Abstract
BACKGROUND
Human papillomavirus (HPV) is a well-established prognostic marker for oropharyngeal squamous cell cancer (OPSCC). Because of the limited numbers of women and nonwhites in studies to date, sex and racial/ethnic differences in prognosis have not been well explored. In this study, survival differences were explored by the tumor HPV status among 1) patients with OPSCCs by sex and race and 2) patients with nonoropharyngeal (non-OP) head and neck squamous cell cancers (HNSCCs).
METHODS
This retrospective, multi-institution study included OPSCCs and non-OP HNSCCs of the oral cavity, larynx, and nasopharynx diagnosed from 1995 to 2012. Race/ethnicity was categorized as white non-Hispanic, black non-Hispanic, Asian non-Hispanic, and Hispanic of any race. Tumors were centrally tested for p16 overexpression and the presence of HPV by HPV16 DNA and high-risk HPV E6/E7 messenger RNA in situ hybridization. Kaplan-Meier and Cox proportional hazards models were used to evaluate overall survival (OS).
RESULTS
The study population included 239 patients with OPSCC and 621 patients with non-OP HNSCC with a median follow-up time of 3.5 years. After adjustments for the tumor HPV status, age, current tobacco use, and stage, the risk of death was lower for women versus men with OPSCC (adjusted hazard ratio, 0.55; P =.04). The results were similar with p16. In contrast, for non-OP HNSCCs, HPV positivity, p16 positivity, and sex were not associated with OS.
CONCLUSIONS
For OPSCC, there are differences in survival by sex, even after the tumor HPV status has been taken into account. For non-OP HNSCC, the HPV status and the p16 status are not of prognostic significance.
Keywords: head and neck squamous cell cancer (HNSCC), human papillomavirus (HPV), oropharyngeal squamous cell cancer (OPSCC), p16, prognosis, sex, race
INTRODUCTION
Oropharyngeal squamous cell cancer (OPSCC) is increasing in incidence in the United States and other countries.1 These epidemiologic changes are driven by increasing oral exposure to human papillomavirus (HPV) infection.2,3 HPV-positive OPSCC patients are more likely than HPV-negative OPSCC patients to be white, to be younger, and to have better survival4–6 both at the time of the primary diagnosis and upon disease recurrence.7–11
To date, in the majority of studies evaluating the prognosis for OPSCC, the patient populations have been composed primarily of male and white non-Hispanic patients; this is a reflection of the demographics of the disease.12 Although the incidence of OPSCC is lower among women and nonwhites, we have recently shown that the majority of OPSCCs in these groups are HPV-related.13 Sex-related differences in the prognosis for OPSCC are poorly understood, but a multivariate analysis in one recent study suggested that men have worse overall survival (OS) than women.14
Studies have focused on racial differences in OPSCC and have specifically compared the prognosis of blacks and whites.15 The worse survival of blacks has been attributed to a reduced prevalence of HPV-related disease in blacks.15–17 After accounting for the tumor HPV status, initial studies have suggested that survival is similar for blacks and whites with HPV-positive OPSCC, but it is significantly worse for blacks versus whites among patients with HPV-negative OPSCC.14 The prognostic impact of the tumor HPV status and race/ethnicity is largely unknown for other groups in the United States such as Asians and Hispanics.
Because the tumor HPV status is being considered as a risk stratification biomarker for patients with head and neck squamous cell cancer (HNSCC), there is a need to establish the prognostic impact of the tumor HPV status for nonoropharyngeal (non-OP) HNSCCs. The most rigorous study to date found that the tumor HPV status according to in situ hybridization (ISH) did not have prognostic significance in non-OP HNSCCs; however, tumor p16 expression did.18 The reasons for the discordance between the tumor HPV status and p16 expression are unknown.
Therefore, this study was designed to investigate survival differences by sex and race/ethnicity among OPSCC patients as well as the effect of HPV within each of these subgroups.
MATERIALS AND METHODS
This was an institutional review board–approved retrospective study of incident HNSCC cases diagnosed between 1995 and 2012 at the Sidney Kimmel Comprehensive Cancer Center (Johns Hopkins Hospital, Baltimore, Md) and the Helen Diller Family Cancer Center (University of California San Francisco, San Francisco, Calif). The study population was composed of patients with HNSCCs of the oropharynx, oral cavity, larynx, and nasopharynx. For each tumor site, cases were randomly sampled from the cancer registry (when there were sufficient numbers to allow this) in sex and race/ethnic groups of interest to oversample nonwhites and women. Medical record abstraction was performed to summarize clinical variables of interest and confirm tumor sites (including American Joint Committee on Cancer [AJCC] 7th edition tumor and nodal stage).
