Abstract
The co-receptors CD4 and CD8 are important in the activation of T cells, primarily because of their ability to interact with the proteins of the MHC, enhancing recognition of the MHC–peptide complex by the T cell receptor (TCR). An antigen-presenting cell presents a small number of antigenic peptides on its MHC molecules, in the presence of a much larger number of endogenous, mostly nonstimulatory, peptides. Recent work has demonstrated that these endogenous MHC–peptide complexes have an important role in modulating the sensitivity of the TCR. But the role of the endogenous nonstimulatory MHC–peptide complexes differs in MHC class I and class II-restricted T cells. This chapter discusses the data on the role of CD4 or CD8 co-receptors in T cell activation at the immunological synapse, and the role of non stimulatory MHC–peptide complexes in aiding antigen recognition.
1 Introduction
The CD4 and CD8 proteins have long been known to be important in antigen recognition, and in the discrimination between antigens presented by MHC class I or class II molecules. Their precise role has been more difficult to elucidate. Recent data suggest that endogenous MHC–peptide complexes are involved in the activation of T cells by antigen, making the T cells more sensitive to low quantities of the antigen. The means by which these endogenous peptides aid in antigen recognition appear to be different in MHC class I and class II-restricted T cells. In this chapter, we review the data on CD4 and CD8 in the immunological synapse, and their apparently different modes of action in aiding TCR activation by limited antigen quantity in the presence of endogenous, nonstimulatory, MHC–peptide complexes.
2 Co-Receptors in the Immunological Synapse
2.1 MHC Recognition by Co-Receptors
CD4 and CD8 can bind to MHC class II or class I respectively, and over-expression of CD4 or CD8 on one cell type allows cell–cell binding to another cell type that overexpresses the relevant MHC molecule (Doyle and Strominger 1987; Norment et al. 1988). However, CD4 and CD8 are not usually thought to have an important role in adhesion in the absence of overexpression. Their main role is believed to be in their ability to act as co-receptors, to bind the MHC at the same time as TCR, and thus stabilize the TCR–MHCp complex. However, while there is clear evidence of this role for CD8, there is no similar evidence for CD4.
Because both CD4 and CD8 bind to the Src-family kinase Lck through their intracellular tails, this causes Lck to be brought into proximity with a TCR that is recognizing antigen, where it kick-starts the signaling cascade. CD4 is believed to be much more efficient at this function by virtue of a stronger interaction with Lck (Hurley et al. 1989).
Although both CD4 and CD8 interact with nonpolymorphic parts of the different MHC molecule classes (König et al. 1992; Moebius et al. 1993; Potter et al. 1989; Salter et al. 1990), they are radically divergent in structure: CD4 has a single polypeptide chain consisting of four immunoglobulin-like domains, of which the most amino-terminal membrane distal domain binds to MHC class II (Wang et al. 2001; Wu et al. 1997). There is some evidence that CD4 molecules can form noncovalent dimers through their membrane-proximal domains, which would result in a protein with the predicted ability to bind and therefore to cross-link two MHC class II proteins (Moldovan et al. 2002; Wu et al. 1997). In contrast, CD8 is an obligate dimer that can consist of ααζor αβζchains, covalently bound to each other. These dimers form a binding site for a single MHC class I protein (Gao et al. 1997; Kern et al. 1998). It is commonly believed that a co-receptor and TCR interact with the same MHC–peptide molecule, but as yet no structure of a complete TCR–MHC–peptide–co-receptor complex has been obtained. The X-ray crystal structures of TCR–MHC–peptide leave room for the TCR to bind; similarly, CD4–MHC–peptide and CD8–MHC–peptide structures also leave room for TCR binding.
The cytoplasmic domain of CD4 has a site for palmitoylation (Crise and Rose 1992), which allows it to associate with lipid microdomains (Balamuth et al. 2004), where Lck is preferentially found. CD8βζalso has a palmitoylation site, but CD8αζdoes not, so the CD8ααζdimer is less likely to associate with lipid microdomains and therefore come into contact with Lck than CD8αβζ(Arcaro et al. 2001). This combination of motif and opportunity may partially explain why CD8αβζis a stronger co-receptor than CD8αα, even though they bind equally well to MHC class I (Garcia et al. 1996).
2.2 Co-Receptor Recruitment to the Immunological Synapse
CD4 and CD8 are recruited to the immunological synapse during antigen recognition (Krummel et al. 2000; Kupfer et al. 1987; Zal et al. 2002) (Fig. 1). This recruitment occurs very fast – within seconds – during antigen recognition, and the movement of co-receptor within the T-cell–APC contact area can be very dynamic (Zal et al. 2002). There is evidence of the co-receptor leaving the synapse while TCR accumulates (Krummel et al. 2000). Although the recruitment of Lck to the synapse requires its interaction with CD4 or CD8, activation of Lck as measured by phosphorylation occurs predominantly at the periphery of the synapse, rather than in the central region (Lee et al. 2002). There is strong evidence that during recognition of strong antigens, TCR forms microclusters in the peripheral synapse. This is where signaling is initiated, with TCR being endocytosed in the central synapse (Varma et al. 2006). However, with weaker stimulation, activated Lck is found in the central regions of the synapse (Cemerski et al. 2008).
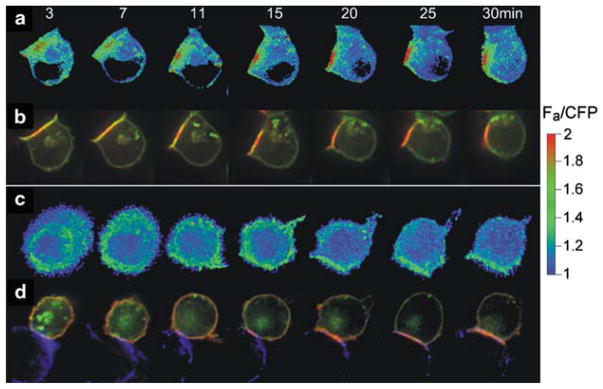
Fig. 1.
