Abstract
Objective:
Metabolic syndrome is a set of cardiac risk factors with increased risk of chronic diseases. The aim of this study is to evaluate the efficacy of crocin of saffron on metabolic syndrome.
Methods:
This double-blind, randomized clinical trial was conducted on metabolic syndrome patients who were randomly assigned to crocin of saffron or control (placebo) groups. The intervention group received 100 mg/day crocin tablets (a constituent of saffron) for 6 weeks. Then, the changes in metabolic syndrome component were compared between two groups. The trial was registered in the Iranian Registry of Clinical Trials. Data were entered to SPSS 15. Chi-square, Fisher's exact, paired t-test, and independent t-test were used to analyze data. P < 0.05 was defined as statistical significant level.
Findings:
Totally, 48 patients included in the trial (24 intervention and 24 placebo participants). There were significant reductions from baseline measurements in the levels of total cholesterol (P < 0.001) and triglyceride (P = 0.003) after the 6-week crocin administration. However, this decrease in lipid profile was not significant when compared with placebo group. There was no significant change in other laboratory values, blood pressure, and anthropometric measures.
Conclusion:
The present study indicated that the dose of about 100 mg crocin of saffron was well tolerated and has no complication for 6 weeks of oral administration. However, the dosage used in our study had no effect on metabolic syndrome. Further studies are required to assess this effect with the higher dosages of crocin as well as long time effects of its administration on metabolic syndrome patients.
KEYWORDS: Crocin, metabolic syndrome, randomized clinical trial, Saffron
INTRODUCTION
Metabolic syndrome is a combination of some disorders and risk factors related to cardiovascular disease and diabetes.[1,2,3] Over the past two decades, different definitions have been proposed for metabolic syndrome.[4] A harmonized definition was published in 2009 by several associations and foundations including International Diabetes Federation (IDF), National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity. This definition contained several factors including the high levels of glucose and triglycerides (TG) and decreased level of high-density lipoprotein cholesterol (HDL) along with high blood pressure (BP) and obesity, particularly central obesity.[5] It is estimated that about 20%–25% of adults in the world are affected by metabolic syndrome.[6]
Historically, some foods and herbs have been used in the prevention, management, and treatment of diseases and related health problems. Saffron (Crocus sativus L.) is a valuable native plant in Iran, especially in Southern Khorasan Province. The stigma of saffron has been widely used as a spice, medicinal plant, and food additive in Iran and other countries. Furthermore, it is used in traditional medicine for treatment of several disorders as antidepressant, sedative, antispasmodic, respiratory decongestant, expectorant, diaphoretic, aphrodisiac, and emmenagogue.[7,8,9] The three major components responsible for the value of saffron are crocin, picrocrocin, and safranal.[9,10] Several animal and human studies were performed to determine the effects of saffron extracts on various kinds of diseases.[11,12,13,14,15] Studies on the effects of saffron extracts on some of the components of metabolic syndrome (hypertension and dyslipidemia) in animals (rats) also have been reported.[12,16,17,18,19] Modaghegh et al. and Mohamadpour et al. evaluated the effects of saffron (C. sativus) tablets in healthy volunteers.[20,21] They showed that saffron tablets may change some hematological and biochemical parameters. However, these alterations were in normal ranges.
Xi et al. studied the effect of crocetin (as crocin) on insulin resistance and its related abnormalities induced by high-fructose diet in male Wistar rats. Compared to the controlled rats fed on normal laboratory diet, fructose-fed rats developed a series of pathological changes including insulin resistance, hyperinsulinemia, dyslipidemia, and hypertension. These disorders were effectively normalized in crocetin-treated rats.[22]
The aim of this trial was to evaluate the efficacy of crocin tablets on metabolic syndrome patients.
METHODS
This double-blind, randomized placebo-controlled clinical trial was conducted between March 2010 and March 2011 on patients referred to Cardiac Outpatient Cardiology Clinic of Birjand University of Medical Sciences. The trial was registered at the Iranian Registry of Clinical trial with the IRCT2016112617756N11.
