Abstract
Context:
Health system is likely to encounter more adolescents with Type 2 diabetes mellitus (T2DM) as a consequence of obesity and sedentary lifestyle. Intervention at various stages of the life cycle is needed as cumulative effect of risk factors accumulated from fetal life to adult increases risk of noncommunicable disease.
Aims:
The aim of this study was to find out awareness regarding T2DM and distribution of risk factor for T2DM in adolescents from the rural areas of Wardha district, India.
Methodology:
A cross-sectional study conducted in the rural area of India involves 412 adolescent boys and girls selected by systematic random sampling technique. Data collected by a face-to-face interview and waist-hip ratio (WHR), body mass index, random capillary blood glucose (RCBG) were estimated.
Results:
65.1% were aware of T2DM. Girls, older adolescents, higher education were associated with awareness (P < 0.001). Totally, 204 (49.51%) had some risk factors for T2DM, of these 191 (46.6%) had sedentary lifestyle, 153 (31.7%) adolescents had nutritional risk factors, 69 (43.4%) boys had WHR >0.90 and 113 (71.1%) girls had WHR >0.85, 103 (25%) adolescents had RCBG ≥110 mg/dl, and 77 (18.7%) participants reported family history of DM.
Conclusions:
Considering the risk factors of T2DM among adolescents from the rural area, there is a need for prevention programs for creating awareness related to T2DM, early identification of risk factor for T2DM, and targeted interventions. The study may serve as a formative research for developing and testing interventions aimed at primary prevention of T2DM among adolescents from rural India.
Keywords: Adolescents, awareness, risk factors, rural area, type 2 diabetes mellitus
Introduction
Diabetes mellitus (DM) has reached epidemic proportions globally.[1] The World Health Organization (WHO) estimated that there were 135 million people living with diabetes in 1995; this number will increase to 300 million by 2025.[2,3,4] The WHO has projected that maximum increase in number of diabetics would occur in India. With a high genetic predisposition and high susceptibility to environmental insults, the Indian population faces a higher risk of diabetes and its associated complications.[5] The number of people living with diabetes in India was 31.7 million in 2002, and it is estimated that the number will be 79.4 million by 2030.[6,7,8] Considering the large population and increasing prevalence of DM in India, burden of diabetes in India will be enormous in coming years.[9]
As an unfortunate consequence of the current epidemic of obesity and sedentary lifestyle among children and adolescents, the health system is likely to encounter increasing numbers of young patients presenting initially with signs and symptoms associated with uncontrolled hyperglycemia and subsequently, relatively advanced cases of diabetes.[10] Control of noncommunicable diseases needs intervention at various stages of life cycle, such as fetal life, infancy and childhood, adolescence, and adult life, as the cumulative effect of risk factors accumulated since birth or childhood increases the risk of noncommunicable disease.[4,10] Type 2 DM (T2DM) in children and adolescents as a consequence of increasing rates of obesity in our society is a growing public health concern.[10,11] Community- and school-based programs may be helpful to educate parents and children about various risk factors of T2DM and to promote behavioral change for primary prevention of T2DM.[1,11] However, to plan for such specific interventions, it is necessary to have a knowledge of the distribution of important risk factors of T2DM in adolescents. With this background, we carried out this study to find out the awareness regarding T2DM and distribution of risk factor for T2DM in adolescents from the rural areas of Wardha district, India.
Methodology
A cross-sectional study was conducted in the rural area of Wardha, India. Wardha district has a population of around 1.2 million, 2 medical colleges, a district hospital, 2 subdistrict hospitals, 6 rural hospitals, and 29 primary health centers (PHCs). As per the recent survey conducted in the rural area of the district, the prevalence of T2DM in adult from rural Wardha was 3.4% (unpublished data). One out of eight blocks in Wardha district was randomly selected for the study. One PHC out of five in the selected block was randomly picked up, and finally, three subhealth centers (covering 16 villages) were randomly selected for the study.
The study participants were adolescents (age group 11–19 years), boys and girls from the selected villages. Considering the prevalence of obesity in the rural area around 6–8% in adolescent age group,[12,13,14] at 95% confidence level and absolute precision of 5% points, the total of 412 participants was included in the study.[15]
The study protocol was approved by the Institutional Ethics Committee. Data were collected by structured interview. The interview schedule was designed to capture sociodemographic information, behavioral, physiological risk factor for T2DM, and family history of T2DM. An eligible participant from household was selected by systematic random sampling. Only one adolescent from the selected household was included in the study. If the selected household does not have a person from adolescent age group, the immediate next household was selected. Written informed consent was taken, and the participant was assured of the confidentiality of the information. Interviews were conducted in the local language, and each interview lasted for 35–45 min.