CD4 co-receptor recruitment to the immunological synapse and FRET between TCR and CD4. (a), (b) show a time course of interaction between T cell and an APC presenting antigenic peptide. (c), (d) show the same with an APC that does not present the antigenic peptide. (a), (c) show the FRET response between CD3ζ–CFP and CD4–YFP, using a heat scale (Zal et al. 2002). (b), (d) show the fluorescence of the CD3ζ–CFP (green) and CD4–YFP (red). Only the antigenic stimulation causes close interaction between TCR and CD4, as reported by FRET between CD3ζ–CFP and CD4–YFP (a versus c), though both APCs recruited CD4 to the immunological synapse (b and d). Recruitment was much slower in the absence (d) versus the presence (d) of antigen. Reproduced with permission from Zal et al. (2002)
In our early experiments on CD4 and TCR movement, we found that CD4 could move to the synapse between a T cell and an antigen presenting cell even when antigen was not available (Fig. 1d) (Zal et al. 2002). Thus the CD4 concentration in the synapse must have been due to the CD4 interaction with class II, irrespective of the peptide presented. This is referred to as noncognate MHC recognition, in contrast to the cognate recognition that is found between TCR and its specific MHC–peptide complex. The noncognate recruitment of CD4 was considerably slower than CD4 recruitment in the presence of antigen (Fig. 1b, d), and did not occur with all antigen-presenting cell types (Gascoigne and Zal 2004; Zal et al. 2002). In contrast, the noncognate recruitment of CD8 showed little difference between the presence and absence of specific antigen (Yachi et al. 2005). This indicated that the noncognate CD8–MHC class I interaction was sufficient to recruit CD8 and presumably MHC class I to the synapse.
3 Co-Receptor Interaction with NonCognate MHC Class I in Antigen Recognition
3.1 NonStimulatory Peptides Aid MHC Class I-Restricted Antigen Recognition by T Cells
Several studies have demonstrated that endogenous nonstimulatory peptides can enhance recognition of antigenic peptides (Krogsgaard et al. 2005; Yachi et al. 2005, 2007). This is particularly noticeable when the antigen is in limiting quantity, and in fact can explain why T cells are sensitive to tiny amounts of antigen – T cells have been reported to respond to a single antigenic peptide (Irvine et al. 2002; Sykulev et al. 1996), with full activation with as little as three (Purbhoo et al. 2004). The mechanism by which the endogenous peptides aid recognition appears to differ between MHC class I and class II-restricted T cells (Gascoigne 2008; Yachi et al. 2005, 2007). Here we will first deal with class I-restricted cells.
The RMA-S cell line is deficient in the Tap2 gene so peptides are not loaded into the MHC class I molecule as it is folded. In the presence of exogenously added peptides, though, the class I is correctly folded (Ljunggren et al. 1989; Townsend et al. 1989), and at low temperature (~30°C) the class I molecules are folded and expressed at the cell surface without peptide. If the temperature is raised to 37°C, they fall apart (Ljunggren et al. 1990). This phenomenon has been used to load specific peptides onto class I molecules in the presence of very few other peptides –the RMA-S cells are cultured for a period at 30°C, the peptide of interest is added, and culture continued. This allows the peptide to associate with the class I molecule. The temperature is then raised to 37°C to destroy the class I molecules that have not bound peptide. We used this method to load RMA-S cells with titrated amounts of an antigenic peptide. We were able to measure the amount of antigenic class I–peptide complexes by using a specific antibody recognizing this complex (Porgador et al. 1997).
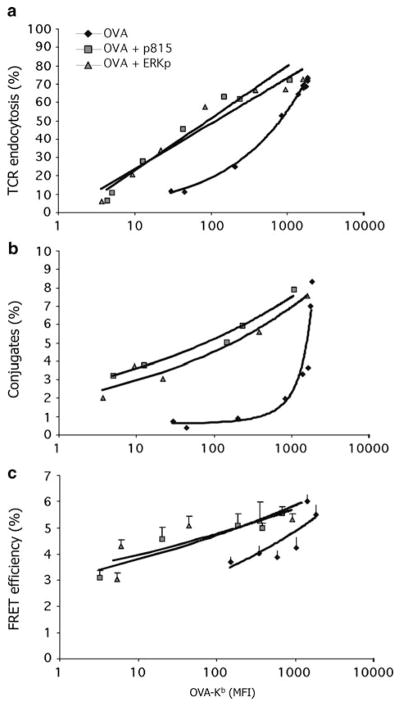
We found that the ability to stimulate T cells, as measured by a number of different parameters, declined steeply as the amount of antigen was reduced (Yachi et al. 2005, 2007). When the titration of antigen was performed in the presence of excess nonstimulatory peptides, the curve was shifted substantially, such that stimulation occurred at much lower concentrations of antigen than in the absence of the nonstimulatory peptides (Fig. 2). Using T hybridoma cells, this was true for the formation of conjugates between the T cells and antigen presenting cells (APCs), for TCR downregulation, and for the induction of close interactions between the TCR–CD3 complex and the co-receptor CD8 using Foerster Resonance Energy Transfer (FRET) microscopy (Yachi et al. 2005). Immature prepositive selection thymocytes and naïve primary CD8+ T cells also showed lower activation by a given amount of antigen on its own, compared to activation in the presence of nonstimulatory peptides (Yachi et al. 2007). We tested a number of different peptides that are known to bind to the MHC class I molecule (H2–Kb), including a peptide from a virus that does not stimulate the TCR that we tested, and several that are natural endogenously produced Kb–binding peptides that do not stimulate T cells or thymocytes bearing this TCR (Santori et al. 2002). Remarkably, each of the ~10 different peptides that we tested showed roughly equivalent ability to aid in antigen recognition (Yachi et al. 2005, 2007). This ability was demonstrated most strikingly when we loaded the RMA-S cells with a very small amount of antigen and then titrated in the nonstimulatory peptides: the stimulation of the responding T cells correlated with the amount of MHC class I expressed on the RMA-S cell surface (Fig. 3). Indeed, all our data led to the conclusion that the important factor in the role of the endogenous/nonstimulatory peptides in aiding antigen recognition is in fact due to the expression of the MHC class I protein, rather than to the specific peptide that it presents.
