Abstract
Introduction:
Delay in the diagnosis of tuberculosis (TB) can lead to an increased infectivity period, delayed treatment, and increased severity of the disease. The objective of this study was to estimate the diagnostic delay and factors associated with the delay in diagnosis among the newly diagnosed smear-positive pulmonary TB patients in Kerala, India.
Materials and Methods:
A cross-sectional study was conducted among TB patients who were in the intensive phase of directly observed treatment short-course treatment in four randomly selected TB units in a district in Kerala during the years 2012–2013. Diagnostic delay was defined as the delay between the onset of symptoms and diagnosis. Data collection using a modified World Health Organization questionnaire was done by interviewing 302 participants.
Results:
Mean age of the participants was 48.6 ± 14.5 years. Males constituted 76.5% of the study population. The mean diagnostic delay was 43.5 ± 29.1 days (median: 37 days). The median patient and health system delays were 16 days and 15 days, respectively. Patient delay (55.6%) contributed more than health system delay (44.4%). Poor knowledge about TB, first consulting a private physician, and increased number of consultations were found to be significantly associated with diagnostic delay.
Conclusion:
The diagnostic delay in tuberculosis reported in this study was lower than other studies in India but it needs further reduction. Both patients and health providers play a role in a delay in diagnosis, and poor knowledge about the disease among the patients was one of the main risk factors. Interventions to improve knowledge and awareness of the disease and to increase the suspicion of chest symptomatic by health-care providers in the private sector are vital to reduce diagnostic delay.
Keywords: Diagnostic delay, factors, Kerala, patient delay, South India, tuberculosis
Introduction
According to the World Health Organization (WHO), one-third of the world's population is estimated to be infected with Mycobacterium tuberculosis. India has more new cases annually than any other country. The WHO estimated that incidence of new tuberculosis (TB) cases in India (2011) was 2.3 million, and there were 320,000 deaths during the same period.[1] The Revised National Tuberculosis Control Programme (RNTCP), based on the internationally recommended directly observed treatment short-course (DOTS) strategy, was launched in 1997 and expanded across the country in a phased manner. A full nationwide coverage was achieved in March 2006.[2] In spite of its impressive performance in terms of case detection and cure rates, the programme has many challenges due to inadequate infrastructure and the different health-seeking behavior pattern and the TB–diabetes comorbidity.
Early diagnosis of TB and prompt initiation of treatment are essential for the effective TB control programme. Patients with undiagnosed pulmonary TB predominantly act as reservoirs for transmission, and delay in the diagnosis may worsen the disease, increases the risk of death and the chances of transmission of TB in the community, as each infectious case will result in 10–15 of the secondary infections.[3] It also increases the patient expenditure on the disease. The objective of this study was to estimate the diagnostic delay and the factors associated with delay among newly diagnosed smear-positive pulmonary TB patients in Kerala in South India.
Materials and Methods
Background
A community-based cross-sectional study was conducted in Kozhikode district in North Kerala during the years 2012–2013. The study participants were newly diagnosed sputum smear-positive pulmonary TB patients aged ≥15 years who were in the intensive phase (Category I) of DOTS treatment under the RNTCP. Seriously ill patients were excluded from the study. Cluster sampling technique was adopted. Each of the tuberculosis unit (TU) was considered as a cluster. Of six TB units in the district, four units were selected by simple random sampling.
Sample size
Total sample size was estimated to be 276 patients. After considering an allowable error of 15% from the mean diagnostic delay in a study conducted by the WHO in Yemen,[4] where the mean diagnostic delay and standard deviation was 57.4 ± 62.3 days. Of the 326 eligible participants during the study period, 302 patients participated in the study, with an overall response rate of 92.64%.
