Abstract
Objective:
The present study was conducted to investigate the antioxidant and hepatoprotective activity of the hydro-alcoholic extract of aerial parts of Artemisia dracunculus (HAAD) against CCl4-induced hepatotoxicity in rats.
Materials and Methods:
The antioxidant activity was evaluated by reducing power, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 20-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. Rats were pre-treated with either 50, 100, and 200 mg/kg of HAAD or silymarin (100 mg/kg; served as the positive control group) for 15 days and they received a single dose of CCl4 on the last day. Hepatoprotective effects were investigated by assessment of serum biochemical enzymes such as alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total protein (TP), total bilirubin (TB), malondialdehyde (MDA), and antioxidant enzymes (SOD, CAT, GST and GSH), along with histopathological studies.
Results:
Total phenolic content was 197.22±3.73 mg gallic acid equivalent/g HAAD dry weight. HAAD indicated powerful activity in FRAP, DPPH and ABTS tests. Acute toxicity study showed that the extract had an LD50 of >5000 mg/kg. Oral treatment with HAAD exhibited a significant decrease in the levels of AST, ALT, ALP and TB and an increase in the level of TP. The extract significantly diminished MDA levels. The activities of the antioxidant enzymes were significantly augmented in rats pretreated with HAAD 200 mg/kg. Histopathological examination demonstrated lower liver damage in HAAD-treated groups as compared to CCl4 groups.
Conclusion:
Our findings indicated hepatoprotective effects of the hydro-alcoholic extract of A. dracunculus on CCl4-induced hepatic damage in rats and suggested that these effects may be produced by reducing oxidative stress.
Key Words: Antioxidant, Artemisia dracunculus, Carbon Tetrachloride, Hepatotoxicity, Oxidative stress, Rats
Introduction
Carbon tetrachloride (CCl4) as a standout amongst the hepatotoxins, is generally utilized to induce experimental models (Cheng et al., 2013 ▶). It is accepted that CCl4 hepatotoxicity occurs due to its reductive dehalogenation reaction which is catalyzed by cytochrome P-450 in hepatocytes (Raj and Gothandam, 2014 ▶). This response actuates the generation of a few sorts of reactive oxygen species (ROS) (Jalali Ghassam et al., 2014 ▶). These ROS can bind to lipids or proteins resulting in creation of distinctive radicals and initiation of lipid peroxidation which end up in membrane injury and consequently damage the liver (Ali et al., 2014 ▶; Kepekçi et al., 2013 ▶).
Today, administration of conventional or synthetic drugs for the treatment of liver disorders is facing serious side effects (Toori et al., 2015 ▶, Manns et al., 2006 ▶). Therefore it is necessary to seek for new medicines for liver disease, notably those originated from natural resources. Medicinal plants are great sources of natural compounds such as phenolic acids and flavonoids, which have been shown good in vitro antioxidative properties and are able to scavenge free radicals and defend liver from CCl4-induced hepatic injury (Azeem et al., 2010 ▶, Gnanadesigan et al., 2011 ▶, Gupta et al., 2011 ▶, Singhal and Gupta, 2012 ▶).
Artemisia dracunculus L. (tarragon) is a perennial plant belonging to the Asteraceae family, which has a long history of use as a spice and remedy (Obolskiy et al., 2011 ▶). A. dracunculus is distributed over most of temperate Asia, western North America and central Europe (Eisenman and Struwe, 2011 ▶). Aerial parts of tarragon which have been used as an antihyperlipidemic and anticoagulant agent, are commonly consumed in fresh or dried form in salads and soups in Iranian cuisine Shahriyary and Yazdanparast, 2007 ▶). Previous studies on A. dracunculus indicated that this plant have bioactive constituents like α-pinene, terpinolene, allo-ocimene, α-trans-ocimene, 7-methoxycoumarin, trans-anethole, methyleugenol, estragole, γ-terpinene and elemicin (Aglarova, 2006 ▶, Khodakov et al., 2009 ▶, Kordali et al., 2005 ▶, Pappas and Sturtz, 2001 ▶, Sayyah et al., 2004 ▶). Moreover, several biological activities of tarragon such as antibacterial, antiplatelet, anti-inflammatory, antihyperglycemic, gastroprotective and antioxidant properties have been confirmed in the literature (Aglarova, 2006 ▶, Benli et al., 2007 ▶, Kheterpal et al., 2010 ▶, Lopes-Lutz et al., 2008 ▶, Shahriyary and Yazdanparast, 2007 ▶).
To our knowledge, there is no report on the antioxidant and hepatoprotective potential of A. dracunculus in the literature. Hence, the aim of this study was to analyze the antioxidant and hepatoprotective activity of hydro-alcoholic extract of aerial parts of A. dracunculus (HAAD) against CCl4-induced hepatotoxicity in rats.
Materials and Methods
This experimental study was carried out in Yasuj University of Medical Sciences, Yasuj, Iran from December 2014 to March 2015. The study was approved by the Ethics Committee of Yasuj University of Medical Sciences (Code: 94/6/2/5) in August 2014 and was exactly performed according to the “Principles of Laboratory Animal Care” (NIH Publication No. 86-23).
Chemicals and reagents
Trichloroacetic acid (TCA), Thiobarbituric acid (TBA), pyrogallol, Folin–Ciocalteu reagent, gallic acid, carbon tetrachloride (CCl4), potassium persulfate, potassium ferricyanide, and diethyl ether were obtained from Merck, Germany. Silymarin, 2, 2-diphenyl-1-picrylhydrazyl, 2, 20-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), 5, 5'-dithiobis-(2-nitrobenzoic acid, and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). The kits for the determination of ALT, AST, ALP, TB and TP were purchased from Pars Azmun, Tehran, Iran.
All chemicals and reagents used for biochemical assays were of analytical grade.
Plant materials
Aerial parts of A. dracunculus, including stems and leaves were collected in August 2014 from the suburbs of Behbahan, Khuzestan province, Iran. The plant was authenticated by Dr. J. Vaezi from Department of Biology, Faculty of Sciences, Shahid Chamran University of Ahvaz, Ahvaz, Iran, and a voucher specimen (herbarium No. 93/135) was deposited. The collected aerial parts of A. dracunculus were shade dried, and pulverized.