The abdominal (waist) circumference was measured in a standing position. An examiner stands behind and palpates hip area for the right iliac crest. The examiner marked a horizontal line at high point of the iliac crest and then crosses the line to indicate the midaxillary line of the body. The examiner then stands on the right side and placed the measuring tape around the trunk in a horizontal plane at this level and marked on the right side of trunk. The recorder walked around to make sure that the tape was parallel to the floor and that the tape is snug but does not compress the skin. The measurement was made at minimal respiration to the nearest 0.1 cm.[16,17]
Measurement of buttocks (hip) circumference: The person stands erect with feet together and weight evenly distributed on both feet. The recorder stands behind the person and gathers the side seams of the pants together above hips and places thumb to make a fold. The recorder holds folded sides of pants snugly while examiner squatted on the right side of the person and places measuring tape around buttocks. The tape was placed at the maximum extension of buttocks. The recorder then adjusted the sides of tape and checks front and sides, so that plane of the tape is horizontal. The tape was held snug but not tight.[16,17]
Waist-hip ratio (WHR) was estimated, and WHR ≥0.90 for male and ≥0.85 for female were considered a substantial risk for metabolic disorders.[17]
Body weight was measured (to the nearest 0.5 kg) with the subject standing motionless on the weighing scale with feet apart and weight equally distributed on both legs. Height will be measured (to the nearest 0.5 cm) with the subject standing in an erect position against a vertical scale with the head position, so that the top of the external auditory meatus was in the level with the inferior margin on the bony orbit. Body proportions normally change during the pubertal development and may vary among adolescents. We, therefore, defined obesity on the basis of a threshold body mass index (BMI), Z score of 2.0 or more, adjusted for age and sex. The subjects were then classified as moderately obese (a Z score of 2.0–2.5) or severely obese (a Z score above 2.5).[16,18]
Random capillary blood glucose (RCBG) was measured by properly calibrated glucometer.
Adolescent was said to be aware of T2DM, if he/she would be able to correctly tell that minimum of three risk factors of T2DM, how T2DM is diagnosed, and minimum three components of diabetes management such as taking regular insulin or oral antidiabetics drugs, dietary modification, regular physical exercise, or regular follow-up with doctors for blood glucose monitoring and early detection of complications.
Sedentary lifestyle was defined as a type of lifestyle with no physical exercise or exercising <45 min in a day and exercising <4 days a week. The nutritional risk factor was considered, if the participant is consuming fruits and vegetables <3 servings in a week.
Data analysis
The outcome variables were the risk factors for T2DM such as sedentary lifestyle or physical inactivity, nutrition risk factors, high WHR, high BMI, RCBG, and family history of T2DM. The association of risk factors with various sociodemographic factors was studied. Chi-square test of significance was used to study the difference in the distribution of risk factors for T2DM across various sociodemographic characteristics of participants.
Results
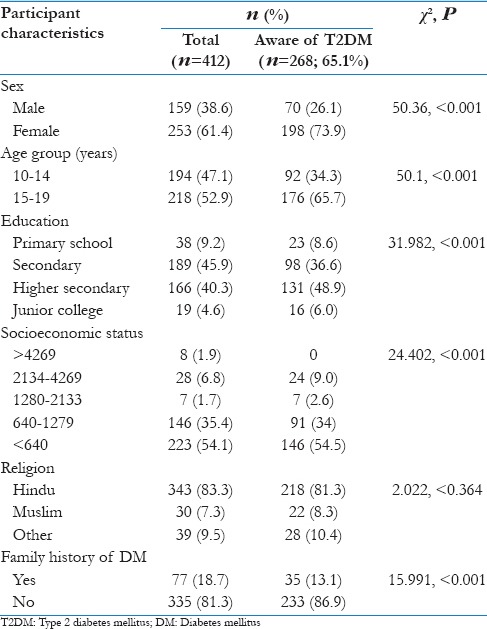
Of 412 adolescents included in the study, 61.4% were girls and 52.9% were in the age range of 15–19 years. The mean age of boys was 14.7 (standard deviation [SD] 2.9) and girls was 14.9 (SD 2.5). The average per capita income of the study participants was Rs. 875 [Table 1]. Overall, 268 (65.1%) were aware of DM. Girls were more aware of T2DM compared to boys (P < 0.001); adolescents in the age group of 15–19 years were more aware compared to those in the age group of 10–14 years (P < 0.001); education till secondary or junior college was significantly associated with more awareness compared to those studied till secondary school only (P < 0.001) [Table 1]. The mean age of participants who were aware of T2DM (15.65 years; 95% confidence interval [CI] 15.41–15.89) was significantly more than the mean age of the participants who were not aware of T2DM (13.54 years; 95% CI 13.3–13.78) (t = 5.83, P < 0.001).