Survey instrument
Details of the eligible patients were obtained from senior treatment supervisors of each TU immediately after treatment initiation for each patient. Data were collected by using a pretested semi-structured questionnaire by personal interview of patients at DOTS centers/patients’ home and by the verification of registers and records at primary health centers such as outpatient card, RNTCP patient card, and discharge card; the patients were asked to recall the duration from the onset of symptoms to the first health-seeking action, the reasons for delay in seeking care, and the number and types of providers consulted. Confirmation of the information was done with the help of their relatives and verification of dates of prescriptions and laboratory investigations. To assess the knowledge of TB, seven questions were asked. Scoring was done based on a number of correct responses. Questions included knowledge regarding the cause of TB, transmissibility, mode of transmission, possibility of cure, symptoms of TB (able to tell at least two of TB-related symptoms), vaccine for TB, and duration of treatment. A total of six questions were asked to assess the stigma about TB, and based on the answers, scoring was done.
Definitions
Diagnostic delay: It is the time interval between the onset of symptoms and confirmation of TB in the patient. This includes patient delay and health system delay. Patient delay: Period from the onset of the first symptom(s) related to pulmonary TB such as cough, fever, and chest pain to the first medical consultation. Health system delay: period from the first consultation to the date of diagnosis. Diagnostic delay was categorized using a cutoff value of 4 weeks by considering an acceptable patient delay and health system delay of 2 weeks each.
Ethical clearance
Permission was obtained from district medical officer(H) and district TB officer to conduct the study, and Ethical Committee approval was taken from the Institutional Ethical Committee of Government Medical College, Kozhikode, Kerala, India. Informed written consent was obtained from all the study participants.
Data analysis
Descriptive statistics was used for the delay in days, and the level of significance was set at ≤0.05. To study the factors associated with various delays, Pearson's Chi-square test was done. All variables which were found important in univariate analysis were put into a multivariate logistic regression model, and adjusted odds ratio (OR) and 95% confidence interval (CI) were obtained.
Results
Among the 302 patients studied, the majority were males, i.e., 231 (76.5%) and 71 (23.5%) were females. The mean age of the participants was 48.6 ± 14.5 years, and majority (65.9%) of the participants were in the age group of 31–60 years. Most of them, i.e., 173 (57.3%) were from urban area and 66% of the participants were Hindus. Half of the patients (50%) were doing the unskilled type of occupation. The median family size was 5, and 57% of the participants were living in pucca houses. The majority of the participants (70.6%) belonged to low socioeconomic status. The majority of the participants (84.1%) were married and living with spouse at the time of the study. History of TB in the family was reported by thirty (9.9%) patients.
The extent of delays
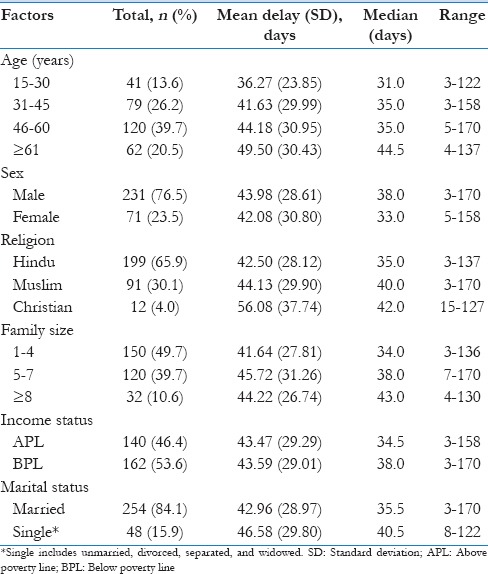
Median diagnostic delay was 37 days, and the median patient and health system delays were 16 and 15 days, respectively [Table 1]. There is a trend toward an increasing diagnostic delay with an increasing age. Males (38 days vs. 33 days) showed higher delay compared to females. Christians showed a longer delay though the sample size is low, and the longer diagnostic delay was noticed among patients with a large family size (≥5 family members). The median diagnostic delay among single patients which include unmarried, divorced, separated, and widowed was higher than married participants (40.5 vs. 35.5) [Table 2].
Table 1.
Extent of diagnostic delay (in days)

Table 2.