Extract preparation
The powdered plant material (200 g) was concentrated for two times using l000 ml mixture of EtOH-H2O (7:3) at 45°C for 48 hr. The extract was separated and organic solvent was totally dissipated under decreased pressure in a rotary evaporator (Hyedolph, type: Heizbad Hei-VAP, Germany) at 60°C. Then, the concentrated extract was dried at room temperature. The average yield of the hydro-alcoholic extract was around 20.3% (Sadeghi et al., 2014 ▶).
Determination of total phenolic contents
The Folin–Ciocalteu method was applied for determination of total phenolic contents in HAAD (Roby et al., 2013 ▶). First, 50 µl of the extract (200 μg/ml) was blended with 5 ml Folin–Ciocalteu reagent and then, 10 ml of saturated soda (Na2CO3) was added to this mixture. The tubes were vortexed or 15 sec and incubated in dark for about 30 min for color emersion. The absorbance of the tubes was then measured at 760 nm using a Cary 5000 UV-Vis-NIR spectrophotometer. Also, in order to prepare the standard curve, gallic acid at different concentrations was used and the absorbance was measured at 760 nm. Trials were carried out in triplicate and the average was reported. The total phenolic amount (presented as mg gallic acid/g HAAD) of the A. dracunculus was obtained from the standard gallic acid curve and following equation:
Absorbance = 0.0008 × gallic acid (mg)
FRAP (ferric reducing antioxidant power) assay
The reducing power of HAAD was determined by the method of Ilhami Gulcin et al. (Gülçin et al., 2010 ▶) with slight modifications. Different concentrations of ascorbic acid (as positive control) and HAAD (100-500 µg/mL) in 1 mL of distilled water were blended with phosphate-buffered saline (PBS) (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide (K3Fe(CN)6) (2.5 mL, 30 mM). Then, the mixture was incubated at 50 oC for 20 min. Afterwards, 2.5 mL trichoroacetic acid (TCA) (0.6 M) was added to the solution and centrifuged at 3000 g for 10 min. After the addition of distilled water (2.5 ml) and 0.5 ml FeCl3 (6 mM) to the upper layer of the mixture, the absorbance of the solution was assayed at 700 nm using a spectrophotometer. All assays were repeated three times. The absorbance of the reaction mixture has a direct correlation with the reduction capacity.
DPPH free radical scavenging activity
DPPH• free radical scavenging ability of the plant extract was determined according to the method described by Dehong Hua et al. (Hua et al., 2014 ▶) with minor modifications. For this purpose, 1 ml of different concentrations of the extract (25, 50, 100, 200 and 400 µg/ml) were added to 1 ml of a 0.1 mM DPPH methanolic solution and the mixture was incubated for 30 min in dark. Next, the absorbance of the solutions was measured at 515 nm. Ascorbic acid was utilized as positive control and the same procedure was carried out. All assays were performed in triplicate. The scavenging activity was calculated using the following equation (Zhang et al., 2015 ▶):
DPPH radical scavenging activity (%) =
Where, As: the absorbance of the test sample (DPPH solution + extract and ascorbic acid), Ab: the absorbance of the blank and Ac: the absorbance of the control (DPPH solution without samples).
ABTS radical scavenging activity
ABTS radical cation scavenging activity of the extract was measured as antioxidant potential (Debbache et al., 2014 ▶). First, ABTS (7 mM) and potassium persulfate (2.45 mM) solutions were mixed and stirred at room temperature for 16 hr. Next, the stock solution was diluted with ethanol to attain an absorbance of 0.7 ± 0.02 at 750 nm. Thereafter, 100 μl of ABTS+• solution was added to 100 μl of different concentrations (100-500 μg/ml) of HAAD and ascorbic acid (as positive control). Afterwards, the samples were vortexed and incubated in dark for 6 min. All assays were done in triplicate. ABTS radical-scavenging activity was evaluated by spectrophotometer at 750 nm and reported using the following equation:
ABTS radical-scavenging activity (%) =
Where, As: the absorbance of the test sample (ABTS solution + extract and ascorbic acid) and Ac: the absorbance of the control (ABTS solution without samples).
Animals and treatment
Adult male Wistar rats weighing 200-250 g were utilized in this study. The experimental procedures were exactly performed according to the “Principles of Laboratory Animal Care” (NIH Publication No. 86-23). The animals were randomly divided into groups of six rats. The rats were kept under 12 hr:12 hr light/dark cycles at 24 ± 2°C and permitted to have free access to normal diet and water. The animals were adapted to laboratory conditions for 7 days prior to initiation of examination.
Acute toxicity test
In order to measure the LD50 of the HAAD, three doses of the extract (1000, 3000, and 5000 mg/kg) were given orally to three groups of the rats (n=6). The animals were supervised for behavioral changes and mortality for 48 hr (Kumar et al., 2012 ▶).
CCl 4 -induced hepatotoxicity
In this study, we used the method of induction of hepatotoxicity using CCl4which was previously described by Raj et al. (Raj and Gothandam, 2014 ▶). The animals were randomly allocated into six groups (six rats per each group) including group I (normal control group), group II (CCl4 group) (both groups I and II received normal saline p.o. every day), group III (positive control group) received silymarin (100 mg/kg per day p.o.) as the standard drug, groups IV, V and VI orally received HAAD 50, 100 and 200 mg/kg BW, respectively. Fifteen days after the onset of HAAD and silymarin administration, liver injury was induced by CCl4 (10 ml/kg of 1:1 v/v mixture of CCl4 and olive oil; i.p.) one hour after the last administration in all groups except for the normal control group. Then, 24 hr post CCl4 administration, all animals were euthanized by diethyl ether and blood samples were collected promptly from the abdominal aorta. The liver was dissected and excised immediately. A section of the left lobe of the liver was used for histopathological studies, and the remaining was frozen quickly and stored at -80 oC for biochemical analysis.
Assessment of liver function parameters
Collected blood samples were centrifuged at 2500 rpm at room temperature for 20 min and serum was separated. The serum levels of biochemical parameters namely, ALT, AST, ALP, TP and TB were evaluated using commercially available diagnostic kits (Roche Diagnostics) and Cobas C 311 autoanalyzer (Roche Hitachi, Manheim, Germany) (Zeashan et al., 2008 ▶).
