Abstract
Objective:
Acute brain injury is one of the leading causes of morbidity and mortality worldwide. Phenytoin has been commonly used as an anticonvulsant agent for the treatment or prophylaxis of seizures following acute brain injury. After a severe head injury, several pharmacokinetic changes occur. The aim of this study is the comparative evaluation of phenytoin serum concentration in patients with traumatic and nontraumatic brain injury (TBI).
Methods:
This prospective observational study was performed on twenty adult brain injury patients who were admitted to an Intensive Care Unit and required phenytoin for the treatment or prophylaxis of postinjury seizures. For all the patients, phenytoin serum concentration was determined in three scheduled time points. Phenytoin serum concentration and pharmacokinetic parameters were compared between patients with TBI and cerebrovascular accident (CVA).
Findings:
The Vmaxand Kmwere significantly higher in head trauma (HT) patients than the CVA group. The phenytoin concentration (Cp) and the Cp/dose ratio were significantly higher in the CVA group patients during the first sampling (P < 0.05). The Acute Physiology and Chronic Health Evaluation П (APACHE П) score was significantly lower than the baseline at the end of the study in each group of patients (P < 0.05). In addition, no significant correlation was observed between Vmax, Km, Cp, Cp/dose ratio, and APACHE II scores at the time of sampling.
Conclusion:
Due to significant differences in phenytoin plasma concentration and pharmacokinetic parameters between HT and CVA patients, close attention must be paid to the pharmacokinetic behavior of phenytoin in the efforts to improve the patient's outcome after a severe HT.
KEYWORDS: Brain injury, Iranian population, pharmacokinetic, Phenytoin
INTRODUCTION
Acute brain injury is one of the leading causes of morbidity and mortality worldwide.[1] Over the last three decades, the understanding of the clinical events after a brain injury has progressed significantly, followed by the understanding of the pathophysiological effects of secondary neurological injury in such patients. Following the initial damage, secondary injury occurs to the viable brain tissue that was not affected by the primary damage. It is possible to reverse the secondary injury through intense effort and using neuroprotective agents, which can attenuate many biochemical processes and/or systemic effects that occur during this period. However, the optimal use of drugs requires the understanding of the pharmacokinetic alterations that may occur in these patients.[2,3]
In the early phase after a brain injury, seizure may cause secondary brain damage as a result of increased metabolic demands, increased intracranial pressure, and excess neurotransmitter release. It has been demonstrated that seizures are an important cause of morbidity.[4] Phenytoin has been commonly used as an anticonvulsant agent for the treatment of or the prophylaxis against seizures for decades. Although the complications of early seizure are the primary therapeutic rational for the use of phenytoin, its neuroprotective effect is the other major factor may be mediated by a voltage-dependent blockade of sodium channels.[5,6]
Phenytoin is 90% bound to albumin and 95% metabolized by the liver. The hepatic clearance of phenytoin primarily depends on the unbound drug fraction and the activity of hepatic drug-metabolizing enzymes.[7]
After a severe head injury, several pharmacokinetic changes occur. These are blood–brain barrier disruption and changes in drug penetration, cytokine release, which can affect the cytochrome P450 enzyme system, alteration in protein binding and drug transportation, and hypothermia.[8,9,10] Although several studies have shown significant metabolism changes in patients after a severe head injury, few studies have focused on the effects of neurotrauma on drug metabolism. The understanding of possible pharmacokinetic alterations is important for the safe and effective drug use in this patient subset. Hence, we focused on the difference between traumatic and nontraumatic brain injury (TBI) in total phenytoin serum concentration to gain a better understanding of phenytoin pharmacokinetic behavior in two subgroups of patients.
METHODS
This was a prospective, observational study conducted from July 2012 to September 2014 at the Intensive Care Unit (ICU) of Sina Hospital, which is affiliated to the Tehran University of Medical Science. Twenty adult brain injury patients who were admitted to the ICU and required phenytoin for the treatment or the prophylaxis of postinjury seizures were enrolled in the study. Patients aged 18 or above, with moderate to severe acute (traumatic or nontraumatic) brain injury who had the Glasgow Coma Scale (GCS) score <13, requiring intravenous phenytoin were chosen. Injuries are classified as severe (GCS: 3–8), moderate (GCS: 9–13), or mild (GCS: 14–15).[11]
Patients who had any of the following conditions were excluded: bradycardia, second- or third-degree heart block, clinically significant hypotension, pregnancy, laboratory evidence of preexisting hepatic or renal disease (i.e. total bilirubin >2 mg/dl, alanine aminotransferase >thrice the normal limit, and serum creatinine >2 mg/dl), a history of phenytoin administration (during current hospitalization), hypersensitivity to phenytoin, and/or concomitant drug therapy with cimetidine, chloramphenicol, phenobarbital, carbamazepine, valproate, theophylline, erythromycin, isoniazid, trimethoprim, sulfonamides, salicylates, or warfarin, which can affect the pharmacokinetic behavior of phenytoin.