Sociodemographic factors and diagnostic delay
Factors associated with diagnostic delay
Sociodemographic and other clinically relevant factors associated with diagnostic delay are shown in Table 3. The study showed an increasing diagnostic delay with an increasing age, particularly after 61 years (72.6%) of age. The proportion of males and females with the delay in diagnosis is almost same (63.6% vs. 62.0%). Diagnostic delay was more among Christians (75% of were delayed), but the sample size was too small to arrive at a definite conclusion. However, more than 60% of the patients showed a delay in diagnosis in all the three groups. Illiterates had a delay in diagnosis than literates (81% vs. 61.9%). The proportion of employed and unemployed in getting a delay in diagnosis is almost same (63.3% vs. 63.1%). This indicates unemployment was not a factor for delay in diagnosis.
Table 3.
Factors associated with diagnostic delay
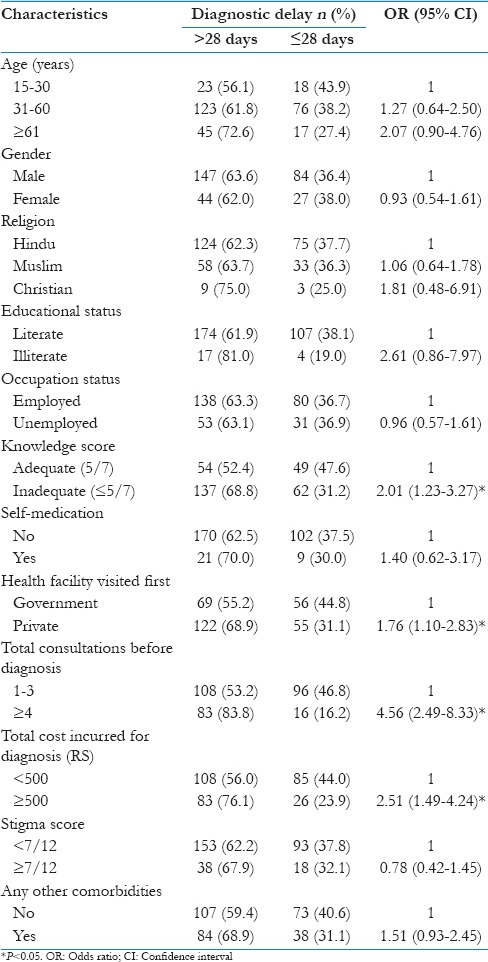
Patients with “inadequate knowledge” (median knowledge score of 5 was taken as a cutoff) had a more diagnostic delay than those with adequate knowledge (68.8% vs. 52.4%) (P = 0.005). This finding is in line with the current literature that indicates inadequate knowledge leads to delay in seeking treatment and consequently diagnosis. The longer diagnostic delay was noticed among those who had self-medicated (70.0% vs. 62.5%) (P = 0.419).
A large proportion of patients had a long diagnostic delay if they had first consulted a private provider as compared to government health provider (68.9% vs. 55.2%) (P = 0.015). This may be because of the appropriate referrals to the government health providers to diagnostic microscopy center and greater awareness regarding RNTCP and the habit of patient holding in the private sector. Patients with long diagnostic delay had a high number of consultations, and the delay could be due to frequent change of doctors.
Patients having long diagnostic delay spent more money (76.1% vs. 23.9%) (P < 0.001). This may be related to the number of consultations. The long diagnostic delay was found among patients who had high stigma score (67.9% vs. 62.2%). History of chronic diseases was reported among the study participants. Among the study participants, 33.1% gave a history of self-reported diabetes mellitus, 6.3% of the participants were hypertensives, and chronic obstructive pulmonary disease was reported among 2.3% of the participants. This suggests high levels of diabetes mellitus in patients with TB. Patients had a long diagnostic delay if they have had any other comorbidity (68.9% vs. 59.4%) [Table 3].
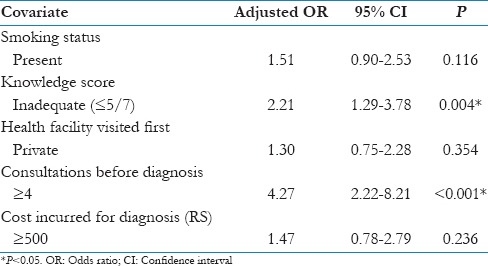
On multivariate analysis, inadequate knowledge score (OR: 2.21; 95% CI: 1.29–3.78) and ≥4 consultations made before diagnosis remained as significant factors for the diagnostic delay [Table 4].