Assessment of lipid peroxidation
Thiobarbituric acid reactive substance (TBARS) assay was used for evaluation of MDA, an end-product of lipid peroxidation, as previously reported with minor changes (Pareek et al., 2013 ▶). The frozen portions of liver were thawed and homogenized in ice-cold phosphate buffer 50 mM (1:9, pH 7.4). Thereafter, the homogenate was centrifuged at 12000 rpm at 4 oC for 15 min and the supernatant was collected in Eppendorf microtubes. Next, 1 mL of ice-cold TCA (75 mg/mL) was mixed with 1 mL of the homogenate and then centrifuged at 1500 rpm. Afterwards, 1 mL of TBA (8 mg/mL) was added to the supernatant and the mixture was kept in boiling water for 45 min. The absorbance of the samples was measured at 535 nm. The results were expressed as nmol MDA per g tissue.
Assay of glutathione (GSH)
The reduced glutathione content of liver tissue was determined by the method of Raj et al. (Raj and Gothandam, 2014 ▶) with slight modifications. In order to precipitate the proteins, 1 ml of liver homogenate was mixed with 1 ml of 10% TCA and incubated for 5 min. Then, the mixture was centrifuged at 2000 rpm at 4°C for 10 min. Afterwards, 0.5 ml of supernatant was mixed with 5 ml of 0.2 M PBS (pH 8) and 2 ml 0.6 mM Ellman's reagent (5, 5'-dithiobis-(2-nitrobenzoic acid)) or DTNB at 4°C for 5 min. The absorbance was measured at 412 nm, and the concentration of GSH was expressed as μg/mg protein.
Assessment of superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST) activities
SOD activity was measured using autoxidation of 2mM pyrogallol in Tris buffer (Raj and Gothandam, 2014 ▶). Results are shown as unit/min/mg protein. One unit (U) of SOD activity was defined as the amount of enzyme required to inhibit the oxidation of 50% pyrogallol. Disintegration of H2O2 in the presence of CAT was recorded at 620 nm (Maheshwari et al., 2011 ▶). Results are expressed as µmole H2O2/min/mg protein. The activity of GST was assessed in PBS (0.1 M, pH 7.6) containing glutathione (0.5 mM) and 1-chloro-2, 4-dinitrobenzene (CDNB) 0.5 mM) (Jalali Ghassam et al., 2014 ▶). The change in absorbance at 344 nm was monitored using a spectrophotometer. Results are presented as mmole CDNB conjugate/min/mg protein.
Histopathological studies
For histological assessment, the liver samples from all groups were taken and fixed in 10% formaldehyde for 1 week. Thereafter, fixed tissues were embedded in paraffin and cut into 3-4 μm thick sections. The sections were stained using hematoxylin and eosin and observed by a blind pathologist.
Statistical analysis
The results are expressed as mean ± standard error of mean (Mean ± SEM). The data were analyzed by ANOVA followed by Tukey as post-hoc test, using SPSS 22.0 software. A p< 0.05 was assumed significant.
Results
Total phenolic content
Total phenolic content was 197.223±3.73 mg gallic acid equivalent/g HAAD dry weight. The result was the Mean±SEM of three times of absorbance reading.
Ferric reducing antioxidant power activity
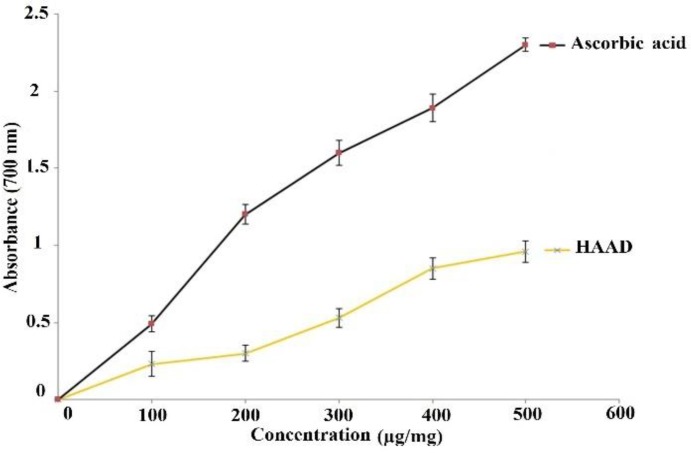
As shown in Figure 1, different concentrations of HAAD (100-500 µg/ml) exhibited powerful reducing power in a dose-dependent manner. Also, ascorbic acid at the same concentrations showed effective reducing power more than that of HAAD.
Figure 1.
In vitro antioxidant activity of HAAD and ascorbic acid. Graph showing ferric reducing antioxidant power activity. Values are presented as mean ± SEM. HAAD: hydro-alcoholic extract of aerial parts of A. dracunculus
DPPH radical scavenging activity
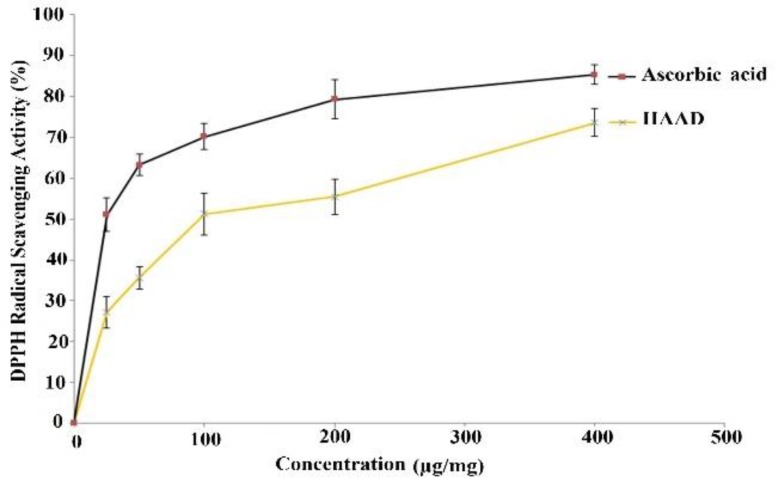
The DPPH radical scavenging activity of the plant is expressed as percentage. As illustrated in Figure 2, disparate concentrations of HAAD (25, 50, 100, 200 and 400 µg/ml) showed marked DPPH radical scavenging activity almost comparable with that of ascorbic acid at the same concentrations of those of samples.
Figure 2.