Fig. 2.
Increased T-cell activation by endogenous nonstimulatory peptides at limiting antigen quantities. (a) shows the amount of TCR endocytosis at differing quantities of antigen OVA–Kb expressed on the cell surface of RMA-S cells, either alone or with added nonstimulatory peptides derived from VSV, Erk, or the P815 tumor antigen. Erk and P815 are natural endogenous Kb–binding peptides (Santori et al. 2002). (b) shows the percentage of T cells in conjugates with RMA-S cells treated as in (a). (c) shows the interaction between TCR and CD8 by the FRET signal between CD3ζ–CFP and CD8β–YFP. Used with permission from Yachi et al. (2005)
Fig. 3.
The ability of nonstimulatory MHC–peptide ligands to enhance antigen recognition depends on their quantity rather than their sequence. A small amount of antigen was added to RMA-S cells (OVA). This resulted in very low expression of the epitope for the anti-OVA–Kb. Other nonstimulatory peptides were titrated in to increase the overall amount of Kb expression. This increased expression of Kb correlated with increased activation of thymocytes. Used with permission from Yachi et al. (2007)
Evidence from other labs also supports these findings. Early data showed an adhesion function of mouse CD8 in binding to noncognate MHC class I, after antigenic stimulation (O’Rourke et al. 1990), and this was recently confirmed for human CD8–MHC class I interactions (Varghese and Kane 2008). Interestingly, the effector memory cells and activated CTL, but not the naïve CD8+ T cells, showed this antigen-enhancement of CD8–class I binding (Varghese and Kane 2008).
In a different experimental system, MHC class I–peptide complexes were bound as arrays of about ten molecules to quantum dots (Anikeeva et al. 2006). These were able to activate CTL as long as at least one of the class I molecules presented the antigenic peptide. If all ten had a nonstimulatory peptide, then there was no activation. The role of the nonstimulatory MHC–peptide complexes was to bind to CD8, as they did not promote recognition of a single antigenic MHC–peptide if they were mutated at the CD8 binding site (Anikeeva et al. 2006).
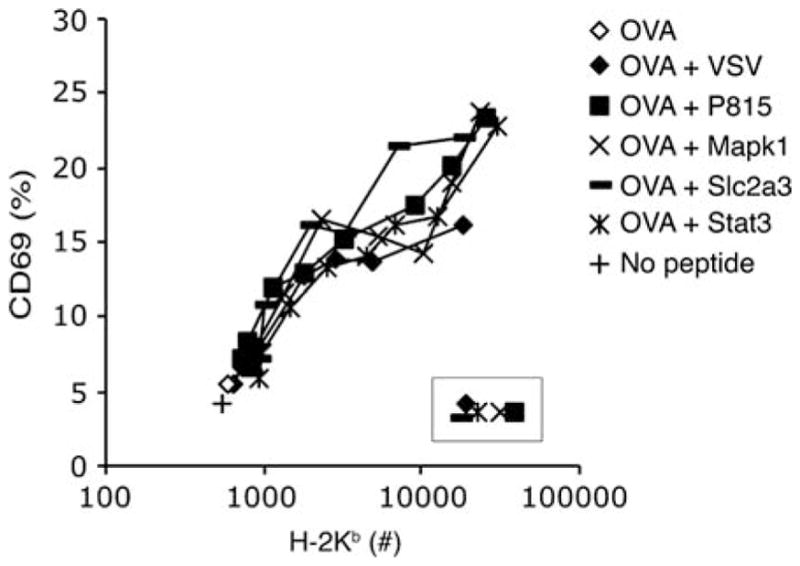
It must be noted that there are some reports that failed to show an effect of the endogenous MHC class I in aiding T-cell activation by antigen. In an experiment similar to our own, Sporri and Reis e Sousa (2002) compared T-cell activation by the Tap-sufficient RMA parental line with Tap-deficient RMA-S. The responses to RMA-S were strong enough and the authors concluded that the lack of endogenous peptides on the RMA-S cells did not affect stimulation by antigenic peptide (Sporri and Reis e Sousa 2002). When we performed the same experiment, we found significant difference between activation by RMA-S and RMA – the RMA cells induced stronger activation of T cells than did the RMA-S cells for the same amount of antigenic peptide presented (Fig. 4) (Yachi et al. 2007). Our main set of experiments, however, was to compare RMA-S cells with antigen plus or minus nonstimulatory peptides. Thus we were comparing the same cells, using the anti-Kb–peptide antibody (Porgador et al. 1997) to measure the amount of antigenic MHC–peptide on the cell surface, comparing the response to the same amount of antigen in the presence or absence of other exogenously added nonstimulatory peptides. This is probably a better way to assay the role of the nonstimulatory peptides than relying on the endogenously produced peptides of the RMA cells. Also, the RMA and RMA-S cells have been separated for over 20 years and may have other, more subtle, differences.
Fig. 4.
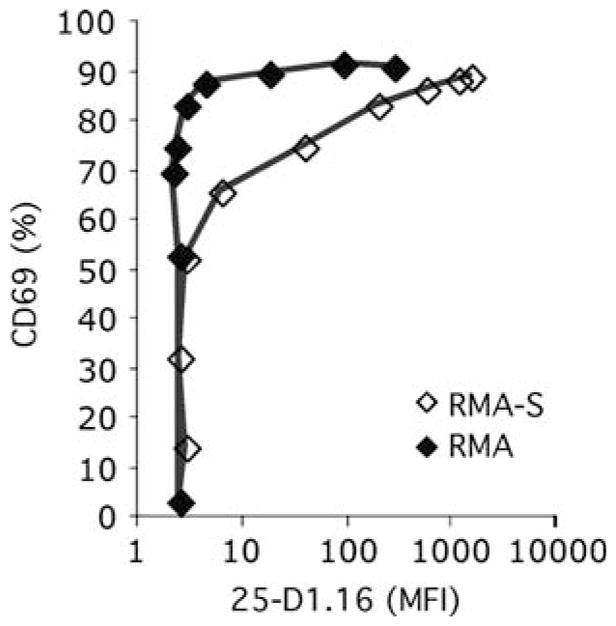
Endogenous peptides on RMA cells support antigen recognition. RMA and RMA-S cells were incubated with titrated amounts of antigen (OVA peptide) and used to stimulate naïve CD8+ T cells expressing the OT-I anti-OVA–Kb TCR. CD69 upregulation was assessed as a function of expression of the OVA–Kb epitope recognized by the 25-D1.16 mAb (Porgador et al. 1997). Used with permission from Yachi et al. (2007)
We conclude that the nonstimulatory peptides have a real effect on MHC class I-antigen recognition. It may have been overlooked in some studies because of the high sensitivity of the responding T cells which can show a response to small quantities of antigenic peptide alone. This may be because of the small but real number of endogenous peptides – derived from signal peptidase activity on nascent transmembrane and secreted proteins – that are expressed on the RMA-S cells.