Table 4.
Risk factors associated with diagnostic delay – multivariate analysis
Discussion
The present study showed the mean diagnostic delay as 43.5 ± 29.1 days (median: 37 days, range: 3 days to 170 days). Similar delays were reported in other studies by Gosoniu et al. at Malawi (33.5 days)[5] and a multicountry study by Bassili et al.[4] at Yemen (35 days) and in Iraq (36 days).
The median diagnostic delay in our study is much lower than other reported studies such as Lacroix et al. at Canada in 2007 (83 days),[6] Lawn and Griffin in Ghana (4 months),[7] Pronyk et al. (2001) from South Africa (10 weeks),[8] Machado et al. at Brazil (68 days),[9] and Ward et al. at Queensland (66 days).[10] A similar finding in India, a study from Tamil Nadu by Rajeswari et al.[11] in the early period of introduction of DOTS program (1997–1998) showed a median diagnostic delay of 60 days. Patient delay (55.6%) contributed more than health system delay (44.4%). Another study conducted in India by Gosoniu et al. also showed a longer median diagnostic delay of 74 days.[5]
However, a study by Phoa et al.[12] in Singapore showed a median diagnostic delay of 4 weeks which was lower than our findings.
Our study showed the effect of increasing diagnostic delays with increasing age, particularly after 61 years (72.6%) (OR: 2.07; 95% CI: 0.90–4.76). Two USA studies also found that patients aged over 65 were misdiagnosed more commonly than younger patients,[13,14] although other studies have not shown any significant association with age.[15,16,17] Males showed higher delay compared to females (OR: 0.93; 95% CI: 0.54–1.61). A study in Nigeria[18] also found no difference in the influence of gender, which shows the absence of gender bias in a delay in the diagnosis of TB. However, a study done by Gosoniu et al. in Bangladesh[5] found increased delay among the female participants. More delay was noticed among patients with ≥5 family members and among single patients. The delay was similar across religion and income status. However, although the sample size is low, longer delay was noticed among Christians (OR: 1.81; 95% CI: 0.48–6.91). Illiterates had a delay in diagnosis than literates (81% vs. 61.9%) (OR: 2.61; 95% CI: 0.86–7.97). There was no difference in the diagnostic delay with respect to the occupational status of the study participants (OR: 0.96; 95% CI: 0.57–1.61). However, a study at Malawi[5] found that diagnostic delay was seen in homemakers.
The main factor contributed to diagnostic delay was inadequate knowledge about TB (OR: 2.01; 95% CI: 1.23–3.27) which is consistent with another study by Bassili et al.[4] that poor knowledge leads to delay in seeking treatment and consequently the diagnosis. Participants with a history of self-medication had higher odds of delay (OR: 1.40; 95% CI: 0.62–3.17), but the difference was not statistically significant may be because of the proportion of patients who self-medicated was less (9.9%) in our study.
Patients had a longer diagnostic delay if they had consulted a private health-care provider (OR: 1.76; 95% CI: 1.10–2.83); this may be because of the easy accessibility of government health-care providers to diagnostic microscopy center and greater awareness regarding RNTCP when compared to private providers. Private health-care providers do not have strong linkages with the government health system. Lack of training of health-care providers in the private sector contributes to delay in diagnosis. Therefore, linkage of private practitioners in RNTCP needs to be stepped up. Our finding was consistent with the study by Rajeswari et al.[11] where longer health system delay was observed among those who approached a private health provider first (30 days vs. 7 days). The same finding was noted by Lawn and Griffin[7] at Ghana that delay in diagnosis was strongly associated with attendance of the patients at private medical clinics. A high number of consultations before diagnosis lead to delay in diagnosis (OR: 4.56; 95% CI: 2.49–8.33), and it could be due to frequent change of doctors.