. In vitro antioxidant activity of HAAD and ascorbic acid. Graph showing DPPH radical scavenging activity. Values are presented as mean ± SEM. HAAD: hydro-alcoholic extract from aerial parts of A. dracunculus
ABTS radical scavenging activity
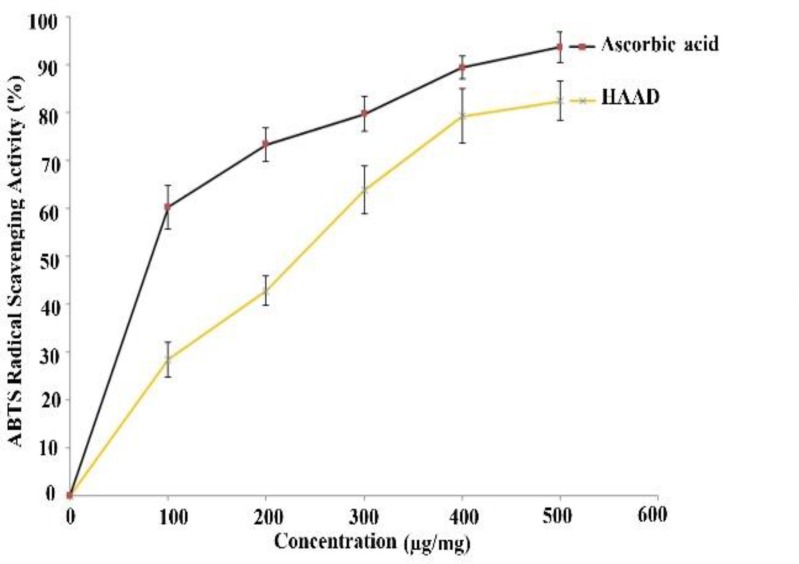
The results of ABTS radical scavenging activity of different concentrations of the extract (100-500 µg/ml) are presented in Figure 3. HAAD exhibited powerful scavenging activity but it was lower than that of ascorbic acid at the same concentrations.
Figure 3.
In vitro antioxidant activity of HAAD and ascorbic acid. Graph showing ABTS radical scavenging activity. Values are presented as mean ± SEM. HAAD: hydro-alcoholic extract of aerial parts of A. dracunculus
Acute toxicity
The HAAD did not cause any behavioral changes or mortality during 48 hr. These results showed that the LD50 of the HAAD was >5000 mg/kg.
Effect of HAAD on serum biochemical enzymes
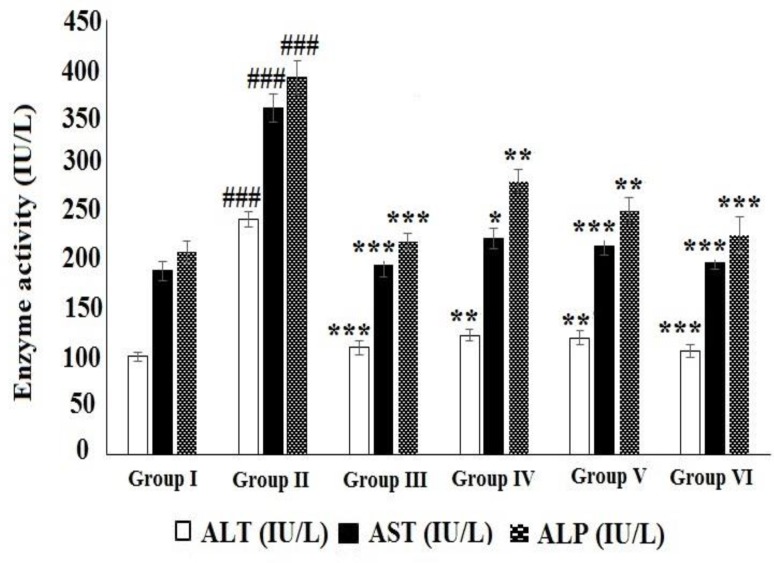
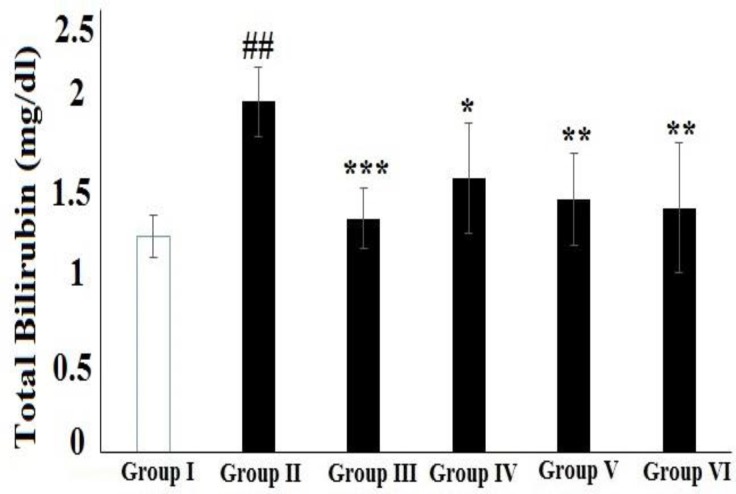
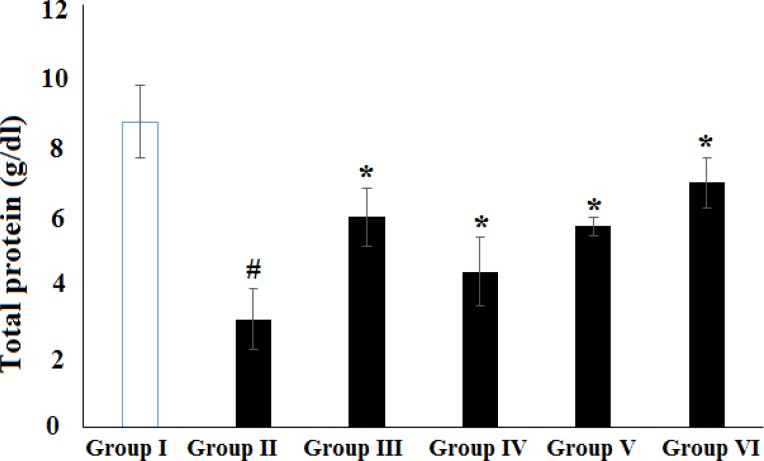
The effects of HAAD on serum biochemical parameters of CCl4-poisoned rats are shown in Figures 4, 5, and 6. The levels of AST, ALT, ALP and TB significantly increased while TP level significantly decreased in CCl4 group compared to normal group. Oral treatment of the rats with HAAD 50, 100 and 200 mg/kg showed a significant decrease in the levels of serum enzymes namely, AST, ALT, ALP and TB (p<0.05) while induced a significant increase in the level of serum TP (p<0.05) compared to the CCl4 group. Oral administration of silymarin (100 mg/kg) also showed a significant decrease in the levels of serum ALT, AST, ALP and TB (p<0.001) while significantly increased the level of serum TP (p<0.05) compared to the CCl4 group.