Table 1.
Characteristics of study participants and awareness regarding Type 2 diabetes mellitus
Overall, 204 (49.51%) adolescents had some risk factors for T2DM, and of these, 191 (46.6%) had a sedentary lifestyle, 153 (31.7%) adolescents had nutritional risk factors. Among adolescents boys, 69 (43.4%) had WHR >0.90 and 113 (71.1%) girls had WHR >0.85. RCBG of ≥110 mg/dl was observed in 103 (25%), and 77 (18.7%) reported family history of DM.
Sedentary lifestyle was seen more in girls compared to boys (χ2 = 7.27, P = 007) [Table 2]. Adolescents in the age group of 15–19 years were more sedentary compared to those in 10–14 years (χ2 = 6.58, P = 0.01), and those having per capita income <1297 were more sedentary compared to higher household per capita income (χ2 = 15.14, P = 004). The mean age of adolescent with sedentary lifestyle 15.1 years (95% CI 14.85–15.35) was more compared to those with nonsedentary lifestyle 14.5 years (95% CI 14.24–14.76).
Table 2.
Distribution of risk factors for Type 2 diabetes mellitus in male and female adolescents
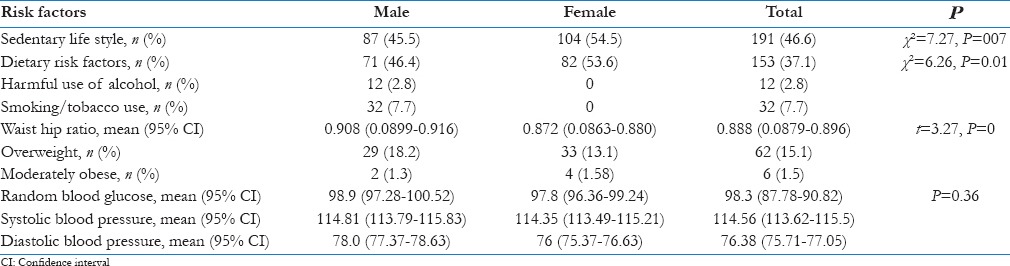
The nutritional risk factors were more in girls compared to boys (χ2 = 6.26, P = 0.01) [Table 2]. Adolescents with higher secondary education have more nutritional risk factors compared to secondary- or primary-educated adolescents (χ2 = 16.13, P = 0.001). The nutritional risk factors were more in adolescents in the age group of 15–19 years compared to younger age group (χ2 = 8.23, P = 0.004) and adolescents with household per capita income <Rs. 640 had more nutritional risk factors compared to higher income (|2 = 15, P = 0.004).
Twelve (2.8%) adolescent boys reported the use of alcohol and 14 (3.4%) boys reported the use of cigarettes or bidi smoking, 18 (4.3%) reported the usage of smokeless tobacco [Table 2].
The mean WHR of study participants was 0.888 (95% CI 0.88–0.9). The mean WHR in boys was significantly more compared to girls (t = 3.27, P = 0.000). The mean BMI among study participants was 18.15 (95% CI 17.85–18.45). The mean BMI in boys was significantly more compared to girls (2.08, P = 0.037). Overall, 6 (1.5%) adolescents were severely obese (BMI Z score above 2.5) and 62 (15.1%) were overweight. None of the adolescents were severely obese (BMI Z score of 2.0–2.5) [Table 2].
The average RCBG was 98.3 (95% CI 96.78–99.82) [Table 2]. Forty-four (27.7%) boys and 60 (23.7%) girls had blood glucose >110 mg/dl, but the difference was not statistically significant (P > 0.05). Similarly, the mean random capillary blood glucose among adolescent boys and girls was not significantly different (P > 0.05). Of those with a family history of T2DM, 22 (28.6%) had RCBG >110 mg/dl. None of the adolescents had blood glucose level >140 mg/dl.
The mean systolic and diastolic blood pressure were 114.56 (95% CI 113.62–115.5) and 76.38 (95% CI 75.71–77.05), respectively. The mean systolic and diastolic bold pressure among adolescent boys and girls were not significantly different (P > 0.05%).