According to the type of brain injury (traumatic or nontraumatic), the patients were divided into two groups: the cerebrovascular accident (CVA) group (had CVA) and the head trauma (HT) group (had a HT).
All the patients received an intravenous loading dose of 15 mg/kg phenytoin sodium (50 mg/ml, Darupakhsh, Iran) through an infusion pump at a maximum rate of 50 mg/min, followed by a maintenance dose of 6 mg/kg/day divided into three equal doses, administered at intervals of 8 h for 3 days. Afterward, the maintenance dose was reduced to 4.5 mg/kg/day and continued. This is the most popular dosing regimen when the drug is used locally. For all the patients, there were three scheduled time points for the phenytoin dose through concentration determination. The first blood sample was taken 30 min before initiating a new maintenance dose (4.5 mg/kg/day). The second and third blood samples were obtained 2 and 4 days after the first sample was taken, respectively. All samples were taken 30 min before the next dose.
The Acute Physiology and Chronic Health Evaluation-П (APACHE П) scoring system was used to grade the disease severity.[12] The APACHE П score and GCS were calculated for each patient separately at the baseline and each sampling time.
The electrocardiogram was continuously monitored with lead II, systolic, diastolic, mean arterial pressure, heart rate, and central venous pressure in accordance with the ICU protocol. Paraclinical parameters, such as arterial blood gas, serum electrolytes, serum creatinine, blood urea nitrogen, bilirubin, glucose, and complete blood count were measured during the study period. Serum albumin concentrations were measured at the baseline and sampling days.
The blood samples were allowed to clot and centrifuged at room temperature for 15 min at 3000 rpm. The serum was aspirated and frozen at −30°C until it was analyzed. Phenytoin plasma concentrations were quantified by high-performance liquid chromatography technique with method of Cwik et al.[13]
The Michaelis–Menten pharmacokinetic model (equation 1) was used to calculate the maximum rate of metabolism (Vmax) and the Michaelis–Menten elimination rate constant (Km).
(S) (F) (Dose/t) = Vmax × Cp/Km + Cp (1)
Where Cp(mg/L) is the Cp, Vmax(mg/day) is the maximum rate of metabolism, Km(mg/L) is the concentration at which metabolism rate is half the maximum rate, F = 1 is phenytoin bioavailability, S = 0.92 shows sodium salt factor, and dose/t (mg/day) is daily dose of phenytoin.
The Winter–Tozer method (equation 2) was used to calculate the adjusted total serum Cp based on the serum albumin concentration (Alb).[14] The adjusted total serum Cp were used to calculate the Vmax and the Km.
Cp = Cmeasured/[(0.9 × Alb/4.4) + 0.1] (2)
Where Cp(mg/mL) is Cp that would be observed if patient had normal protein binding, Cmeasured(mg/mL) is Cp measured by laboratory, and Alb (g/dL) is patient's serum albumin concentration.
Statistical analysis was performed using Statistical Package for the Social Sciences software version 22 for windows (SPSS Inc., IBM Co., Chicago, IL, USA). A nonparametric method (Wilcoxon signed-rank test) was used to compare the pharmacokinetic parameters, APACHE II, the GCS scores, and the albumin serum concentrations at the baseline and the sampling days. These data were compared between the two groups of patients (traumatic and nontraumatic) using the Mann–Whitney test. The results were expressed as the mean ± standard deviation (SD) The Spearman rank test was used for the evaluation of correlation between Vmax, Km, Cp, Cp/Dose ratio, and APACHE II scores at the time of sampling. P < 0.05 was considered statistically significant.