Figure 4.
Effect of HAAD and silymarin on ALT, AST, and ALP activity in CCl4-induced hepatotoxicity. Groups – I: normal saline; II: CCl4 (CCl4 group); III: Silymarin (100 mg/kg) + CCl4 (10 ml/kg); IV: HAAD (50 mg/kg) + CCl4 (10 ml/kg); V: HAAD (100 mg/kg) + CCl4 (10 ml/kg); VI: HAAD (200 mg/kg) + CCl4 (10 ml/kg). Values are mean ± S.E.M. (n=6). ###p<0.001 vs. group (I); *p<0.05, **p<0.01, and ***p<0.001 vs. CCl4 group (II).
Figure 5.
Effect of HAAD and silymarin on total bilirubin (TB) in CCl4-induced hepatotoxicity. Groups – I: normal saline; II: CCl4 (CCl4 group); III: Silymarin (100 mg/kg) + CCl4 (10 ml/kg); IV: HAAD (50 mg/kg) + CCl4 (10 ml/kg); V: HAAD (100 mg/kg) + CCl4 (10 ml/kg); VI: HAAD (200 mg/kg) + CCl4 (10 ml/kg). Values are mean ± S.E.M. (n=6). ##p<0.01 vs. group (I); *p<0.05, **p<0.01, and ***p<0.001 vs. CCl4 group (II
Figure 6.
Effect of HAAD and silymarin on total protein (TP) in CCl4-induced hepatotoxicity. Groups – I: normal saline; II: CCl4 (CCl4 group); III: Silymarin (100 mg/kg) + CCl4 (10 ml/kg); IV: HAAD (50 mg/kg) + CCl4 (10 ml/kg); V: HEAD (100 mg/kg) + CCl4 (10 ml/kg); VI: HAAD (200 mg/kg) + CCl4 (10 ml/kg). Values are mean ± S.E.M. (n=6). #p<0.05 vs. group (I); *p<0.05 vs. CCl4 group (II
Effect of HAAD on liver lipid peroxidation and antioxidant enzymes
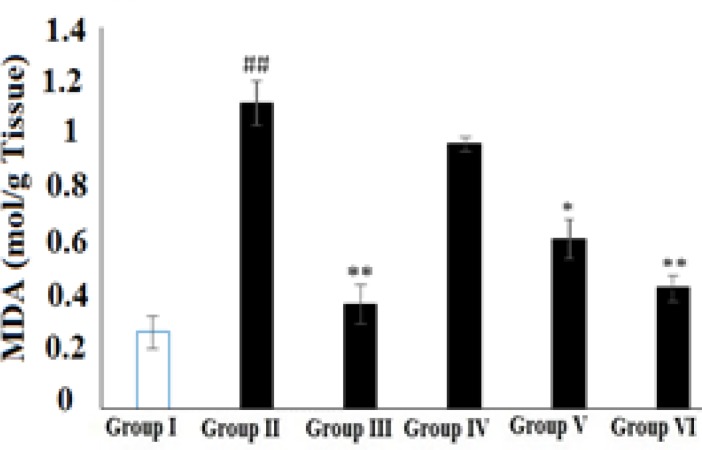
As illustrated in Figure 7, the level of tissue MDA significantly increased in the CCl4 group as compared to the normal group (p<0.01). The elevated level of tissue MDA was diminished significantly by the pretreatment of rats with HAAD at doses of 100 and 200 mg/kg as compared to the CCl4 group (p<0.05 and p<0.01, respectively). Silymarin 100 mg/kg also produced a significant reduction in MDA level compared to the CCl4 group (p<0.01).
Figure 7.
Effect of HAAD and silymarin on total MDA levels in CCl4-induced hepatotoxicity. Groups – I: normal saline; II: CCl4 (CCl4 group); III: Silymarin (100 mg/kg) + CCl4 (10 ml/kg); IV: HEAD (50 mg/kg) + CCl4 (10 ml/kg); V: HAAD (100 mg/kg) + CCl4 (10 ml/kg); VI: HAAD (200 mg/kg) + CCl4 (10 ml/kg). Values are mean ± S.E.M. (n=6). ##p<0.01 vs. group (I); **p<0.01 and *p<0.05 vs. CCl4 group (II
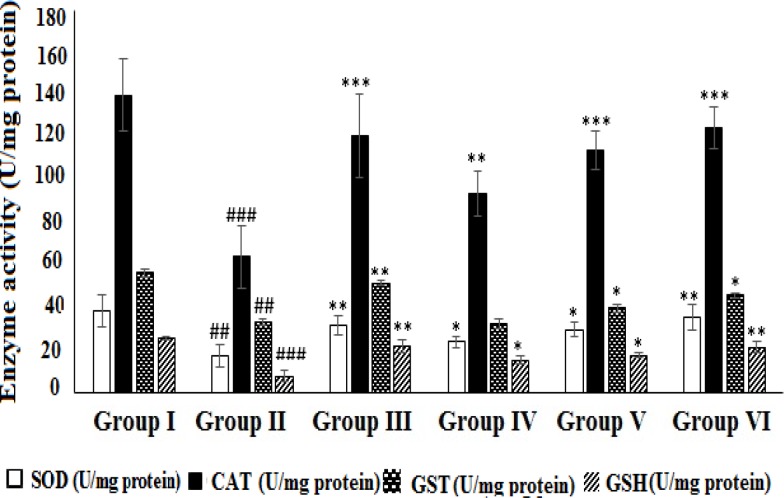
The activities of the hepatic antioxidant enzymes (SOD, CAT and GST) and the level of glutathione were found to be drastically decreased (p<0.01 and p<0.001) in CCl4 group as compared to normal group (Figure 8). The decreased activities of the enzymes in CCl4-intoxicated rats were augmented more effectively (p<0.05, p<0.01 and p<0.01) in rats pretreated with HAAD 200 mg/kg , as compared to those pretreated with HEAD 50 and 100 mg/kg. This protective effect was more marked (p<0.01 and p<0.001) in animals pretreated with silymarin 100 mg/kg.
Figure 8.