3.2 The FRET Response Between CD8 and TCR–CD3, and What It Tells Us About the Role of Endogenous Peptides in T-Cell Activation
Our studies on T-cell activation by low amounts of antigen in the presence or absence of endogenous/nonstimulatory peptides included the rather surprising result that FRET between the fluorescently labeled CD3ζ–CFP and CD8β–YFP was enhanced by the presence of these nonstimulatory peptides (Fig. 2) (Yachi et al. 2005). This FRET response, like that between CD3ζ–CFP and CD4–YFP in an MHC class II-restricted T cell, is a measure of the close apposition of the CD4 or CD8 co-receptor to the TCR/CD3 complex – presumably the TCR that is interacting with the antigenic MHC–peptide complex (Gascoigne and Zal 2004; Yachi et al. 2005, 2006; Zal and Gascoigne 2004a,b; Zal et al. 2002). This means that the noncognate CD8–MHC class I interaction was somehow enhancing the cognate TCR–CD8 interaction induced by antigen (Fig. 2) (Yachi et al. 2005).
This suggested to us that the role of the noncognate CD8–MHC class I interaction is to concentrate the MHC class I and the CD8 (and therefore also Lck) proteins (“Pre-concentration model,” Fig. 5). Either of these would have the overall effect of increasing antigen recognition and the cognate TCR–CD3–CD8 interaction. Concentration of MHC class I molecules would make it quicker and more efficient for the TCR to “find” the antigenic MHC class I–peptide amongst the mass of the nonstimulatory class I proteins. The on rate of the interaction is concentration- dependent, and the kon of a TCR–MHC–peptide interaction (as measured in solution) can have a significant effect on the biological outcome of TCR recognition, even if the koff has a larger influence overall (Alam et al. 1996, 1999; Gascoigne et al. 2001; Rosette et al. 2001; Stone et al. 2009). Thus the TCR would bind to MHC–peptide at a faster rate, the off rate remaining unchanged, so the TCR would sort through the available MHC–peptide complexes until it associates with one to which it binds more strongly. Looking at this pre-concentration model from the point of view of concentration of the CD8–Lck, the CD8 is more available to stabilize the TCR interaction with antigenic MHC–peptide, and the Lck similarly is more available to start the signaling cascade. Obviously, these two mechanisms are not mutually exclusive. We have some preliminary data indicating that the concentration of CD8/Lck is the more important aspect (JH, PPY and NRJG, unpublished).
Fig. 5.
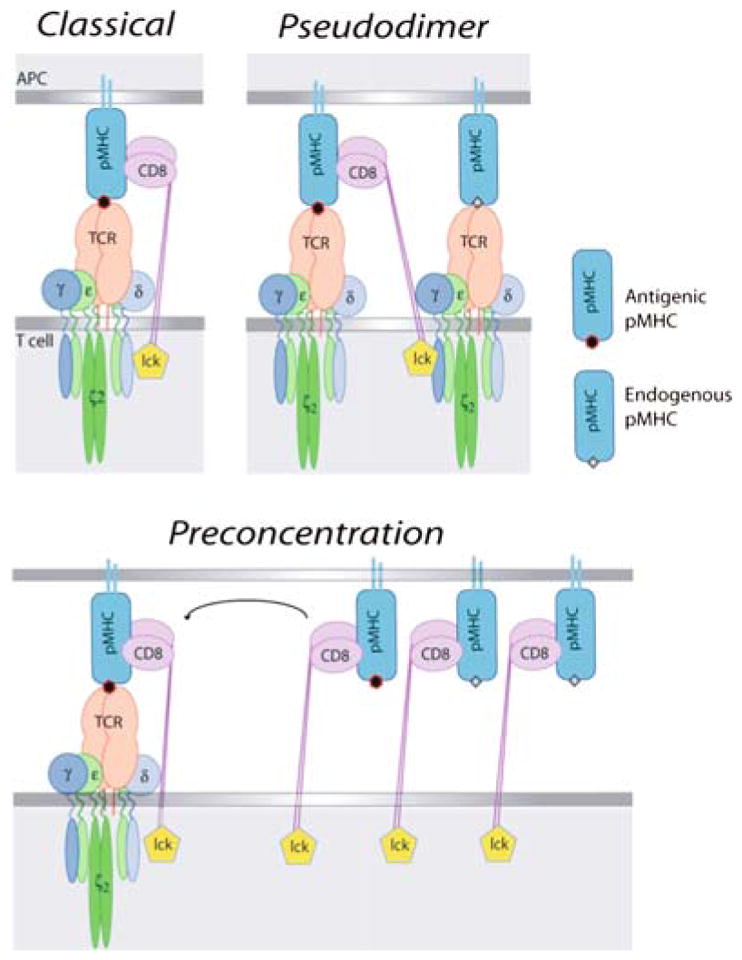
Models of co-receptor function in T-cell activation. For consistency, these are all drawn using CD8 as the co-receptor. However, the pseudodimer model (b) is derived from studies of CD4+ MHC class II-restricted T cells (Krogsgaard et al. 2005). (a) The “classical” model where the co-receptor stabilizes the interaction between TCR and antigenic MHC–peptide. (b) The “pseudodimer” model, where co-receptor cross-links two TCRs, one interacting with antigenic MHC–peptide, and the other interacting with endogenous MHC–peptide. (c) The “pre-concentration” model, where the co-receptor interaction with antigenic or nonstimulatory MHC–peptide causes concentration of MHC–peptide, co-receptor, and Lck to the synapse
We believe that this pre-concentration model (Fig. 5) is sufficient to explain our data on endogenous/nonstimulatory peptides in helping T-cell activation by small amounts of peptide. However, the situation with MHC class II-restricted cells is different, as described in the following sections.