Testing Methods
The sampling and tumor HPV detection methods have been previously described in detail in a separate article.13 In brief, tumor HPV detection was performed centrally in 2014 and 2015 and was interpreted by a single pathologist (W.H.W.). Testing included p16 immunohistochemistry (MTM Laboratories, Heidelberg, Germany) and HPV16 DNA ISH (GenPoint; Dako, Carpinteria, Calif) for all samples. Tumors that were p16-positive but HPV16 DNA ISH–negative were tested with an RNA ISH probe (RNAscope; Advanced Cell Diagnostics, Hayward, Calif) targeting E6/E7 messenger RNA for 18 high-risk HPV genotypes.19 Algorithms that combine the high sensitivity of p16 expression with visualization of high-risk HPV, including confirmation of transcriptionally active high-risk HPV for the subset of p16-positive/HPV16 DNA–negative cases, are highly accurate for determining the tumor HPV status.19–22 p16 expression was considered positive if a ≥70% strong and diffuse nuclear and cytoplasmic staining pattern was observed. Cases were considered to be HPV-positive if they were positive for either HPV16 DNA ISH or high-risk HPV RNA ISH.
Statistical Analysis
Race and ethnicity (called race hereafter) were categorized as white non-Hispanic, black non-Hispanic, Asian non-Hispanic, and Hispanic of any race (hereafter white, black, Asian, and Hispanic, respectively). Non-Hispanic patients of other races were not sampled because of insufficient numbers. For most analyses, tumors of the oral cavity, larynx, and nasopharynx were combined for analysis as non-OP HNSCCs.
The characteristics of HNSCCs were compared by the tumor HPV status with a chi-square test for categorical variables and with a nonparametric equality-of-medians test for continuous variables. The effect of HPV on survival was evaluated with Kaplan-Meier, log-rank, and Cox methods. OS was defined as the time from the date of diagnosis to death due to any cause. Patients were censored at death, analytic censoring, or loss to follow-up.
RESULTS
The study population (n = 860) included 311 women (36.2%), 276 blacks (32.1%), 170 Asians (19.8%), and 99 Hispanics (11.5%; Table 1). There were 239 cancers of the oropharynx (27.8%), 253 cancers of the oral cavity (29.4%), 243 cancers of the larynx (28.3%), and 125 cancers of the nasopharynx (14.5%). As previously reported, the prevalence of HPV-positive tumors among cancers of the oropharynx, oral cavity, larynx, and nasopharynx was 56%, 2%, 5%, and 10%, respectively.13 The majority of men (58%) and women (52%) with OPSCCs were HPV-positive.13 Because of our interest in sex- and race-based differences in survival, the characteristics of the study population were compared by sex and race (Supporting Tables 1 and 2 [see online supporting information]). Men and women were similar in most characteristics, but women were more likely to be white (P = .005) and were less likely to have ever been tobacco users (P < .001) or to be current alcohol users (P = .008). When we compared patients across race groups, there were more significant differences observed, including differences in sex, tobacco and alcohol use, tumor stage, and anatomic site (P < .001 for each).
TABLE 1.