Effect of HAAD and silymarin on SOD, CAT, GST, and GSH activity in CCl4-induced hepatotoxicity. Groups – I: normal saline; II: CCl4 (CCl4 group); III: Silymarin (100 mg/kg) + CCl4 (10 ml/kg); IV: HAAD (50 mg/kg) + CCl4 (10 ml/kg); V: HAAD (100 mg/kg) + CCl4 (10 ml/kg); VI: HAAD (200 mg/kg) + CCl4 (10 ml/kg). Values are mean ± S.E.M. (n=6). ##p<0.01 and ###p<0.001 vs. group (I); *p<0.05, **p<0.01, and ***p<0.001 vs. CCl4 group (II
Histopathological studies
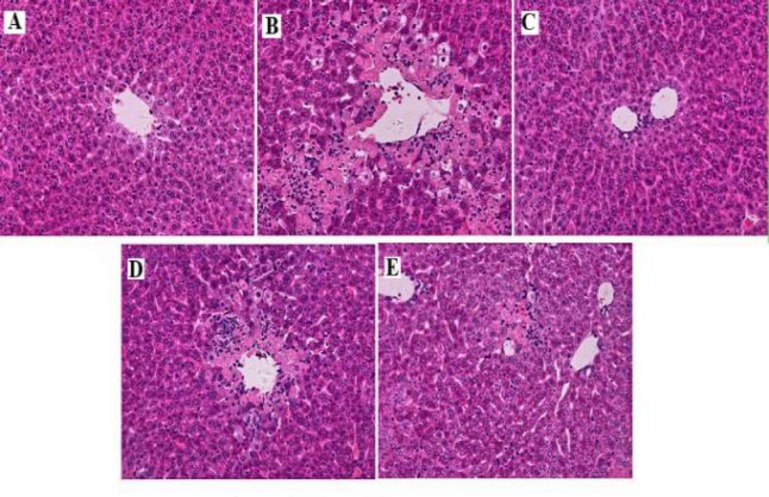
Histopathological examination of normal group (Figure 9A) animals showed normal layout with distinct cells, visible central vein and sinusoidal spaces. In contrast, the CCl4 group showed liver injuries such as moderate to severe necrosis around the central vein, neutrophils infiltration, ballooning degeneration and loss of cellular bourgeons (Figure 9B). Rats treated with silymarin showed almost normal architecture with uniform sinusoids (Figure 4C). Treatment with HAAD (100 mg/kg (Figure. 9D) and 200 mg/kg (Figure 9E)) revealed a relatively normal pattern with a mild degree of necrosis and inflammatory cells infiltration compared to CCl4 group.
Figure 9.
Effect of HAAD on the histopathological morphology of rats liver as assessed by hematoxylin and eosin (H & E) staining (magnification X400). (A) Normal control, (B) CCl4 group, (C) Silymarin 100 mg/kg b.w. + CCl4, (D) HEAD 100 mg/kg b.w. + CCl4, and (E) HAAD 200 mg/kg b.w. + CCl4
Discussion
Previous studies on A. dracunculus indicate that this plant has antibacterial, antiplatelet, anti-inflammatory, antihyperglycemic, gastroprotective and antioxidant activities (Aglarova, 2006 ▶, Benli et al., 2007 ▶, Kheterpal et al., 2010 ▶, Lopes-Lutz et al., 2008 ▶, Shahriyary and Yazdanparast, 2007 ▶).
In the present experiment, the antioxidant activity of HAAD and the possible mechanisms were investigated by investigation of HEAD reducing power, as well as its DPPH and ABTS radicals scavenging activity. The hepatoprotective effect was also estimated using a model of hepatotoxicity induced by CCl4 in rats. In addition, an attempt was made to realize the correlation between antioxidant and hepatoprotective activity.
FRAP is one mechanism for operation of antioxidants and may serve as a significant marker of potential antioxidant activity of antioxidants (Canabady-Rochelle et al., 2015 ▶). In this study, the extract showed a good concentration-dependent reducing power, which was consistent with the findings of Rajabian et al. (Rajabian et al., 2016 ▶).
DPPH and ABTS radical scavenging methods are general spectrophotometric procedures for assessment of antioxidant capacities of chemicals (Schaich et al., 2015 ▶). In this assay, HAAD showed pronounced DPPH radical and ABTS radical scavenging activity, and it was almost as effective as ascorbic acid at similar concentrations. These results are similar to those of Bahramkia et al. (Bahramkia et al., 2008)
CCl4 is a potent hepatotoxin known for inducing hepatotoxicity features in animals that are similar to those of acute hepatitis in humans (Li et al., 2015 ▶). CCl4 is metabolized by cytochrome P-450 and converted to trichloromethyl and trichloromethyl peroxy radicals which initiate peroxidation of polyunsaturated fatty acid constituents of various membranes with secondary damage, severe enzymatic dispensations, and increased MDA production (Zhou et al., 2010 ▶). Elevation in serum ALT, AST and ALP activity induced by CCl4 have been ascribed to hepatic structural damage because these enzymes are generally localized in the cytosol and released into the blood circulation after cellular damage (Huang et al., 2012 ▶. Shah et al., 2015 ▶). In the present study, the levels of all these enzymes increased in CCl4 group (CCl4-treated group) indicating liver damage induced by CCl4. Bilirubin, a product of heme degradation, is a most helpful clinical indicator of necrosis severity (Vuda et al., 2012 ▶). As shown in results, total bilirubin level significantly elevated in CCl4 group exhibiting severe injury induced by CCl4. Therefore, pretreatment of rats with 50, 100 and 200 mg/kg of HAAD decreased elevated levels of ALT, AST, ALP and TB suggesting that the plant extract effectively protected the animals against CCl4-induced hepatotoxicity. Restoration of serum enzyme activities to normal amounts in rats after treatment with HAAD shows prohibition of the leakage of intracellular enzymes by maintaining the integrity of liver cells membrane. In this experiment, CCl4 also decreased serum TP while HAAD 50, 100 and 200 mg/kg of HAAD significantly restored the protein synthesis. The hepatoprotective effect of HAAD was further investigated by the histopathological examinations. HAAD at different doses offered hepatoprotection, but 200 mg/kg of HEAD was more effective than the other doses.