4 Differing Co-Receptor Roles in Recognition of Endogenous MHC Class I– and II–Peptide?
4.1 NonStimulatory Peptides Aid MHC Class II-Restricted Antigen Recognition by T Cells
The initial idea of aid for antigen recognition caused by endogenous peptides came from studies showing that freshly isolated T cells show partial phosphorylation of the CD3ζchain (Van Oers et al. 1994). This is caused by interactions with endogenous MHC–peptide complexes (Witherden et al. 2000), and enhances recognition of antigen (Stefanova et al. 2002). A study of the immunological synapse found that during antigen recognition, endogenous as well as antigenic MHC class II–peptide complexes became concentrated at the synapse (Wulfing et al. 2002).
This finding was greatly extended by showing that a set of endogenous non-stimulatory peptides were concentrated to the synapse during antigen recognition (Krogsgaard et al. 2005). Soluble dimers of MHC class II molecules bound to these endogenous peptides did not stimulate the T cells to flux Ca2+, whereas dimers of the antigenic peptide did stimulate the T cells. When mixed MHC class II dimers were made with one antigenic peptide and one endogenous peptide, stimulation was achieved by a subset of the endogenous peptides, indicating that these endogenous peptides were able to enhance recognition of the antigen. Similar results were obtained with peptides added to cells expressing “empty” MHC class II molecules, to which peptides were added in a manner analogous to the RMA-S experiments described for class I experiments above (Krogsgaard et al. 2005). These data, like those obtained for the MHC class I-restricted response, indicated that recognition of endogenous nonstimulatory peptides aids antigen recognition. However, there is a fundamental difference in that only a subset of the endogenous nonstimulatory MHC class II–peptide complexes worked in this way (Krogsgaard et al. 2005), whereas all of the tested endogenous nonstimulatory MHC class I–peptide complexes functioned to help antigen recognition (Yachi et al. 2005; Yachi et al. 2007). There synapse-recruitment of the endogenous nonstimulatory MHC class II–peptide complexes was TCR rather than CD4-dependent (Wulfing et al. 2002), whereas our data show it to be CD8 rather than TCR-dependent for the class I system.
Krogsgaard also tested the importance of the CD4–MHC class II interaction in stimulation by antigen plus endogenous peptide, finding that when they mutated the CD4-binding site of MHC class II for the molecule presenting antigen, stimulation was abolished. When they mutated this site on the endogenous peptide-presenting molecule, stimulation was not abolished. Taking account of these data and the finding that a single antigenic peptide can stimulate T cells (in the presence of endogenous peptides on an APC) (Irvine et al. 2002), these authors proposed a “pseudodimer” model for T-cell activation (Fig. 5). In this model, the extracellular, membrane-distal, domains of CD4 bind to the antigenic MHC peptide complex, which is bound by a TCR. The intracellular region of CD4, with Lck, is associated with the intracellular portion of another TCR molecule, in this case interacting with the nonstimulatory endogenous MHC–peptide complex. Thus the CD4 bridges two TCRs bound to two different species of MHC class II–peptide complexes. This model suggests that the TCR that is bound to the endogenous MHC–peptide, rather than the one bound to the antigenic MHC–peptide, is the one that will be phosphorylated.
4.2 Predictions and Tests of the Pseudodimer and Pre-Concentration Models
The pseudodimer model of T-cell activation predicts that the strength of agonist affects the ability of the endogenous nonstimulatory peptides to aid its recognition. Thus, the weaker the agonist (i.e., the faster the off rate of the TCR–MHC–peptide interaction), the smaller the proportion of the different endogenous peptides that would be able to act as co-agonists (Krogsgaard et al. 2005; Li et al. 2004). In contrast, the pre-concentration model predicts that the ability of the endogenous nonstimulatory peptides to aid recognition will not be reduced as the agonist strength decreases, as pre-concentration requires only that the co-receptor–MHC–peptide interaction be active.
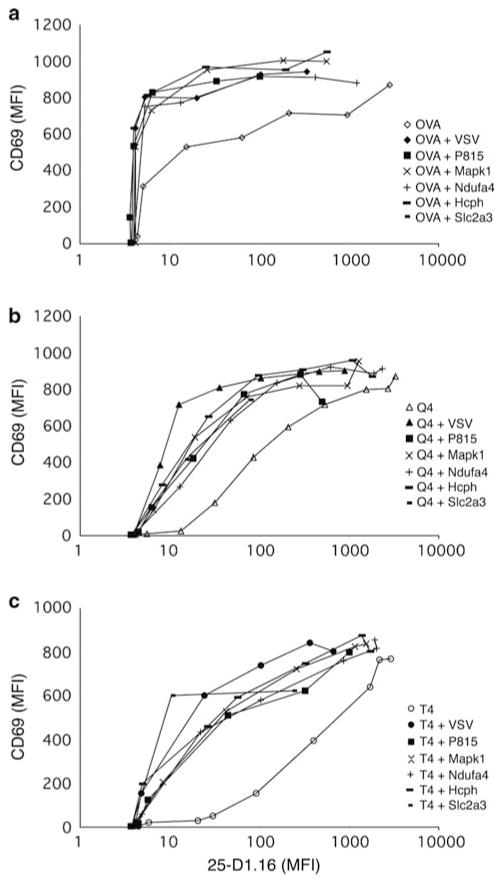
Evidence has been forthcoming to support the prediction of the pseudodimer model in an MHC class II-restricted system. More of the tested endogenous peptides were able to help recognition of a strong agonist than were able to help recognition of a weaker agonist (Krogsgaard et al. 2005; Li et al. 2004). We performed a similar experiment in our MHC class I-restricted system. Recognition of the original antigen (as the strong agonist) was compared to three weaker agonists of varying strengths. We found that the recognition of each of the weaker agonists was aided by all of the different nonstimulatory peptides that we tested (Fig. 6) (Yachi et al. 2007). Indeed, we found that recognition of weak ligands was more reliant on recognition of the nonstimulatory MHC–peptides. These data indicate that for CD8+ cells, the role of the endogenous nonstimulatory peptides is not through formation of a TCR pseudodimer, although we have not formally ruled out any contribution from the TCR interaction with the nonstimulatory MHC–peptide complexes. As noted above (Fig. 3), the endogenous nonstimulatory peptides seem to work in RMA-S cells by their ability to stabilize expression of MHC class I proteins.
