the neuraxial scanning was done by a different operator, and the results were communicated to the person performing the procedure. In this study, it was done by the same person performing spinal anaesthesia. Fourth, both groups received paramedian spinal anaesthesia. In this study, it was compared with midline conventional spinal anaesthesia as it is still considered as the default technique. Finally, Lim et al. used one of the three interspinous spaces L2–3, L3–4 or L4–5 and did not use L5–S1. We only used L5-S1 in our study for paramedian approach. In spite of the differences, the outcomes were similar as there was no difference in the number of passes between the groups.
Studies using a paramedian approach to spinal anaesthesia utilising ultrasound are a recent development.[3,4,10] The earlier study using this approach[3] in 100 patients undergoing elective knee and hip replacement (mean age 63.4 years) showed a significant reduction in the number of passes and attempts to achieve successful dural tap.
Our study attempted to further refine the paramedian approach using only the L5-S1 interspace. In spite of L5-S1 being the widest interlaminar space that is least affected by flexion or extension in a patient, we still found no difference between the two groups. In addition, the L5-S1 group had lower first pass success rates (one attempt and one pass) compared to the conventional midline group. We can only speculate on possible reasons for this outcome.
In spite of being the widest interlaminar space, the L5-S1 interspace has a very high incidence of facet joint osteoarthritis and spondylolisthesis[11,12,13]
Anatomical variations such as sacralisation of lumbar vertebrae and lumbarisation of sacral vertebrae can occur in up to 12% of general population[14]
During ultrasound scanning, L5-S1 is the most commonly misidentified interspace due to a combination of these factors
In our previous study, using a paramedian approach, the interspace with best views of anterior and posterior complexes was used[3] whereas in this study, L5-S1 was used in all patients irrespective of their visibility
Although the study population was older, it only included elective joint replacements. Positioning them in sitting position was not challenging. On the other hand, the use of L5-S1 inter-spinous space might be more appropriate in elderly patients needing trauma surgery, for example, surgery for hip fracture, where it can be challenging to obtain good positioning for administration of spinal anaesthesia.
This study also showed that no spinal was performed at or above L2–3 level in the ultrasound group compared to 10 in landmark-guided group. This is clinically important as a needle inserted at or above L2–3 level has a 4%–20% possibility of reaching the conus.[15]
The negative results of the study further help delineate the role of 'routine' pre-procedure neuraxial scanning in patients receiving spinal anaesthetic. Routine pre-procedure scanning guided paramedian spinal, by selecting the interspace with the best ultrasound image of the anterior and posterior complexes, reduces the number of passes and attempts.[3] Limiting the paramedian spinal to L5-S1 interspace does not offer any benefit compared to conventional midline approach. In any case, the use of ultrasound significantly reduced the incidence of needle insertion at or above L2–3 interspinous space.
This study has its limitations. First, although the patients were unaware of their group allocation, it is still possible that by the use of ultrasound before versus after spinal injection might make the blinding less robust. In addition, it was difficult to blind the observers due to the use of paramedian approach and skin markings in the ultrasound group. Second, the number of attempts and passes before the use of an alternate technique was left to the discretion of the anaesthesiologist. This does reflect day-to-day practice but introduces the possibility of bias. This might be countered to a certain degree by having three different experienced anaesthesiologists administer spinal anaesthesia. Third, as discussed earlier, neuraxial ultrasound has its own limitations in correctly identifying the L5-S1 interspinous space. However, all three anaesthesiologist performing the procedure were experienced in neuraxial ultrasound, having performed more than 100 neuraxial scans in this patient population before the study. Finally, this was a study looking at paramedian approach involving only L5–S1 interspinous space. Care should be taken to not extrapolate the results to compare the utility of neuraxial ultrasound against conventional approach for lumbar puncture.
CONCLUSION
The routine use of paramedian spinal anaesthesia performed at the L5-S1 level guided by pre-procedure ultrasound does not reduce the number of passes or attempts in achieving successful dural tap.
Financial support and sponsorship
Study was funded by local department funds. No external funding was used.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to acknowledge Ms. Margaret Cole (Statistician) for her valuable contribution to the study.
REFERENCES
- 1.Chin KJ, Perlas A, Chan V, Brown-Shreves D, Koshkin A, Vaishnav V. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115:94–101. doi: 10.1097/ALN.0b013e31821a8ad4. [DOI] [PubMed] [Google Scholar]
- 2.Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: A prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. doi: 10.1155/2013/525818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallidaikurichi Srinivasan K, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;121:1089–96. doi: 10.1213/ANE.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 4.Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42:191–8. doi: 10.1177/0310057X1404200205. [DOI] [PubMed] [Google Scholar]
- 5.Tan Y, Aghdasi BG, Montgomery SR, Inoue H, Lu C, Wang JC. Kinetic magnetic resonance imaging analysis of lumbar segmental mobility in patients without significant spondylosis. Eur Spine J. 2012;21:2673–9. doi: 10.1007/s00586-012-2387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SH, Daffner SD, Wang JC. Does lumbar disk degeneration increase segmental mobility in vivo? Segmental motion analysis of the whole lumbar spine using kinetic MRI. J Spinal Disord Tech. 2014;27:111–6. doi: 10.1097/bsd.0b013e3182a1ddef. [DOI] [PubMed] [Google Scholar]
- 7.Patil AD, Bapat M, Patil SA, Gogna RL. Spinal anesthesia using Taylor's approach helps avoid general anesthesia in short stature asthmatic patient. Saudi J Anaesth. 2015;9:474–6. doi: 10.4103/1658-354X.159481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherif A, Abdelhamid M. Ultrasound-guided intrathecal anesthesia: Does scanning help? Egypt J Anaesth. 2013;29:389–4. [Google Scholar]
- 9.Gnaho A, Nguyen V, Villevielle T, Frota M, Marret E, Gentili ME. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62:520–30. doi: 10.1016/S0034-7094(12)70150-2. [DOI] [PubMed] [Google Scholar]
- 10.Chin KJ, Perlas A, Chan V. The ultrasound-assisted paraspinous approach to lumbar neuraxial blockade: A simplified technique in patients with difficult anatomy. Acta Anaesthesiol Scand. 2015;59:668–73. doi: 10.1111/aas.12502. [DOI] [PubMed] [Google Scholar]
- 11.Ko S, Vaccaro AR, Lee S, Lee J, Chang H. The prevalence of lumbar spine facet joint osteoarthritis and its association with low back pain in selected Korean populations. Clin Orthop Surg. 2014;6:385–91. doi: 10.4055/cios.2014.6.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suri P, Miyakoshi A, Hunter DJ, Jarvik JG, Rainville J, Guermazi A, et al. Does lumbar spinal degeneration begin with the anterior structures? A study of the observed epidemiology in a community-based population. BMC Musculoskelet Disord. 2011;12:202. doi: 10.1186/1471-2474-12-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleem S, Aslam HM, Rehmani MA, Raees A, Alvi AA, Ashraf J. Lumbar disc degenerative disease: Disc degeneration symptoms and magnetic resonance image findings. Asian Spine J. 2013;7:322–34. doi: 10.4184/asj.2013.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bron JL, van Royen BJ, Wuisman PI. The clinical significance of lumbosacral transitional anomalies. Acta Orthop Belg. 2007;73:687–95. [PubMed] [Google Scholar]
- 15.Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia. 2001;56:238–47. doi: 10.1046/j.1365-2044.2001.01422-2.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of number of passes between the groups
Comparison of number of attempts between the groups