Lipid peroxidation is a major index of oxidative stress. Elevated levels of liver MDA induced by CCl4 imply enhanced lipid peroxidation which leads to hepatocellular damage and failure of natural antioxidant defense system to prevent overproduction of free radicals (Pareek et al., 2013 ▶). One of the important antioxidant mechanisms is free radical scavenging activity that interferes with the chain reaction of lipid peroxidation (Kepekçi et al., 2013 ▶). In this study, treatment with 100 and 200 mg/kg of HEAD reduced lipid peroxidation by diminishing MDA levels, exhibiting the free radical scavenging activity of tarragon under in vivo conditions. This claim was supported by the results of the free radical scavenging activity of HAAD under in vitro conditions using DPPH and ABTS tests as mentioned above.
It has been presumed that one of the main underlying mechanisms of CCl4-induced liver damage is formation of lipid peroxides by free radicals produced by CCl4. Hence, the antioxidant activity or the prohibition of the production of free radicals is important in the defense against CCl4-induced hepatotoxicity (Zeashan et al., 2008 ▶). The body has an impressive defense system to hinder and nullify the free radicals-induced harm. This system possesses a collection of endogenous antioxidant enzymes like SOD and CAT. These enzymes establish a supportive group of defense system against ROS (Amresh et al., 2007 ▶). In hepatotoxicity induced by CCl4, the balance between ROS formation and these antioxidant defenses may be vanished and oxidative stress occurs; oxidative stress during a series of events, disturbs the cellular functions leading to hepatic damage. Diminished activities of SOD, CAT and GST indicate the hepatic injury in the rats administered with CCl4 but treatment with 50, 100 and 200 mg/kg of HAAD showed significant elevation in the level of these enzymes, which reflects the antioxidant activity of A. dracunculus extract. Concerning non-enzymatic antioxidants, GSH is a crucial determinant of tissue proneness to oxidative damage and the depletion of hepatic GSH has been shown to be related with an enhanced toxicity to chemicals, including CCl4 (Jiang et al., 2015 ▶). In this study, a decrease in liver tissue GSH level was observed in CCl4-treated groups. The increase in hepatic GSH level in the animals treated with 50, 100 and 200 mg/kg of HAAD may be due to de novo GSH synthesis or GSH regeneration.
The in vitro and in vivo antioxidant activities of HAAD may be due to the presence of phenolic compounds and flavonoids in the extract for which antioxidant and hepatoprotective activities have been recognized (Fuda et al., 2015 ▶). However, further studies on the active compounds and their biochemical mechanisms which may attribute to the antioxidant and hepatoprotective effects of Artemisia dracunculus L., are necessary to be done.
In the present study, hydro-alcoholic extract of A. dracunculus restored the increased serum enzyme levels, diminished liver antioxidant markers, and exerted effective antioxidant activity under in vitro conditions suggesting that it has hepatoprotective and antioxidant capacities in CCl4-intoxicated rats. The hepatoprotective effect of HAAD may be due to decreased lipid peroxidation and ameliorated defense of the hepatocytes against ROS. The histopathological studies also confirmed the activity of the plant extract. However, the protective, curative and antioxidant characteristics of A. dracunculus need to be verified using larger numbers of animals, and determining the active ingredient(s) of this plant as well as recognizing their mechanism(s) of action.
Acknowledgment
We thank Behbahan Faculty of Medical Sciences for financial support, and all the persons who helped us in this work.
Conflicts of interest
Authors declare no conflict of interest.
References
- Aglarova AM. Comparative Analysis of Secondary Metabolites of Artemisia dracunculus L., Russian and French cultivars. Ph. D. thesis. Mahachkala: 2006. [Google Scholar]
- Ali H, Kabir N, Muhammad A, Shah MR, Musharraf SG, Iqbal N, Nadeem S. Hautriwaic acid as one of the hepatoprotective constituent of Dodonaea viscosa. Phytomedicine. 2014;21P:131–140. doi: 10.1016/j.phymed.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Amresh G, Rao CV, Singh PN. Antioxidant activity of Cissampelos pareira on benzo (a) pyrene-induced mucosal injury in mice. Nutr Res. 2007;27:625–632. [Google Scholar]
- Azeem A, Mathew M, Nair C. Hepatoprotective effect of Averrhoea carambola fruit extract on carbon tetrachloride induced hepatotoxicity in mice. Asian Pac J Trop Med. 2010;3:610–613. [Google Scholar]
- Benli M, Kaya I, Yigit N. Screening antimicrobial activity of various extracts of Artemisia dracunculus L. Cell Biochem Funct. 2007;25:681–686. doi: 10.1002/cbf.1373. [DOI] [PubMed] [Google Scholar]
- Canabady-Rochelle LLS, Harscoat-Schiavo C, Kessler V, Aymes A, Fournier F, Girardet JM. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015;183:129–135. doi: 10.1016/j.foodchem.2015.02.147. [DOI] [PubMed] [Google Scholar]
- Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol. 2013;55:234–240. doi: 10.1016/j.fct.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Debbache N, Atmani D, Atmani D. Chemical analysis and biological activities of Populus nigra, flower buds extracts as source of propolis in Algeria. Ind Crop Prod. 2014;53:85–92. [Google Scholar]
- Eisenman SW, Struwe L. The global distribution of wild tarragon (Artemisia dracunculus L; Asteraceae) cytotypes with twenty-seven new records from North America. Genet Resour Crop Ev. 2011;58:1199–1212. [Google Scholar]
- Fuda H, Watanabe M, Hui SP, Joko S, Okabe H, Jin S, Takeda S, Miki E, Watanabe T, Chiba H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015;176:226–233. doi: 10.1016/j.foodchem.2014.12.066. [DOI] [PubMed] [Google Scholar]
- Gnanadesigan M, Ravikumar S, Inbaneson SJ. Hepatoprotective and antioxidant properties of marine halophyte Luminetzera racemosa bark extract in CCL4 induced hepatotoxicity. Asian Pac J Trop Med. 2011;4:462–465. doi: 10.1016/S1995-7645(11)60126-0. [DOI] [PubMed] [Google Scholar]
- Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43–53. [Google Scholar]