RESULTS
A total of twenty critically ill patients with moderate-to-severe acute brain injury (10 HT and 10 CVA) were enrolled in the study. Table 1 shows the demographic and clinical data of the patients. Both groups (HT and CVA) were similar in baseline characteristics (age, weight, GCS, APACHE П score, and serum albumin). The mean values (± SD) of the adjusted phenytoin serum concentration at different time points and pharmacokinetic parameters (Vmax and Km) are shown in Table 2 and Figure 1, respectively. The phenytoin serum concentration per dose ratios (Cp/Dose ratio) are shown in Figure 2. Table 2 shows the total Cp in all twenty patients.
Table 1.
Patients' characteristics
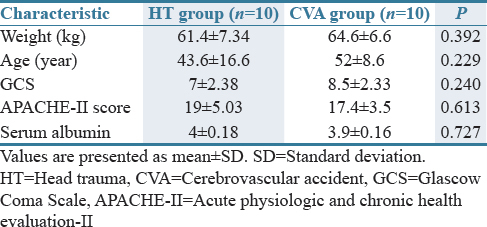
Table 2.
Phenytoin serum concentrations in study patients
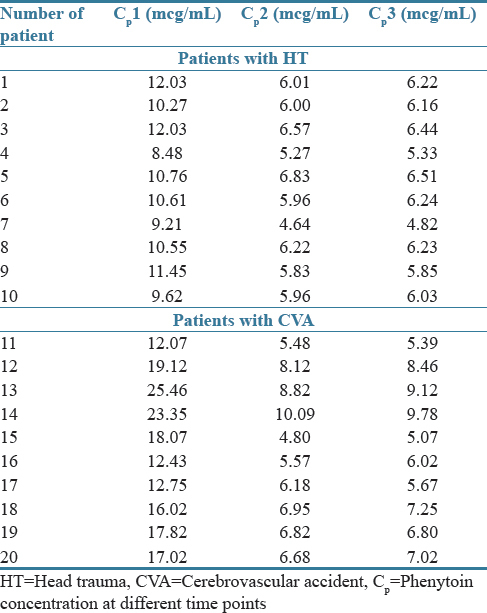
Figure 1.
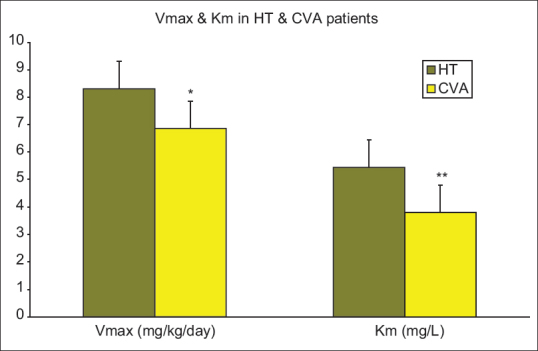
Vmax and Km in head trauma and cerebrovascular accident patients. Data are presented as mean ± standard deviation; HT=Head trauma, CVA=Cerebrovascular accident, Vmax=Maximum rate of drug metabolism, Km=Concentration at which the rate of drug metabolism is 50% of Vmax. *Difference with HT patients was significant (P < 0.05). **Difference with HT patients was statistically significant (P < 0.05)
Figure 2.
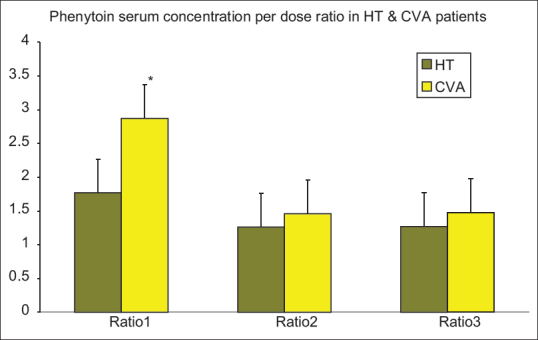
Phenytoin serum concentration per dose ratio in HT and CVA patients. Data are presented as mean ± standard deviation; HT=Head trauma, CVA=Cerebrovascular accident, Vmax=Maximum rate of drug metabolism, Km=Concentration at which the rate of drug metabolism is 50% of Vmax. *Difference with HT patients was statistically significant (P < 0.05
The analysis of the phenytoin serum concentrations (Cp) at different time points indicated that the difference between Vmax and Km in the two groups of patients (HT and CVA) was significant (P< 0.05). The Vmax and Km were significantly higher in HT patients than the CVA group. The Cp and the Cp/Dose ratio were significantly higher in the CVA group patients during the first sampling (P< 0.05). The difference between the Cp and the Cp/Dose ratio in the two groups of patients (HT and CVA) was not significant during second and third sampling. Plasma concentration of phenytoin did not differ significantly in patients with severe TBI in comparison with patients with moderate TBI while there was no significant difference between the CVA and the HT groups for APACHE II scores at the baseline and the sampling days, a decreasing trend was observed for these scores. The APACHE П score was significantly lower than the baseline at the end of the study in each group of patients (P< 0.05). In addition, no significant correlation was observed between Vmax, Km, Cp, Cp/Dose ratio, and APACHE II scores at the time of sampling.