Fig. 6.
The ability of nonstimulatory peptides to enhance antigen-recognition is independent of agonist strength. RMA-S cells were loaded with titrated amounts of antigen (OVA; a), a weaker agonist (Q4; b) or a very weak agonist (T4; c) in the absence or presence of various endogenous Kb-binding, OT-I nonstimulatory peptides (Santori et al. 2002). The upregulation of CD69 on prepositive selection thymocytes from OT-I transgenic Tap−/− mice was assessed and expressed in relation to the expression of the OVA–Kb epitope of mAb 25-D1.16 mAb (Porgador et al. 1997). Used with permission from Yachi et al. (2007)
We are now taking an approach to studying the nonstimulatory peptides where single-chain MHC class I–peptide complexes (Yu et al. 2002) are used in the absence of other class I molecules. This allows us to mutate the CD8-binding site or a TCR-binding site in the antigenic or the nonstimulatory MHC class I–peptide complex. Our preliminary data indicate that the nonstimulatory MHC class I–peptide complex must be able to interact with CD8 for it to aid in antigen recognition, and also that reactivity to antigenic peptide on a nonCD8–binding MHC class I molecule can occur with high expression of a nonstimulatory CD8–binding class I–peptide complex (JH, PPY, NRJG, in progress).
This information leads us to the conclusion that for MHC class I-restricted T cells, at least, the data are adequately explained by the pre-concentration model (Yachi et al. 2005, 2007). As this is simpler than the pseudodimer model, Ockham’s razor causes us to prefer the pre-concentration model. In any event, data from the MHC class I-restricted system do not follow the predictions of the pseudodimer model. Results from an MHC class II system seem to support the pseudodimer model, however. This suggests a fundamental difference between the role of the co-receptors in the MHC class I and class II-restricted cells.
4.3 Different Roles for CD4 and CD8 Co-Receptors in Endogenous Peptide Recognition
CD8 has a higher affinity for MHC class I than CD4 has for MHC class II. Most workers have been unable to measure the CD4–class II interaction, while that of CD8–class I is relatively well defined (Gao et al. 1997; Garcia et al. 1996; Kern et al. 1998; van der Merwe and Davis 2003). Experiments on binding of MHC tetramers to T cells find that the CD8–MHC class I interaction enhances tetramer-binding, but no CD4–class II interaction is detectable in this manner (Boniface et al. 1998; Bosselut et al. 2000; Crawford et al. 1998; Daniels and Jameson 2000; Kerry et al. 2003). Can this explain the difference in the role of the endogenous non-stimulatory peptides?
In thymocyte development, the tipping-point of affinity where the weakest negative-selecting ligands turn into positive selecting ligands (Alam et al. 1996) appears to be similar to the affinity of the CD8–MHC class I interaction (Daniels et al. 2006; Naeher et al. 2007). This has led to the suggestion that the affinity of the CD8–MHC class I interaction, being higher than that of the TCR interaction with nonstimulatory endogenous ligands, is the “affinity driver” for the molecular interactions in the synapse, with the implication that it occurs before the TCR–class I interaction (Gascoigne 2008). In contrast, the affinity of the class II-restricted TCR for the nonstimulatory MHC class II–peptide (being stronger than the CD4–class II interaction) is the affinity driver, implying that the TCR–class II interaction would occur before CD4–class II. Certainly, the TCR has some affinity for MHC proteins that is encoded in the CDR1 and CDR2 of the α- and β-chains (Dai et al. 2008; Sim et al. 1996, 1998; Zerrahn et al. 1997).
4.4 Does Co-Receptor–MHC Interaction Precede or Follow TCR Recognition of pMHC?
The noncognate CD8–MHC class I interaction has been shown to be enhanced by initial recognition of cognate antigen-MHC by the TCR (O’Rourke et al. 1990; Varghese and Kane 2008). However, we found that CD8 became concentrated at the synapse between a T cell and an APC in the absence of any antigenic stimulation (Yachi et al. 2005, 2006). We could even find CD8 recruitment to the synapse when we used a T-cell hybridoma lacking TCR (P.P.Y., unpublished). When we titrated the amount of peptide on the RMA-S cells - whether antigenic or non-stimulatory - we found that the amount of CD8 concentrated to the synapse correlated with the number of MHC class I molecules (Yachi et al. 2005). This data suggested that the CD8 interaction with MHC class I occurs independently of TCR recognition of antigen. Structural data (Gao et al. 1997; Kern et al. 1998) and the fact that noncognate MHC class I tetramers can bind to T cells, albeit weakly (Bosselut et al. 2000; Daniels et al. 2006), support this idea. Recent fluorescence correlation measurements of lateral diffusion rates indicate that the TCR interaction with antigenic MHC class I–peptide is preceded by the CD8–MHC class I interaction and that this aids in binding of MHC class I–peptide to TCR (Gakamsky et al. 2005).
In the case of the CD4 class II interaction, we also demonstrated that CD4 becomes concentrated at the immunological synapse and that this occurred without the presence of antigen, although its recruitment was more efficient when antigen was present (Fig. 1b, d) (Zal et al. 2002).