Characteristics of the Study Population Overall and by HPV Tumor Status at Diagnosis
| Overall (n = 860) | HPV-Negative (n = 695)a | HPV-Positive (n = 164)a | P | |
|---|---|---|---|---|
| Age, median (interquartile range), y | 58 (51-68) | 58 (51-69) | 57.5 (50-64) | .19 |
| Sex | ||||
| Men | 549 (63.8%) | 63.0% | 67.1% | .33 |
| Women | 311 (36.2%) | 37.0% | 32.9% | |
| Race and ethnicity | ||||
| White non-Hispanic | 315 (36.6%) | 32.7% | 53.7% | |
| Black non-Hispanic | 276 (32.1%) | 33.8% | 25.0% | <.001 |
| Asian non-Hispanic | 170 (19.8%) | 21.3% | 12.8% | |
| Any race, Hispanic | 99 (11.5%) | 12.2% | 8.5% | |
| Ever tobacco use | ||||
| No | 159 (18.5%) | 16.5% | 26.8% | |
| Yes | 502 (58.4%) | 58.8% | 56.1% | .004 |
| Unknown | 199 (23.1%) | 24.6% | 17.1% | |
| Current tobacco use | ||||
| Nob | 406 (47.2%) | 44.0% | 60.4% | |
| Yes | 244 (28.4%) | 30.1% | 21.3% | .001 |
| Unknown | 210 (24.4%) | 25.9% | 18.3% | |
| Alcohol use ever | ||||
| No | 199 (23.1%) | 21.4% | 29.9% | |
| Yes | 440 (51.2%) | 51.7% | 49.4% | .05 |
| Unknown | 221 (25.7%) | 26.9% | 20.7% | |
| Current alcohol use | ||||
| No | 324 (37.7%) | 37.3% | 39.0% | |
| Yes | 311 (36.2%) | 35.4% | 39.6% | .27 |
| Unknown | 225 (26.2%) | 27.3% | 21.3% | |
| Tumor stage | ||||
| T1 | 217 (25.2%) | 23.6% | 32.3% | |
| T2 | 220 (25.6%) | 23.2% | 36.0% | |
| T3 | 154 (17.9%) | 19.4% | 11.6% | <.001 |
| T4 | 222 (25.8%) | 27.8% | 17.1% | |
| Indeterminate/unknown | 47 (5.5%) | 6.0% | 3.0% | |
| Nodal stage | ||||
| N0 | 331 (38.5%) | 44.3% | 14.0% | |
| N1, N2a, N2b | 342 (39.8%) | 33.8% | 64.6% | <.001 |
| N2c, N3 | 131 (15.2%) | 14.8% | 17.1% | |
| Indeterminate/unknown | 56 (6.5%) | 7.1% | 4.3% | |
| Anatomic site | ||||
| Oropharynx | 239 (27.8%) | 15.1% | 81.7% | |
| Oral cavity | 253 (29.4%) | 35.7% | 3.0% | <.001 |
| Nasopharynx | 125 (14.5%) | 16.0% | 7.9% | |
| Larynx | 243 (28.3%) | 33.2% | 7.3% | |
| Study site | ||||
| Johns Hopkins Hospital | 434 (50.5%) | 49.1% | 56.1% | .11 |
| University of California San Francisco | 426 (49.5%) | 50.9% | 43.9% | |
| Vital status | ||||
| Death due to any cause | 444 (51.6%) | 56.4% | 31.1% | <.001 |
| Death due to HNSCCc | 185 (25.0%) | 27.8% | 13.0% | <.001 |
| Second primary | ||||
| No | 681 (79.2%) | 79.1% | 79.3% | |
| Yes | 49 (5.7%) | 5.8% | 5.5% | .99 |
| Unknown | 130 (15.1%) | 15.1% | 15.2% | |
| Recurrence | ||||
| No | 536 (62.3%) | 60.0% | 72.6% | |
| Persistent disease | 70 (8.1%) | 8.8% | 5.5% | |
| No treatment | 31 (3.6%) | 3.7% | 3.0% | .02 |
| Yes | 159 (18.5%) | 20.1% | 11.0% | |
| Unknown | 64 (7.4%) | 7.3% | 7.9% |
Abbreviations: HNSCC, head and neck squamous cell cancer; HPV, human papillomavirus.
Any high-risk HPV infection as determined by the in situ hybridization test detailed in the Materials and Methods section. Note that 1 participant did not have HPV ISH results and was therefore excluded from the stratified columns of this table.
This includes former and never smokers.
One hundred nineteen patients with an unknown cause of death were excluded.
The median follow-up time for the study population was 3.5 years (interquartile range, 1.3-6.9 years), with similar follow-up among OPSCC and non-OPSCC patients (3.5 vs 3.5 years; P = .82). During follow-up, 444 patients (51.6%) died of any cause, and 185 (25.0%) died of HNSCC (Table 1).