DISCUSSION
Little is known about phenytoin pharmacokinetic behavior in critically ill patients, especially those with neurotrauma.
Griebel et al. studied the alteration in phenytoin protein binding in pediatric patients with acute head injury. They compared children with HT with epileptic patients receiving chronic phenytoin therapy. They observed that despite a reduction in the total Cp over the 10-day study period, the free fraction of phenytoin in the trauma patients increased.[15]
Two other studies have been done on phenytoin pharmacokinetics in adult trauma patients. Boucher et al. observed an increased free fraction of phenytoin between days 1 and 7 in nine out of ten trauma patients. During the follow-up period, three out of four patients had substantially lower total plasma concentrations than the predicted values. They explained the result by increased metabolism and decreased protein binding, which can decrease the total Cp.[16]
In a subsequent study, the same group gained the same results. The urinary metabolite excretion data were consistent with an increase in the systemic clearance of phenytoin, which can be followed by a decrease in the total Cp.[17] Markowsky et al. noted that during the acute phase, there was a trend of decreasing serum concentrations. They concluded that the true steady-state serum concentrations may not occur.[18]
Similarly, Shohrati et al. found that TBI is associated with lower total plasma concentration of phenytoin, increase in free concentration of phenytoin, and increase in Vm. They concluded that patients with TBI require higher dose of phenytoin to achieve target plasma concentration.[19]
Our results are in agreement with these studies. We noted that the HT patients had lower total phenytoin serum concentrations compared with the CVA patients in the first sampling, which was conducted on the 4th day of the study period. And so, the Vmax and Km were significantly higher in HT patients than the CVA group. Although the Vmax and Km were higher in the HT patients, the albumin serum concentrations were not significantly different in the two groups. This finding may show us the importance of an alteration in the metabolism of phenytoin after a HT. In contrast to the previous studies, we obtained plasma concentration of phenytoin sequentially in three-time points after brain injury. While we found that the plasma concentration of phenytoin was significantly lower in HT patients during the first sampling, there were not significant differences in Cp between two groups during second and third sampling. The results demonstrated that the effects of HT on pharmacokinetic of phenytoin are more during early phase (within 72 h) compared with late phase (after 72 h). Age, weight, GCS, APACHE П score, and serum albumin were some characteristics may affect the outcomes of the study. In our study, these characteristics were similar at baseline in both groups (HT and CVA).
The lower total Cp in HT patients, whose severity of illness was shown by their APACH II and GCS scores, suggests that a steady state may not truly occur in critically ill patients as a result of time-dependent changes in drug clearance.
Some of the studies have determined therapeutic free Cp despite low total Cp. In one study, conducted by Bauer et al., Cp in HT patients was compared with epileptic patients. They showed that though the patients with HT had statistically lower and subtherapeutic total Cp, the free concentrations were similar and therapeutic compared with those concentrations in adult epileptic patients. It was suggested that a higher percentage of unbound phenytoin was the result of hypoalbuminemia in the HT patients. In spite of a greater clearance of total phenytoin in HT patients, the clearance of the unbound drug was similar between the two groups.[20] Although the second and third total Cp were subtherapeutic (<10 μg/ml), we did not measure free Cp because serum albumin concentrations in all the samples were within the normal range. Hence, it is unlikely that the changes in protein binding are entirely responsible for the dramatic increase in dosage requirement in this population. Moreover, a better in vivo–in vitro correlation can be attained by applying a total concentration versus an unbound fraction for some drugs metabolized in human hepatocytes and microsoms. Sadeghi et al. demonstrated the lack of correlation between total and free concentration of phenytoin in TBI patients. They concluded free portion of drug should be monitored along with total concentration in this population to prevent both toxicity and treatment failure.[21]
Phenytoin is exclusively metabolized by the liver, and its clearance depends on the unbound fraction and intrinsic clearance. The intrinsic clearance of phenytoin seems to be increased either by drug interactions, or it is secondary to a stress-related increase in the hepatic metabolism of phenytoin.[22] We excluded the patients who took the medications interacting with phenytoin, thereby minimizing this type of interaction.