All patients were provided written informed consent. The eligible participants were individuals with metabolic syndrome defined by the IDF as central obesity (waist circumference >94 and 80 cm in men and women, respectively) plus any two of the following: reduced HDL cholesterol (<40 and 50 mg/dL in men and women, respectively), previously diagnosed type II diabetes or raised fasting blood sugar (FBS) (≥100 mg/dL), treatment of previously diagnosed hypertension or raised BP (systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg), raised TG level (≥150 mg/dL), or specific treatment for hypertriglyceridemia.[5] The exclusion criteria were any history of cardiac, pulmonary, hepatic, gastrointestinal, neurologic, psychiatric, or renal disease. Furthermore, pregnant women were excluded from the study. Sixty adult outpatients who met the inclusion criteria participated in the trial.
Stigmas of C. sativus L. by Novin Zaferan Co. (collected from Ghaen, South Khorasan province, East of Iran) was obtained and analyzed in accordance with the ISO/TS 3632-2). The crocin tablets were prepared as follow: at first, an aqueous saffron extract was made using the modified method by Hadizadeh et al.[23] The extract was standardized by crocin using method defined by Hosseinzadeh et al.,[11] and crocin content of saffron extract was measured 19.7 (~20) mg/100 mg of saffron extract. Hence, each crocin tablet was prepared with 100 mg of crocin. In addition, the placebo, tablets were similar in size and color to the crocin tablets.
According to this method to quantify crocin in an aqueous saffron extract, a modified method was used (Sujata et al., 1992; Hadizadeh et al., 2007). The extract of authentic stigmata was passed through a 0.2 μm Millipore filter (Millipore, Bedford, MA, USA) and eluted with 100% methanol. This quantification was carried out by Shimadzu HPLC LC-10ADvp system integrated with a Shimadzu SCL-10Avp system controller and a SPD-10Avp ultraviolet–visible spectrophotometric on a reversed-phase Shim-pack C18, VP-ODS analytical column (25 cm × 4.6 mm I.D. with a 12.0 ± 1.0 nm pore size and 4.6 ± 0.3 μm particle size), using an isocratic mobile phase of acetonitrile: water (76:24) at a flow rate of 1.2 ml/min. A Rheodyne Shimadzu Model 7725i injector was used to inject 25 μl of the sample from a 25 μl Hamilton straight-edge needle syringe onto the column. All data were recorded and analyzed on a chromatography workstation using Shimadzu Class-VP™ 6.10 software.
After producing saffron extract, pure crocin powder was extracted by the adept in charge in Booali research center affiliated with Mashhad University of Medical Sciences. Then, tablets 1 cm in diameter, 3 mm in thickness, and weighing 400 ± 20 mg each consisting of 20% crocin and 80% starch excipient were manufactured, and an industrial pharmacist carried out quality tests on them. Besides, placebos which had all the face value of the real tablets were made using starch and artificial coloring specific number of the tablets (both real ones and placebos) were packed up considering the number of the days they were to be taken.
All the boxes were numbered so that all even numbers contained real tablets and all odd numbers had placebos, so the study was blinded for two groups. All tablets (real ones and placebos) were kept in a cool place which was not exposed to light.
All participants underwent a clinical evaluation including standard diagnostic interview and medical history. Height and weight were measured according to a standard protocol by a trained health-care professional with participants wearing lightweight clothing and no shoes using a Seca 200 scale with attached stadiometer (Seca, Hamburg, Germany). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Waist, hip, and thigh circumferences were measured using a standard tape measure, and the waist-to-hip ratio and the waist-to-thigh ratio were calculated to obtain the anthropometric indices for the pattern of body fat distribution. A mercury sphygmomanometer (Rudolf Riester GmbH and Co. Jungingen, Germany) was used to measure the BP with the participant sitting and after 10 min of rest. A blood sample was taken after 12 h overnight fasting and sent to laboratory for the measurement of FBS, serum lipids including TG, total cholesterol (Chol), HDL, and low-density lipoprotein (LDL). All measurements and laboratory tests were recorded at the onset of the study and after 6 weeks of intervention. Participants were requested to have no change in their lifestyle patterns during the study period.