Factors Associated With OS in OPSCC
When we considered the prognostic significance of HPV for OPSCCs, each of the evaluated tumor detection methods was associated with significantly improved OS (Fig. 1 and Table 2). As expected, both HPV positivity (hazard ratio [HR], 0.34; 95% confidence interval [CI], 0.22-0.51) and p16 positivity (HR, 0.35; 95% CI, 0.24-0.53) were associated with significantly improved OS (P < .001 for each). At 5 years, 78.6% of HPV-positive patients and 42.0% of HPV-negative patients were alive (P < .001). This survival advantage remained at 10 years (57.6% vs 28.8%; P < .001). Similarly, survival was significantly improved for p16-positive OPSCC patients versus p16-negative OPSCC patients at 5 and 10 years (Fig. 1).
Figure 1.
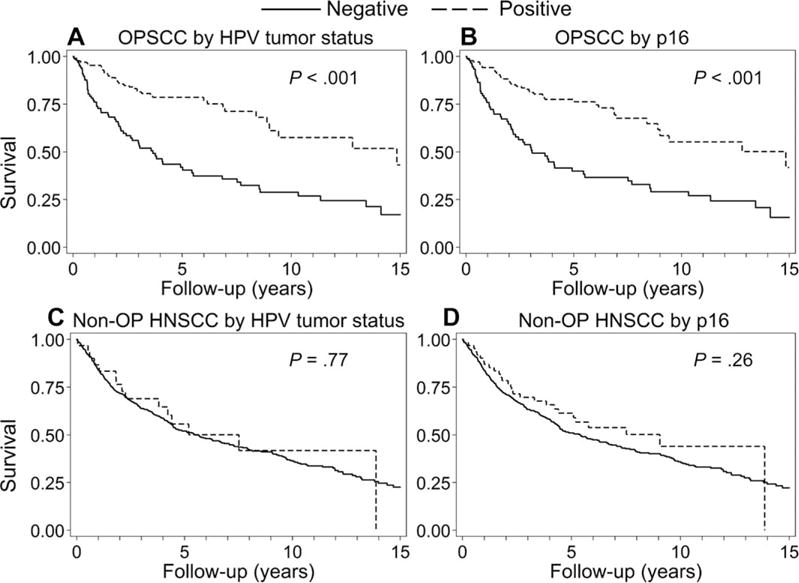
Overall survival by the tumor HPV status and p16 immunohistochemistry for OPSCC patients (n = 239) and non-OP HNSCC patients (n = 621). (A) Overall survival for HPV-positive patients with OPSCC (n = 134) and HPV-negative patients with OPSCC (n = 105) was 78.6% and 42.0%, respectively, at 5 years and 57.6% and 28.8%, respectively, at 10 years. (B) Overall survival for patients with p16-positive OPSCC (n = 144) and patients with p16-negative OPSCC (n = 95) was 77.5% and 39.9%, respectively, at 5 years and 55.2% and 29.1%, respectively, at 10 years. (C) Overall survival for HPV-positive patients with non-OP HNSCC (n = 30) and HPV-negative patients with non-OP HNSCC (n = 590) was 55.7% and 52.0%, respectively, at 5 years and 41.8% and 36.4%, respectively, at 10 years. (D) Overall survival for patients with p16-positive non-OP HNSCC (n = 62) and patients with p16-negative non-OP HNSCC (n = 559) was 61.4% and 51.0%, respectively, at 5 years and 44.0% and 35.8%, respectively, at 10 years. HNSCC indicates head and neck squamous cell cancer; HPV, human papillomavirus; non-OP, nonoropharyngeal; OPSCC, oropharyngeal squamous cell cancer.
TABLE 2.
Univariate Association of the Tumor HPV Status With the Risk of Death Among Patients With OPSCC and Non-OP HNSCC
| HR (95% CI)a
|
|||||
|---|---|---|---|---|---|
| OPSCC (n = 239) | All Non-OP HNSCC (n = 621) | Oral Cavity (n = 253) | Larynx (n = 243) | Nasopharynx (n = 125) | |
| Tumor HPV statusb | 134/239 (56%) | 30/620 (5%) | 5/253 (2%) | 12/243 (5%) | 13/124 (10%) |
| Negative | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 0.34 (0.22-0.51) | 0.93 (0.55-1.55) | 1.76 (0.72-4.31) | 1.15 (0.54-2.46) | 0.49 (0.15-1.58) |
| HPV16 tumor statusc | 114/239 (48%) | 23/619 (4%) | 5/253 (2%) | 8/243 (3%) | 10/123 (8%) |
| Negative | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 0.38 (0.25-0.58) | 1.18 (0.69-2.01) | 1.76 (0.72-4.31) | 1.50 (0.66-3.40) | 0.72 (0.23-2.31) |
| p16 status | 144/239 (60%) | 62/621 (10%) | 15/253 (6%) | 32/243 (13%) | 15/125 (12%) |
| Negative | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 0.35 (0.24-0.53) | 0.80 (0.54-1.18) | 0.91 (0.45-1.86) | 0.81 (0.47-1.38) | 0.74 (0.29-1.84) |
Abbreviations: CI, confidence interval; HNSCC, head and neck squamous cell cancer; HPV, human papillomavirus; HR, hazard ratio; non-OP, nonoropharyng-eal; OPSCC, oropharyngeal squamous cell cancer.