It is possible that recognition of antigen causes a qualitatively different interaction between CD8 and MHC class I to occur. In the past we suggested that the CD4–MHC class II interaction could set up an energetic barrier to TCR interaction with the class II molecule, such that only a TCR with a higher affinity than the CD4–class II interaction would be able to displace CD4 and therefore make the antigen-specific interaction (Gascoigne and Zal 2004; Zal et al. 2002). This could explain data showing that CD4 becomes excluded from the synapse even while TCR becomes concentrated within the synapse (Krummel et al. 2000).
4.5 Adhesion and TCR Cross-Linking in T-Cell Activation
There are several studies that showed that T cells could be activated by monomeric antigenic MHC–peptide complexes (Delon et al. 1998; Doucey et al. 2003; Ma et al. 2008; Randriamampita et al. 2003), in marked contrast to other studies showing that cross-linking was necessary (Boniface et al. 1998; Cochran et al. 2000). These data can be reconciled by the observation that all studies showing activation by monomeric MHC–peptide used systems where the T cells were stimulated on immobilized substrates, whereas the studies demonstrating a requirement for cross-linking all used soluble MHC–peptide complexes (Randriamampita et al. 2003). The mechanism by which this works is that adhesion leads to a transient increase in cyclic AMP, which in turn leads to Erk activation, sensitizing the T cell for the monomeric MHC–peptide stimulation (Conche et al. 2009). Immobilization of MHC–peptide has also been shown to occur as a result of an interaction between MHC class I molecules and ICAM1, causing concentration of both antigenic and nonstimulatory MHC class I–peptide complexes in the immunological synapse, and leading to increased T cell activation (Segura et al. 2008). These data suggest that part of the role of the nonstimulatory MHC–peptide complexes is to aid in the cell adhesion, which in turn aids the priming of the T cells.
5 Concluding Remarks
The emergence of T-cell recognition of endogenous peptides in the activation of T cells by antigen is a fascinating aspect of the immune system’s importance in distinguishing self from nonself. In the old “needle in the haystack” metaphor, it shows the importance of the haystack in the search for the needle, in that the individual straws of hay appear to enhance the ability of the T cell to be stimulated by the needle, when it is finally encountered. The mechanism by which this occurs appears to be different in MHC class I and class II-restricted T cells. In the former, the CD8–MHC interaction appears to drive the formation of complexes that allow faster scanning through the MHC–peptide complexes by the TCR, or better concentration of co-receptor and Lck, or both. In the latter, the TCR–MHC–peptide interaction seems to be stable enough to drive cross-linking of TCRs by CD4 in a pseudodimer.
Acknowledgments
Work from this lab was funded by the NIH (R01GM065230 and AI074074 to N.R.J.G. and K22AI065688 to T.Z.). P.P.Y was supported by T32HL07195 and T.Z by T32AI07290. J.H. was supported by NIH T32AI007244 and the Irving S. Sigal Fellowship. This is TSRI manuscript number 20016.
References
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T cell receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NRJ, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- Anikeeva N, Lebedeva T, Clapp AR, Goldman ER, Dustin ML, Mattoussi H, Sykulev Y. Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response. Proc Natl Acad Sci USA. 2006;103:16846–16851. doi: 10.1073/pnas.0607771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A, Gregoire C, Bakker TR, Baldi L, Jordan M, Goffin L, Boucheron N, Wurm F, van der Merwe PA, Malissen B, et al. CD8βζendows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med. 2001;194:1485–1495. doi: 10.1084/jem.194.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol. 2004;172:5887–5892. doi: 10.4049/jimmunol.172.10.5887. [DOI] [PubMed] [Google Scholar]
- Boniface JJ, Rabinowitz JD, Wulfing C, Hampl J, Reich Z, Altman JD, Kantor RM, Beeson C, McConnell HM, Davis MM. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- Bosselut R, Kubo S, Guinter T, Kopacz JL, Altman JD, Feigenbaum L, Singer A. Role of CD8b domains in CD8 coreceptor function: importance for MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity. 2000;12:409–418. doi: 10.1016/s1074-7613(00)80193-4. [DOI] [PubMed] [Google Scholar]
- Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- Conche C, Boulla G, Trautmann A, Randriamampita C. T cell adhesion primes antigen receptor-induced calcium responses through a transient rise in adenosine 3′, 5′-cyclic mono-phosphate. Immunity. 2009;30:33–43. doi: 10.1016/j.immuni.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Crise B, Rose JK. Identification of palmitoylation sites on CD4, the human immunodeficiency virus receptor. J Biol Chem. 1992;267:13593–13597. [PubMed] [Google Scholar]
- Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for αβζT cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NRJ, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- Delon J, Gregoire C, Malissen B, Darche S, Lemaitre F, Kourilsky P, Abastado J-P, Trautmann A. CD8 expression allows T cell signaling by monomeric peptide-MHC complexes. Immunity. 1998;9:467–473. doi: 10.1016/s1074-7613(00)80630-5. [DOI] [PubMed] [Google Scholar]
- Doucey MA, Legler DF, Faroudi M, Boucheron N, Baumgaertner P, Naeher D, Cebecauer M, Hudrisier D, Ruegg C, Palmer E, et al. The β1 and β3 integrins promote T cell receptor-mediated cytotoxic T lymphocyte activation. J Biol Chem. 2003;278:26983–26991. doi: 10.1074/jbc.M302709200. [DOI] [PubMed] [Google Scholar]
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Gakamsky DM, Luescher IF, Pramanik A, Kopito RB, Lemonnier F, Vogel H, Rigler R, Pecht I. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J. 2005;89:2121–2133. doi: 10.1529/biophysj.105.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Bell JI, Jones EY, Jakobsen BK. Crystal structure of the human CD8ααζand HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne NRJ. Do T cells need endogenous peptides for activation? Nat Rev Immunol. 2008;8:895–900. doi: 10.1038/nri2431. [DOI] [PubMed] [Google Scholar]
- Gascoigne NRJ, Zal T. Molecular interactions at the T cell-antigen-presenting cell interface. Curr Opin Immunol. 2004;16:114–119. doi: 10.1016/j.coi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Gascoigne NRJ, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Exp Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. http://www.expertreviews.org/01002502h.htm. [DOI] [PubMed] [Google Scholar]