Changes in Phase I oxidative hepatic metabolism in adult HT patients have been explained by Boucher and Hanes. They determined the clearance of antipyrine, an agent that is metabolized by oxidative microsomal metabolism, in 10 patients with severe head injury. They observed that antipyrine clearance increased significantly over the 14-day study period. Somehow, the majority of the patients received drugs that are known to induce oxidative microsomal metabolism. Whereas phenytoin is also mainly metabolized by the oxidative microsomal hepatic system, the clearance of phenytoin may be similarly influenced.[22] Srisaeng et al. evaluated pharmacokinetic of phenytoin in 122 patients with early TBI and showed that values of Vm in TBI patients are slightly higher. They concluded that the slightly higher value of Vm was associated with increased metabolism rate of phenytoin during early phase of acute brain injury.[8]
The free fraction of phenytoin should be measured if seizures or toxicity occur despite a total Cp within the normal range, or when the protein binding of phenytoin changes due to alterations in serum albumin, concomitant drug therapy, or uremia. Many factors can alter drug metabolism in patients with neurotrauma. These range from the influence of acute phase reactants, diet, and hypothermia to drug interactions.[22] There is an obvious possibility of a time-dependent element in these alterations. Practically, no data are available on renal drug elimination changes following a head injury even though the monitoring of the renal function is less important for these patients compared to other critically ill patients. Put together; these factors pose a major challenge for clinicians to optimize drug therapy in neurotrauma patients.
Hence, due to significant differences in phenytoin plasma concentration and pharmacokinetic parameters between HT and CVA patients, close attention must be paid to the pharmacokinetic behavior of phenytoin in the efforts to improve a patient's outcome after a severe HT. Although the exact mechanism of decreased plasma concentration of phenytoin in HT patients is unclear, it is hypothesized that an increase in hepatic metabolism and unbound plasma concentration may involve in this phenomenon.[8,16,22] The previous studies demonstrated that the clearance of drugs may also be increased as a consequence of potential drug–drug interaction, high daily intake of protein (by increase in oxidative drug metabolism), fluid shifts, or pH changes after severe brain injury.[22,23]
In addition to the small sample size, this study has several other limitations. We included both hemorrhagic and ischemic CVA patients in nontraumatic group. In addition, we did not measure free Cp.
Although the exact mechanism of an increased clearance of phenytoin in patients with neurologic illness is unknown, it is clear that they need doses significantly higher than the recommended ones to gain therapeutic plasma concentrations. A close monitoring of the total Cp is substantial to individualize the dose. Additional investigations are needed to further elucidate the mechanisms responsible for the observed changes in Cp and to describe the time course of these changes.
AUTHORS' CONTRIBUTION
Shahnaz Alimardani contributed in data acquisition, literature search, data analysis, and manuscript preparation. Sima Sadrai contributed in study design, definition of intellectual content, and statistical analysis. Hamidreza Taghvaye Masoumi contributed in literature search, manuscript preparation, manuscript editing, and manuscript review. Pooneh Salari contributed in manuscript preparation, and manuscript editing. Atabak Najafi contributed in study design, and definition of intellectual content. Behzad Eftekhar contributed in study design, definition of intellectual content, and literature search. Mojtaba Mojtahedzadeh contributed in concept, study design, definition of intellectual content, and manuscript editing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Souza RS, Pinheiro PP, de Lima Silva JM, Neto ML, Machado Filho JA. Traumatic brain injury (TBI): Morbidity, mortality and economic implications. Int Arch Med. 2015;8:4–9. [Google Scholar]