Discussion
The study revealed that nearly half of the adolescents had some risk factors for T2DM, and the most common risk factor was a sedentary lifestyle, followed by higher WHR, nutritional risk factors, RCBG level of 110 mg/dl or more, and family history of DM. Nearly, two-third were aware of T2DM. Adolescents between 15 and 19 years, adolescent girls, and those with a family history of T2DM were better informed about T2DM.
Understanding risk factors for Type 2 diabetes among adolescents from the rural area will facilitate to plan targeted interventions to prevent the emergence of risk factors for T2DM or control of existing risk factors, especially in younger age groups. Increase in prevalence of T2DM among adolescent may likely to increase the incidence of co-morbidities or complications associated with diabetes, such as hypertension, dyslipidemia, retinopathy and cardiovascular diseases.[19,20,21,22,23,24] Moreover, the development and progression of clinical complications of T2DM at an early age due to early onset of DM may pose a public health challenge in coming years.[24]
Changing lifestyle and increase in the prevalence of various modifiable risk factors among adolescents and young adults have been documented by various studies from developing as well as developed countries.[19,20,21,22,23,24] Our study revealed that nearly half of the adolescents had a sedentary lifestyle and this was more prevalent among adolescents in the age group of 15–19 years compared to younger age group. More girls had sedentary lifestyle compared to boys, and this may be attributed to social and cultural factors.
With regard to nutrition, it was observed that around one-third adolescents were consuming fruits and vegetables <3 servings in a week in spite of the fact that around two-third were vegans. Studies had shown that nonvegetarians are at a greater risk of developing T2DM as compared to people with pure vegetarian diet. Tonstad et al., in their study, found that the mean BMI was the lowest in vegans (23.6 kg/m2) and incrementally higher in semivegetarians (27.3 kg/m2) and nonvegetarians (28.8 kg/m2). The prevalence of T2DM increases from 2.9% in vegans to 7.6% in nonvegetarians.[25] Our study revealed that around one-third of adolescents were having nutritional risk factors and therefore may be at risk of T2DM in future life, if dietary habits are not modified.
Many studies have identified that the prevalence of obesity is increasing, even in rural area.[26,27,28] The persons in both these groups are at increased risk of T2DM in future life.[29] Our study revealed that none of the adolescents from the study area were severely obese (BMI Z score above 2.5); however, <2% were moderately obese (BMI Z score of 2.0–2.5) and therefore may be increased risk of developing T2DM.
Males with WHR ≥0.90 and females with WHR ≥0.85 are considered to have substantial risk for metabolic disorders.[17] The mean WHR in our study was 0.89. WHR was significantly more in boys (0.91) compared to adolescent girls (0.87). Nearly half of adolescent boys and three-quarter of adolescent girls had WHR more than 0.90 and 0.85, respectively, and therefore at increased risk of developing T2DM. Waist circumference and WHR correlate more closely to abdominal adipose tissue and abdominal obesity, an important risk factor for T2DM.[17] Therefore, as per the WHR criteria, both adolescent boys and girls may have a substantial risk for developing T2DM in future life. However, the WHR measures have some limitations as a result of change in body composition in adolescent age group and therefore to be interpreted cautiously.
Our study reports that the average RCBG among adolescents was 98.3 mg/dl (95% CI 87.78–90.82). A study conducted in India by Somannavar et al. stated that for Asian Indian, all those with RCBG values >110 mg/dl (6.1 mmol/l) are at increased risk for developing prediabetes and diabetes and therefore should receive more definitive test for diabetes and prediabetes.[29] Our study found that around a quarter adolescents have RCBG >110 mg/dl and can, therefore, at increased risk of T2DM in later life. More boys than girls and over one-fourth adolescents with family history of T2DM were having RBCG >110 mg/dl. We referred these adolescents for the definitive tests at the higher center. Furthermore, we intend to follow-up this cohort of adolescents to study the trends in risk factor over time and documenting the incidence of T2DM in this group over 5 years.