- Gupta RK, Hussain T, Panigrahi G, DasA , Singh GN, Sweety K Faiyazuddin M, Rao CV. Hepatoprotective effect of Solanum xanthocarpum fruit extract against CCl4 induced acute liver toxicity in experimental animals. Asian Pac J Trop Med. 2011;4:964–968. doi: 10.1016/S1995-7645(11)60227-7. [DOI] [PubMed] [Google Scholar]
- Hua D, Zhang D, Huang B, Yi P, Yan C. Structural characterization and DPPH· radical scavenging activity of a polysaccharide from Guara fruits. Carbohyd Polym. 2014;103:143–147. doi: 10.1016/j.carbpol.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang S, Zheng L, He M, Huang R, Lin X. Hepatoprotective effects of total saponins isolated from Taraphochlamys affinis against carbon tetrachloride induced liver injury in rats. Food Chem Toxicol. 2012;50:713–718. doi: 10.1016/j.fct.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Jalali Ghassam B, Ghaffari H, Prakash HS, Kini KR. Antioxidant and hepatoprotective effects of Solanum xanthocarpum leaf extracts against CCl4-induced liver injury in rats. Pharm Biol. 2014;52:1060–1068. doi: 10.3109/13880209.2013.877490. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Fan X, Wang Y, Tan H, Chen P, Zeng H, Huang M, Bi H. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem Biol Interact. 2015;231:83–89. doi: 10.1016/j.cbi.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD. Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem. 2013;141:1972–1979. doi: 10.1016/j.foodchem.2013.04.107. [DOI] [PubMed] [Google Scholar]
- Kheterpal I, Coleman L, Ku G, Wang ZQ, Ribnicky D, Cefalu WT. Regulation of insulin action by an extract of Artemisia dracunculus L in primary human skeletal muscle culture: a proteomics approach. Phytother Res. 2010;24:1278–1284. doi: 10.1002/ptr.3093. [DOI] [PubMed] [Google Scholar]
- Khodakov GV, Kotikov IV, Pankovetskii VN. Component composition of essential oil from Artemisia abrotanum and A dracunculus. Chem Nat Compd. 2009;45:905–908. [Google Scholar]
- Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agr Food Chem. 2005;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- Kumar D, Bhat ZA, Shah MY. Effect of successive extracts of Stachys tibetica Vatke (Lamiaceae) in anxiety. Orient Pharm Exp Med. 2012;12:247–253. [Google Scholar]
- Li C, Yi LT, Geng D, Han YY, Weng Lj. Hepatoprotective effect of ethanol extract from Berchemia lineate against CCl4-induced acute hepatotoxicity in mice. Pharm Biol. 2015;53:767–772. doi: 10.3109/13880209.2014.941506. [DOI] [PubMed] [Google Scholar]
- Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Maheshwari DT, Yogendra Kumar MS, Verma SK, Singh VK, Singh SN. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L) leaves. Food Chem Toxicol. 2011;49:2422–2428. doi: 10.1016/j.fct.2011.06.061. [DOI] [PubMed] [Google Scholar]
- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. Artemisia dracunculus L (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agr Food Chem. 2011;59:11367–11384. doi: 10.1021/jf202277w. [DOI] [PubMed] [Google Scholar]
- Pappas RS, Sturtz G. Unusual alkynes found in the essential oil of Artemisia dracunculus L var dracunculus from the Pacific Northwest. J Essent Oil Res. 2001;13:187–188. [Google Scholar]
- Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150:973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]
- Raj S, Gothandam KM. Hepatoprotective effect of polyphenols rich methanolic extract of Amorphophallus commutatus var wayanadensis against CCl4 induced hepatic injury in swiss albino mice. Food Chem Toxicol. 2014;67:105–112. doi: 10.1016/j.fct.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Rajabian A, Khayyat MH, Emami SA, Tayarani-Najaran Z, Oskooie RR, Asili J. Phytochemical Evaluation and Antioxidant Activity of Essential Oil, and Aqueous and Organic Extracts of Artemisia dracunculus Jundishapur. J Nat Pharm Prod. 2016 Nov (Inpress) [Google Scholar]
- Roby MHH, Sarhan MA, Selim KAH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L), sage (Salvia officinalis L), and marjoram (Origanum majorana L) extracts. Ind Crop Prod. 2013;43:827–831. [Google Scholar]
- Sadeghi H, Zarezade V, Sadeghi H, Akbartabar Toori M, Jafari Barmak M, Azizi A, Ghavamizadeh M, Mostafazadeh M. Anti-inflammatory Activity of Stachys Pilifera Benth. Iran Red Crescent Med J. 2014;16:9. doi: 10.5812/ircmj.19259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyah M, Nadjafnia L, Kamalinejad M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L essential oil. J Ethnopharmacol. 2004;94:283–287. doi: 10.1016/j.jep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Schaich KM, Tian X, Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J Funct Foods. 2015;14:111–125. [Google Scholar]
- Shah MD, Gnanaraj C, Haque ATME, Iqbal M. Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats. Pharm Biol. 2015;53:31–39. doi: 10.3109/13880209.2014.909502. [DOI] [PubMed] [Google Scholar]
- Shahriyary L, Yazdanparast R. Inhibition of blood platelet adhesion, aggregation and secretion by Artemisia dracunculus leaves extracts. J Ethnopharmacol. 2007;114:194–198. doi: 10.1016/j.jep.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Singhal KG, Gupta GD. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4–induced liver injury in rats. Asian Pac J Trop Med. 2012;5:677–685. doi: 10.1016/S1995-7645(12)60106-0. [DOI] [PubMed] [Google Scholar]
- Toori MA, Joodi B, Sadeghi H, Sadeghi H, Jafari M, Talebianpoor MS, Mehraban F, Mostafazadeh M, Ghavamizadeh M. Hepatoprotective activity of aerial parts of Otostegia persica against carbon tetrachloride-induced liver damage in rats. Avicenna J Phytomed. 2015;5.3:238–246. [PMC free article] [PubMed] [Google Scholar]
- Vuda M, D'Souza R, Upadhya S, Kumar V, Rao N, Kumar V, Boillat C, Mungli P. Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in rats. Exp Toxicol Pathol. 2012;64:855–859. doi: 10.1016/j.etp.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem Toxicol. 2008;46:3417–3421. doi: 10.1016/j.fct.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li XM, Wang JN, Li X, Wang BG. New butenolide derivatives from the marine-derived fungus Paecilomyces variotii with DPPH radical scavenging activity. Phytochem Lett. 2015;11:85–88. [Google Scholar]
- Zhou D, Ruan J, Cai Y, Xiong Z, Fu W, Wei A. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J Ethnopharmacol. 2010;129:232–237. doi: 10.1016/j.jep.2010.03.016. [DOI] [PubMed] [Google Scholar]