- 2.Algattas H, Huang JH. Traumatic brain injury pathophysiology and treatments: Early, intermediate, and late phases post-injury. Int J Mol Sci. 2013;15:309–41. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 4.Englander J, Cifu DX, Diaz-Arrastia R, Center MS. Seizures after traumatic brain injury. Arch Phys Med Rehabil. 2014;95:1223–9. doi: 10.1016/j.apmr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torbic H, Forni AA, Anger KE, Degrado JR, Greenwood BC. Use of antiepileptics for seizure prophylaxis after traumatic brain injury. Am J Health Syst Pharm. 2013;70:759–66. doi: 10.2146/ajhp120203. [DOI] [PubMed] [Google Scholar]
- 6.Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen YW, Smith KE, et al. Traumatic brain injury and epilepsy: Underlying mechanisms leading to seizure. Seizure. 2015;33:13–23. doi: 10.1016/j.seizure.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wu MF, Lim WH. Phenytoin: A guide to therapeutic drug monitoring. Proc Singapore Healthc. 2013;14:198–202. [Google Scholar]
- 8.Srisaeng K, Kanjanasilp J, Sriphong P, Kittivaravach C, Wongsrikaew P, Nopsiri W. Population pharmacokinetics of phenytoin in patients with traumatic brain injury. CMU J Nat Sci. 2015;14:231–43. [Google Scholar]
- 9.Kalsotra A, Turman CM, Dash PK, Strobel HW. Differential effects of traumatic brain injury on the cytochrome p450 system: A perspective into hepatic and renal drug metabolism. J Neurotrauma. 2003;20:1339–50. doi: 10.1089/089771503322686139. [DOI] [PubMed] [Google Scholar]
- 10.Pokorna P, Wildschut ED, Vobruba V, van den Anker JN, Tibboel D. The impact of hypothermia on the pharmacokinetics of drugs used in neonates and young infants. Curr Pharm Des. 2015;21:5705–24. doi: 10.2174/1381612821666150901110929. [DOI] [PubMed] [Google Scholar]
- 11.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 12.Wagner DP, Draper EA. Acute physiology and chronic health evaluation (APACHE II) and medicare reimbursement. Health Care Financ Rev. 1984;(Annual Suppl):91–105. [PMC free article] [PubMed] [Google Scholar]
- 13.Cwik MJ, Liang M, Deyo K, Andrews C, Fischer J. Simultaneous rapid high-performance liquid chromatographic determination of phenytoin and its prodrug, fosphenytoin in human plasma and ultrafiltrate. J Chromatogr B Biomed Sci Appl. 1997;693:407–14. doi: 10.1016/s0378-4347(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 14.Murphy J. Clinical Pharmacokinetics. 6th ed. Bethesda: ASHP; 2017. [Google Scholar]
- 15.Griebel ML, Kearns GL, Fiser DH, Woody RC, Turley CP. Phenytoin protein binding in pediatric patients with acute traumatic injury. Crit Care Med. 1990;18:385–91. doi: 10.1097/00003246-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Boucher BA, Rodman JH, Fabian TC, Cupit GC, Ludden TM, West ME, et al. Disposition of phenytoin in critically ill trauma patients. Clin Pharm. 1987;6:881–7. [PubMed] [Google Scholar]
- 17.Boucher BA, Rodman JH, Jaresko GS, Rasmussen SN, Watridge CB, Fabian TC, et al. Phenytoin pharmacokinetics in critically ill trauma patients. Clin Pharmacol Ther. 1988;44:675–83. doi: 10.1038/clpt.1988.211. [DOI] [PubMed] [Google Scholar]
- 18.Markowsky SJ, Skaar DJ, Christie JM, Eyer SD, Ehresman DJ. Phenytoin protein binding and dosage requirements during acute and convalescent phases following brain injury. Ann Pharmacother. 1996;30:443–8. doi: 10.1177/106002809603000501. [DOI] [PubMed] [Google Scholar]
- 19.Shohrati M, Rouini MR, Mojtahedzadeh M, Firouzabadi M. Evaluation of phenytoin pharmacokinetics in neurotrauma patients. DARU J Pharm Sci. 2007;15:34–40. [Google Scholar]
- 20.Bauer LA, Edwards WA, Dellinger EP, Raisys VA, Brennan C. Importance of unbound phenytoin serum levels in head trauma patients. J Trauma. 1983;23:1058–60. doi: 10.1097/00005373-198312000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Sadeghi K, Hadi F, Ahmadi A, Hamishehkar H, Beigmohammadi MT, Mahmoodpoor A, et al. Total phenytoin concentration is not well correlated with active free drug in critically-ill head trauma patients. J Res Pharm Pract. 2013;2:105–9. doi: 10.4103/2279-042X.122376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher BA, Hanes SD. Pharmacokinetic alterations after severe head injury. Clinical relevance. Clin Pharmacokinet. 1998;35:209–21. doi: 10.2165/00003088-199835030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255–71, vi. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]