All participants were randomized to receive 42 tablets of crocin or placebo (one tablet per day) using a computer-generated code. They were assessed at baseline and at the end of 6 weeks from medication started. Throughout the study, the person who assessed the participants and patients were blind to assignments.
Descriptive statistics include frequency distribution tables and mean ± standard deviation were generated with the SPSS version 15 (SPSS, Chicago, Illinois, USA) statistical software. A Chi-square or Fisher's exact test was used to compare categorical variables. Paired t-test (Wilcoxon signed-rank test) was used to analyze changes in parametric data before and after the intervention in the same group, and independent t-test (Mann–Whitney U-test) was used to compare between mean changes in two groups. The statistical significance level was defined as P ≤ 0.05.
RESULTS
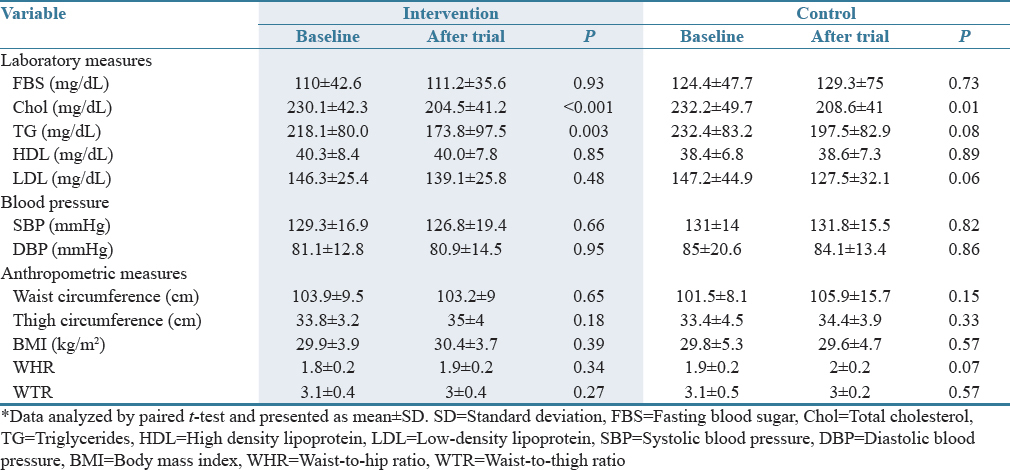
Of the sixty included patients, 48 (24 in intervention and 24 in control group) completed the study. The number of dropouts was six in each group because they have not completed the period of treatment. The mean age of participants was 53.8 ± 9.2 and 50.9 ± 8.8 in intervention and control group, respectively. There were 21 (80.8%) and 20 (76.9%) women among the intervention and control groups, respectively. There was no significant difference between two groups with regard to basic demographic data including age and sex. Furthermore, no significant differences were found between two groups in baseline characteristics including laboratory data, BP, and anthropometric measurements.
The changes of measurements at the endpoint compared to baseline were analyzed with paired t-test and showed a significant reduction in Chol and TG levels among crocin group. Furthermore, a significant reduction in Chol was found among placebo group after 6 weeks [Table 1]. There was no complication or side effect that could be related to the treatment with crocin tablets.
Table 1.