If the amount spent on the diagnosis is more, there is a higher chance of getting a delay in diagnosis (OR: 2.51; 95% CI: 1.49–4.24) with statistically significant results. Our finding consistent with a study by Tobgay et al.[19] at Sikkim found that the patient delay was more among those who spent Rupees 400 than those who spent <Rupees 100 (OR: 2.52; 95% CI: 1.17–5.38). Social stigma still prevails in our societies; many believe that being diagnosed with TB may socially exempt them from all activities, hence do not seek care. Stigma also affects marriage prospects and family life of females; this may be an important factor for delay among them. In our study, as the stigma score increases, there is a chance for the delay in diagnosis (OR: 0.78; 95% CI: 0.42–1.45). Studies showed that TB carries a strong stigma, and the fear of being diagnosed with TB might prevent some patients from seeking the diagnosis.[4,20,21,22] In South India, a study by Balasubramanian et al.[23] found that women faced significantly greater stigma than men in terms of inhibitions, and in a study by Mesfin et al.,[24] they suspect that fear of stigmatization to TB and HIV coinfection may be contributing to delay. However, in a study by Godfrey-Faussett et al.[25] among patients with a chronic cough, they found stigmatizing was not associated with delays in seeking care for a chronic cough.
In our study, prevalence of diabetes among pulmonary TB was found to be high. According to a study by Balakrishnan et al.[26] at Kerala in 2011, it was found that 44% of the TB patients had self-reported diabetes mellitus. This proportion was higher than that found in our study. Though not significant, patients presented with other co-morbidities had higher odds of delay in diagnosis (OR: 1.51; 95% CI: 0.93-2.45). This is an important finding as TB incidence is increasing among diabetic patients, which may lead to resistance.
Conclusion
The median delay of 37 days is lower compared to other studies in the literature. Patient delay for the first consultation was the main reason for the diagnostic delay. Low knowledge score and increased number of consultations were found to be the risk factors associated with diagnostic delay. The delay was more in those who consulted private facilities compared to government facilities. These risk factors for delay can be the subject of future interventions to reduce the delay in diagnosis in patients with TB and hence transmission of the disease in the community.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. World Health Organization Global Tuberculosis Control. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.The Government of India. Revised National Tuberculosis Control Programme (RNTCP). Training Module for Medical Practitioners. Ministry of Health and Family Welfare, Government of India, New Delhi; December. 2010. [Last accessed on 2013 Oct 26]. Available from: http://www.tbcindia.nic.in/WriteReadData/l892s/5949760355Training%20Module%20for%20Medical%20Practitioners.pdf .
- 3.Park K. Tuberculosis, Park's Text Book of Preventive and Social Medicine. 20th ed. Jabalpur (M.P.), India: M/s Banarsidas Bhanot; 2013. pp. 159–75. [Google Scholar]
- 4.Bassili A, Seita A, Baghdadi S, AlAbsi A, Abdilai I, Agboatwalla M, et al. Diagnostic and treatment delay in tuberculosis in 7 countries of the Eastern Mediterranean region. Infect Dis Clin Pract. 2008;16:23–35. [Google Scholar]
- 5.Gosoniu GD, Ganapathy S, Kemp J, Auer C, Somma D, Karim F, et al. Gender and socio-cultural determinants of delay to diagnosis of TB in Bangladesh, India and Malawi. Int J Tuberc Lung Dis. 2008;12:848–55. [PubMed] [Google Scholar]
- 6.Lacroix C, Martin P, Turcotte S, DeRoche S, Magluilo V, Lacroix C. The delay in diagnosis of tuberculosis in the Monteregie region of Quebec, Canada. Mcgill J Med. 2008;11:124–31. [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Griffin GE. The irreversible cost of delayed diagnosis of tuberculosis in HIV co-infected persons in sub-Saharan Africa. Int J Tuberc Lung Dis. 2001;5:200–1. [PubMed] [Google Scholar]