Conclusions
The study identified considerable risk factors of T2DM among adolescents from rural area, and consequently, these adolescents may be at high risk of developing T2DM in future life. Therefore, there is a need for prevention programs for creating awareness related to T2DM, early identification of risk factor for T2DM, and targeted interventions, if the risk factors are present. Our study may serve as a formative research for developing and testing an intervention for the prevention of emergency of risk factor and behavior change for primary prevention of T2DM among adolescents from the rural area in India. Further cohort studies or randomised control trails at population level may be needed to study the effectiveness of programs targeting risk factors in adolescent age group on reduction in prevalence of T2DM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Menon VU, Kumar KV, Gilchrist A, Sugathan TN, Sundaram KR, Nair V, et al. Prevalence of known and undetected diabetes and associated risk factors in central Kerala – ADEPS. Diabetes Res Clin Pract. 2006;74:289–94. doi: 10.1016/j.diabres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Iyer SR. Type 2 diabetes express highway, where is the ‘U’ turn? J Assoc Physicians India. 2003;51:495–500. [PubMed] [Google Scholar]
- 3.Rao CR, Kamath VG, Shetty A, Kamath A. A study on the prevalence of type 2 diabetes in coastal Karnataka. Int J Diabetes Dev Ctries. 2010;30:80–5. doi: 10.4103/0973-3930.62597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A. Epidemiology of diabetes in India – Three decades of research. J Assoc Physicians India. 2005;53:34–8. [PubMed] [Google Scholar]
- 6.Gupta R, Misra A, Vikram NK, Kondal D, Gupta SS, Agrawal A, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord. 2009;9:28. doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 8.Khatib NM, Quazi ZS, Gaidhane AM, Waghmare TS, Goyal RC. Risk factors of type-2 diabetes mellitus in rural Wardha: A community based study. Int J Diabetes Dev Ctries. 2008;28:79–82. doi: 10.4103/0973-3930.44077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 10.Copeland KC, Becker D, Gottschalk M, Hale D. Type 2 diabetes in children and adolescents: Risk factors, diagnosis, and treatment. Clin Diabetes. 2005;23:181–5. [Google Scholar]
- 11.Kesavachandran CN, Bihari V, Mathur N. The normal range of body mass index with high body fat percentage among male residents of Lucknow city in North India. Indian J Med Res. 2012;135:72–7. doi: 10.4103/0971-5916.93427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhatwal J, Verma M, Riar SK. Obesity among pre-adolescent and adolescents of a developing country (India) Asia Pac J Clin Nutr. 2004;13:231–5. [PubMed] [Google Scholar]
- 13.Raj M, Sundaram KR, Paul M, Deepa AS, Kumar RK. Obesity in Indian children: Time trends and relationship with hypertension. Natl Med J India. 2007;20:288–93. [PubMed] [Google Scholar]
- 14.Deshmukh PR, Gupta SS, Bharambe MS, Dongre AR, Maliye C, Kaur S, et al. Nutritional status of adolescents in rural Wardha. Indian J Pediatr. 2006;73:139–41. doi: 10.1007/BF02820204. [DOI] [PubMed] [Google Scholar]
- 15.Lawaga SK, Lameshow S. Sample Size Determination in Health Studies. Geneva: World Health Organization; 1991. [Google Scholar]
- 16.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Technical Report Series. Report No. 854. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 17.Geneva: World Health Organization 2008; 2008. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. 8-11 December. [Google Scholar]
- 18.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 19.Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, et al. Exercise dose and diabetes risk in overweight and obese children: A randomized controlled trial. JAMA. 2012;308:1103–12. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deoke A, Hajare S, Saoji A. Prevalence of overweight in high school students with special reference to cardiovascular efficiency. Glob J Health Sci. 2012;4:147–52. doi: 10.5539/gjhs.v4n2p147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: A systematic review. Int J Obes (Lond) 2012;36:1–11. doi: 10.1038/ijo.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musaiger AO, Al-Hazzaa HM. Prevalence and risk factors associated with nutrition-related noncommunicable diseases in the Eastern Mediterranean region. Int J Gen Med. 2012;5:199–217. doi: 10.2147/IJGM.S29663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson S, Yardy K, Carter V. A narrative literature review of the development of obesity in infancy and childhood. J Child Health Care. 2012;16:339–54. doi: 10.1177/1367493512443908. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 25.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2003;32:791–6. doi: 10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra S, Unnikrishnan AG. Obesity in India: The weight of the nation. J Med Nutr Nutraceut. 2012;1:37–41. [Google Scholar]
- 27.Siddiqui NI, Bose MS. Prevalence and trends of obesity in Indian school children of different socioeconomic class. Indian J Basic Appl Med Res. 2012;2:393–8. [Google Scholar]
- 28.Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP the ICMR-INDIAB Collaborative Study Group. Prevalence of generalized and abdominal obesity in urban and rural India- the ICMR - INDIAB Study (Phase-I) [ICMR - INDIAB-3] The Indian J Med Res. 2015;142:139–50. doi: 10.4103/0971-5916.164234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somannavar S, Ganesan A, Deepa M, Datta M, Mohan V. Random capillary blood glucose cut points for diabetes and pre-diabetes derived from community-based opportunistic screening in India. Diabetes Care. 2009;32:641–3. doi: 10.2337/dc08-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]