Baseline and outcome measures (after 6 weeks) in two groups*
DISCUSSION
Our findings indicated that crocin tablet consumption (~100 mg/day for 6 weeks) may change some laboratory parameters in patients with metabolic syndrome. Although the decrease in lipid profile including Chol and TG levels was significant after the trial, the reductions were nonsignificant when compared with placebo group. A clinical trial by Modaghegh et al. was conducted on thirty healthy volunteers in three groups of placebo, 200, and 400 mg saffron tablets for 7 days.[20] Similarly, they found no change in lipid profile after administration of saffron tablets. In another study by Zheng et al. on experimental hyperlipidemic animals, although the levels of plasma lipids remained unchanged after administration of saffron, there was a significant reduction in the progression of atherosclerosis and LDL oxidation.[18] However, some other animal studies reported the antilipidemic effect of saffron extract. In a study by Sheng et al. showed that the absorption of fat and cholesterol can be inhibited by crocin which was closely related to the hydrolysis of fat. Furthermore, they found that crocin raised the fecal excretion of fat and cholesterol in rats.[16] Furthermore, He et al. demonstrated that after 9 weeks of saffron consumption, the serum levels of Chol, TG, and LDL were decreased and the formation of aortic plaque was prevented.[19] These finding were more distinguished besides the elevation of HDL levels in Xu's study after 2-month treatment with saffron in rats.[17]
Our study found no change in BP after the administration of ~100 mg crocin for 6 weeks. However, some animal studies demonstrated the antihypertensive effect of saffron in a dose-dependent manner. So that intravenous administration of aqueous saffron extract in rats as 200 mg/kg of crocin, 10 and 1 mg/kg of safranal reduced the mean arterial pressure about 51, 60, and 51 mmHg, respectively. They concluded that the aqueous extract of saffron stigma has hypotensive properties, which appear to be attributable, in part, to the actions of two major constitutes of this plant, crocin and safranal.[12] Furthermore, in Modaghegh's et al. study on healthy volunteers, a higher dose of saffron compared with a lower dose (400 vs. 200 mg) significantly reduced the systolic BP as well as mean arterial pressure.[20]
In this study, we did not find significant change on BMI and abdominal obesity after the 6-week administration of ~100 mg crocin. Although research on the relationship between body weight and saffron is currently not conclusive, there are several theories about the relationship between overweight/obesity and metabolic disorders with saffron antioxidant properties. Gout et al. study results showed that the extract of saffron capsules reinforces the sense of satiety and weight loss of sixty overweight women. In this case–control study within 2 months, a significant reduction observed in snaked-food consumption and weight compared with controls.[24] Several studies have shown that ethanol extract of saffron reduces body weight in rats. However, loss of appetite due to saffron therapy showed clinical problems and side effects.[25] Similar studies showed that taking two capsules of saffron per day (176.5 mg/day) without restrictions on food consumption for 2 consecutive months show a significant reduction in snaked-food consumption and weight loss in the intervention group.[24] Despite the lack of reliable sources about the effects of saffron on weight loss, current knowledge about the properties of saffron showed that saffron at least has a beneficial effect on reducing the risk of obesity and overweight, although there are few studies about its clinical potential properties.[26] Studies have shown that saffron and its active components have significant effects on obesity in animals and humans. In Mashmoul et al. animal study, saffron extract (40 and 80 mg/kg) showed a significant increase on food intake in obese rats. In addition, crocin significantly reduces the rate of weight gain.[27] According to various researches, antiobesity effect of saffron and its active components is as mild to moderate.[28] Kermani et al. studied the effect of saffron supplements in patients with metabolic syndrome. The results of this study showed that saffron can modulate balance on prooxidant–antiprooxidant in subjects with metabolic syndrome.[29]
The present study indicated that the dose of about 100 mg crocin was well tolerated and has no complication for 6 weeks of oral administration. However, the dosage used in our study had no effect on the components of the metabolic syndrome. It may be due to quantity of saffron consumed in our study or duration of consumption. Therefore, it is suggested that further studies are conducted regarding the higher dosages of saffron as well as long-time effects of its administration on metabolic syndrome patients. Since our patients would be at least three components of the metabolic syndrome, our sample was low. Confounding factors such as dietary intake and physical activity can have an impact on the results, while we were unable to adjust them. This is one of the limitations of this study.
AUTHORS' CONTRIBUTION
All authors contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the article.
Financial support and sponsorship
This study was financially supported by the Birjand University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to all cooperation and Clinical Research Development Center of Valiasr Hospital in Birjand.