- 8.Pronyk RM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. Int J Tuberc Lung Dis. 2001;5:619–27. [PubMed] [Google Scholar]
- 9.Machado AC, Steffen RE, Oxlade O, Menzies D, Kritski A, Trajman A. Factors associated with delayed diagnosis of pulmonary tuberculosis in the state of Rio de Janeiro, Brazil. J Bras Pneumol. 2011;37:512–20. doi: 10.1590/s1806-37132011000400014. [DOI] [PubMed] [Google Scholar]
- 10.Ward J, Siskind V, Konstantinos A. Patient and health care system delays in Queensland tuberculosis patients, 1985-1998. Int J Tuberc Lung Dis. 2001;5:1021–7. [PubMed] [Google Scholar]
- 11.Rajeswari R, Chandrasekaran V, Suhadev M, Sivasubramaniam S, Sudha G, Renu G. Factors associated with patient and health system delays in the diagnosis of tuberculosis in South India. Int J Tuberc Lung Dis. 2002;6:789–95. [PubMed] [Google Scholar]
- 12.Phoa LL, Teleman MD, Wang YT, Chee CB. Characteristics of patients with delayed diagnosis of infectious pulmonary tuberculosis. Respirology. 2005;10:196–200. doi: 10.1111/j.1440-1843.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 13.Mathur P, Sacks L, Auten G, Sall R, Levy C, Gordin F. Delayed diagnosis of pulmonary tuberculosis in city hospitals. Arch Intern Med. 1994;154:306–10. [PubMed] [Google Scholar]
- 14.Counsell SR, Tan JS, Dittus RS. Unsuspected pulmonary tuberculosis in a community teaching hospital. Arch Intern Med. 1989;149:1274–8. [PubMed] [Google Scholar]
- 15.Hooi LN. Case-finding for pulmonary tuberculosis in Penang. Med J Malaysia. 1994;49:223–30. [PubMed] [Google Scholar]
- 16.Mori T, Shimao T, Jin BW, Kim SJ. Analysis of case-finding process of tuberculosis in Korea. Tuber Lung Dis. 1992;73:225–31. doi: 10.1016/0962-8479(92)90091-W. [DOI] [PubMed] [Google Scholar]
- 17.Pirkis JE, Speed BR, Yung AP, Dunt DR, MacIntyre CR, Plant AJ. Time to initiation of anti-tuberculosis treatment. Tuber Lung Dis. 1996;77:401–6. doi: 10.1016/s0962-8479(96)90111-2. [DOI] [PubMed] [Google Scholar]
- 18.Odusanya OO, Babafemi JO. Patterns of delays amongst pulmonary tuberculosis patients in Lagos, Nigeria. BMC Public Health. 2004;4:18. doi: 10.1186/1471-2458-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobgay KJ, Sarma PS, Thankappan KR. Predictors of treatment delays for tuberculosis in Sikkim. Natl Med J India. 2006;19:60–3. [PubMed] [Google Scholar]
- 20.Jaramillo E. Pulmonary tuberculosis and health-seeking behaviour: How to get a delayed diagnosis in Cali, Colombia. Trop Med Int Health. 1998;3:138–44. doi: 10.1046/j.1365-3156.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- 21.Johansson E, Long NH, Diwan VK, Winkvist A. Gender and tuberculosis control: Perspectives on health seeking behaviour among men and women in Vietnam. Health Policy. 2000;52:33–51. doi: 10.1016/s0168-8510(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 22.Liefooghe R, Michiels N, Habib S, Moran MB, De Muynck A. Perception and social consequences of tuberculosis: A focus group study of tuberculosis patients in Sialkot, Pakistan. Soc Sci Med. 1995;41:1685–92. doi: 10.1016/0277-9536(95)00129-u. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian R, Garg R, Santha T, Gopi PG, Subramani R, Chandrasekaran V, et al. Gender disparities in tuberculosis: Report from a rural DOTS programme in South India. Int J Tuberc Lung Dis. 2004;8:323–32. [PubMed] [Google Scholar]
- 24.Mesfin MM, Tasew TW, Tareke IG, Kifle YT, Karen WH, Richard MJ. Delays and care seeking behavior among tuberculosis patients in Tigray of Northern Ethiopia. Ethiopian J Health Dev. 2005;19:7. [Google Scholar]
- 25.Godfrey-Faussett P, Kaunda H, Kamanga J, van Beers S, van Cleeff M, Kumwenda-Phiri R, et al. Why do patients with a cough delay seeking care at Lusaka urban health centres? A health systems research approach. Int J Tuberc Lung Dis. 2002;6:796–805. [PubMed] [Google Scholar]
- 26.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]