- Hurley TR, Luo K, Sefton BM. Activators of protein kinase C induce dissociation of CD4, but not CD8, from p56lck. Science. 1989;245:407–409. doi: 10.1126/science.2787934. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- Kern PS, Teng MK, Smolyar A, Liu JH, Liu J, Hussey RE, Spoerl R, Chang HC, Reinherz EL, Wang JH. Structural basis of CD8 coreceptor function revealed by crystallographic analysis of a murine CD8ααζectodomain fragment in complex with H-2Kb. Immunity. 1998;9:519–530. doi: 10.1016/s1074-7613(00)80635-4. [DOI] [PubMed] [Google Scholar]
- Kerry SE, Buslepp J, Cramer LA, Maile R, Hensley LL, Nielsen AI, Kavathas P, Vilen BJ, Collins EJ, Frelinger JA. Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement. J Immunol. 2003;171:4493–4503. doi: 10.4049/jimmunol.171.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König R, Huang L-Y, Germain RN. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–798. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Sjaastad MD, Wülfing C, Davis MM. Differential clustering of CD4 and CD3ζduring T cell recognition. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Singer SJ, Janeway CA, Jr, Swain SL. Coclustering of CD4 (L3T4) molecule with the T cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci USA. 1987;84:5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Paabo S, Cochet M, Kling G, Kourilsky P, Karre K. Molecular analysis of H-2-deficient lymphoma lines. Distinct defects in biosynthesis and association of MHC class I heavy chains and β2-microglobulin observed in cells with increased sensitivity to NK cell lysis. J Immunol. 1989;142:2911–2917. [PubMed] [Google Scholar]
- Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius U, Pallai P, Harrison SC, Reinherz EL. Delineation of an extended surface contact area on human CD4 involved in class II major histocompatibility complex binding. Proc Natl Acad Sci USA. 1993;90:8259–8263. doi: 10.1073/pnas.90.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan MC, Yachou A, Levesque K, Wu H, Hendrickson WA, Cohen EA, Sekaly RP. CD4 dimers constitute the functional component required for T cell activation. J Immunol. 2002;169:6261–6268. doi: 10.4049/jimmunol.169.11.6261. [DOI] [PubMed] [Google Scholar]
- Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988;336:79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- O’Rourke AM, Rogers J, Mescher MF. Activated CD8 binding to class I protein mediated by the T cell receptor results in signalling. Nature. 1990;346:187–189. doi: 10.1038/346187a0. [DOI] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide–MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Potter TA, Rajan TV, Dick RF, II, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Boulla G, Revy P, Lemaitre F, Trautmann A. T cell adhesion lowers the threshold for antigen detection. Eur J Immunol. 2003;33:1215–1223. doi: 10.1002/eji.200323844. [DOI] [PubMed] [Google Scholar]
- Rosette C, Werlen G, Daniels MA, Holman PO, Alam SM, Travers PJ, Gascoigne NRJ, Palmer E, Jameson SC. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TPJ, Clayberger C, Krensky AM, Norment AM, Littman DR, Parham P. A binding site for the T-cell co-receptor CD8 on the α3 domain of HLA-A2. Nature. 1990;345:41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC. Rare, structurally homologous self-peptides promote thy-mocyte positive selection. Immunity. 2002;17:131–142. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- Segura JM, Guillaume P, Mark S, Dojcinovic D, Johannsen A, Bosshard G, Angelov G, Legler DF, Vogel H, Luescher IF. Increased mobility of major histocompatibility complex I-peptide complexes decreases the sensitivity of antigen recognition. J Biol Chem. 2008;283:24254–24263. doi: 10.1074/jbc.M803549200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim B-C, Zerva L, Greene MI, Gascoigne NRJ. Control of MHC restriction by TCR VαζCDR1 and CDR2. Science. 1996;273:963–966. doi: 10.1126/science.273.5277.963. [DOI] [PubMed] [Google Scholar]
- Sim B-C, Lo D, Gascoigne NRJ. Preferential expression of TCR Vαζregions in CD4/CD8 subsets: class discrimination or co-receptor recognition? Immunol Today. 1998;19:276–282. doi: 10.1016/s0167-5699(98)01257-2. [DOI] [PubMed] [Google Scholar]
- Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur J Immunol. 2002;32:3161–3170. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- Townsend ARM, Öhlén C, Bastin J, Ljunggren H-G, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- Van Oers NSC, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR z in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Varghese JC, Kane KP. TCR complex-activated CD8 adhesion function by human T cells. J Immunol. 2008;181:6002–6009. doi: 10.4049/jimmunol.181.9.6002. [DOI] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci USA. 2001;98:10799–10804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med. 2000;191:355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- Wu H, Kwong PD, Hendrickson WA. Dimeric association and segmental variability in the structure of human CD4. Nature. 1997;387:527–530. doi: 10.1038/387527a0. [DOI] [PubMed] [Google Scholar]
- Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–47. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- Yachi PP, Ampudia J, Gascoigne NRJ, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachi PP, Ampudia J, Zal T, Gascoigne NRJ. Altered peptide ligands induce delayed and reduced CD8-TCR interaction – a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Yachi PP, Lotz C, Ampudia J, Gascoigne NRJ. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007;204:1747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168:3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- Zal T, Gascoigne NRJ. Photobleaching-corrected FRET efficiency imaging of live cells. Biophys J. 2004a;86:3923–3939. doi: 10.1529/biophysj.103.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T, Gascoigne NRJ. Using live FRET imaging to reveal early protein-protein interactions during T cell activation. Curr Opin Immunol. 2004b;16:418–427. doi: 10.1016/j.coi.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Zal T, Zal MA, Gascoigne NRJ. Inhibition of T-cell receptor-coreceptor interactions by antagonist ligands visualized by live FRET imaging of the T-hybridoma immunological synapse. Immunity. 2002;16:521–534. doi: 10.1016/s1074-7613(02)00301-1. [DOI] [PubMed] [Google Scholar]
- Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]