REFERENCES
- 1.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–81. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 2.Kazemi T, Sharifzadeh GR, Zarban A, Fesharakinia A, Rezvani MR, Moezy SA, et al. Risk factors for premature myocardial infarction: A matched case-control study. J Res Health Sci. 2011;11:77–82. [PubMed] [Google Scholar]
- 3.Kazemi T, Sharifzadeh G, Zarban A, Fesharakinia A. Comparison of components of metabolic syndrome in premature myocardial infarction in an Iranian population: A case -control study. Int J Prev Med. 2013;4:110–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Tanner RM, Brown TM, Muntner P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep. 2012;14:152–9. doi: 10.1007/s11906-012-0254-y. [DOI] [PubMed] [Google Scholar]
- 7.Bostan HB, Mehri S, Hosseinzadeh H. Toxicology effects of saffron and its constituents: A review. Iran J Basic Med Sci. 2017;20:110–21. doi: 10.22038/ijbms.2017.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khazdair MR, Boskabady MH, Hosseini M, Rezaee R, M Tsatsakis A. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J Phytomed. 2015;5:376–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Kamalipour M, Akhondzadeh S. Cardiovascular effects of saffron: An evidence-based review. J Tehran Heart Cent. 2011;6:59–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15:491–5. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–4. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 13.Khorasany AR, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: A review. Iran J Basic Med Sci. 2016;19:455–69. [PMC free article] [PubMed] [Google Scholar]
- 14.Mollazadeh H, Emami SA, Hosseinzadeh H. Razi's al-hawi and saffron (Crocus sativus): A review. Iran J Basic Med Sci. 2015;18:1153–66. [PMC free article] [PubMed] [Google Scholar]
- 15.Rahaiee S, Moini S, Hashemi M, Shojaosadati SA. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J Food Sci Technol. 2015;52:1881–8. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of Crocin in rats: Crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–22. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Xu GL, Yu SQ, Gong ZN, Zhang SQ. Study of the effect of crocin on rat experimental hyperlipemia and the underlying mechanisms. Zhongguo Zhong Yao Za Zhi. 2005;30:369–72. [PubMed] [Google Scholar]
- 18.Zheng S, Qian Z, Sheng L, Wen N. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J Cardiovasc Pharmacol. 2006;47:70–6. doi: 10.1097/01.fjc.0000194686.11712.02. [DOI] [PubMed] [Google Scholar]
- 19.He SY, Qian ZY, Wen N, Tang FT, Xu GL, Zhou CH, et al. Influence of crocetin on experimental atherosclerosis in hyperlipidamic-diet quails. Eur J Pharmacol. 2007;554:191–5. doi: 10.1016/j.ejphar.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 20.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–7. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 22.Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, et al. Beneficial impact of Crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem. 2007;18:64–72. doi: 10.1016/j.jnutbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Hadizadeh F, Mohajeri SA, Seifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak J Biol Sci. 2010;13:691–8. doi: 10.3923/pjbs.2010.691.698. [DOI] [PubMed] [Google Scholar]
- 24.Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. 2010;30:305–13. doi: 10.1016/j.nutres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S, et al. Comparison of petal of Crocus sativus L. And fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:439–42. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Mashmoul M, Azlan A, Khaza'ai H, Yusof BN, Noor SM. Saffron: A Natural potent antioxidant as a promising anti-obesity drug. Antioxidants (Basel) 2013;2:293–308. doi: 10.3390/antiox2040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mashmoul M, Azlan A, Mohtarrudin N, Mohd Yusof BN, Khaza'ai H, Khoo HE, et al. Protective effects of saffron extract and Crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement Altern Med. 2016;16:401. doi: 10.1186/s12906-016-1381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razavi BM, Hosseinzadeh H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97:1679–85. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- 29.Kermani T, Mousavi SH, Shemshian M, Norouzy A, Mazidi M, Moezzi A, et al. Saffron supplements modulate serum pro-oxidant-antioxidant balance in patients with metabolic syndrome: A randomized, placebo-controlled clinical trial. Avicenna J Phytomed. 2015;5:427–33. [PMC free article] [PubMed] [Google Scholar]