Bolding indicates statistical significance.
Any high-risk HPV infection as detailed in the Materials and Methods section.
As determined by HPV16 in situ hybridization.
In the univariate analysis, differences in survival were also observed with race and sex as well as increasing age, tumor stage, and current tobacco use (HR, 1.22-2.74; Table 3). Survival was better for women than men (HR, 0.68; 95% CI, 0.43-1.05; 5-year survival, 73.1% vs 58.3%; 10-year survival, 58.1% vs 39.6%; P = .08; Fig. 2A). In comparison with white patients, survival improved among Asian patients (HR, 0.48; 95%, 0.19-1.23; P = .13) but not Hispanic patients (HR, 0.89; 95%, 0.41-1.90; P = .76), and it was significantly worse among black patients (HR, 1.55, 95% CI, 1.01-2.37; P = .04; Fig. 2B).
TABLE 3.
Univariate and Multivariate Risk Factors for Death Among Patients With Oropharyngeal Squamous Cell Cancer
| Characteristic at Diagnosis | Univariate Analysis (n = 239)a
|
Multivariate Analysis (n = 183)a,b
|
||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Age (per 10-y increase) | 1.22 (1.01-1.48) | .04 | 1.23 (0.95-1.59) | .11 |
| Tumor stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2 | 1.20 (0.66-2.20) | .55 | 0.90 (0.43-1.85) | .77 |
| T3 | 2.39 (1.32-4.32) | .004 | 0.92 (0.43-1.97) | .84 |
| T4 | 2.74 (1.50-4.99) | .001 | 1.06 (0.47-2.38) | .89 |
| P for trend | <.001 | .92 | ||
| Nodal stage | ||||
| N0 | 1.00 | 1.00 | ||
| N1-N2b | 1.07 (0.62-1.87) | .80 | 1.85 (0.86-3.97) | .12 |
| N2c- N3 | 1.77 (0.94-3.33) | .08 | 2.92 (1.16-7.36) | .02 |
| P for trend | .07 | .01 | ||
| Sex | ||||
| Men | 1.00 | 1.00 | ||
| Women | 0.68 (0.43-1.05) | .08 | 0.48 (0.26 -0.88) | .02 |
| Race and ethnicity | ||||
| White Non-Hispanic | 1.00 | 1.00 | ||
| Black Non-Hispanic | 1.55 (1.01-2.37) | .04 | 0.99 (0.54-1.81) | .97 |
| Asian Non-Hispanic | 0.48 (0.19-1.23) | .13 | 0.12 (0.02-0.92) | .04 |
| Any race, Hispanic | 0.89 (0.41-1.90) | .76 | 0.79 (0.23-2.69) | .71 |
| Current tobacco use | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.31 (1.42-3.75) | .001 | 1.77 (0.98-3.20) | .06 |
| Current alcohol use | ||||
| No | 1.00 | – | ||
| Yes | 1.21 (0.74-1.98) | .46 | ||
| Tumor p16 status | ||||
| Negative | 1.00 | – | ||
| Positive | 0.35 (0.24-0.53) | <.001 | ||
| Tumor HPV status | ||||
| Negative | 1.00 | 1.00 | ||
| Positive | 0.34 (0.22-0.51) | <.001 | 0.34 (0.18-0.64) | .001 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HPV, human papillomavirus; HR, hazard ratio.
Bolding indicates statistical significance.
The multivariate model was restricted to patients for whom the current tobacco-use status, tumor and nodal stage.
Figure 2.
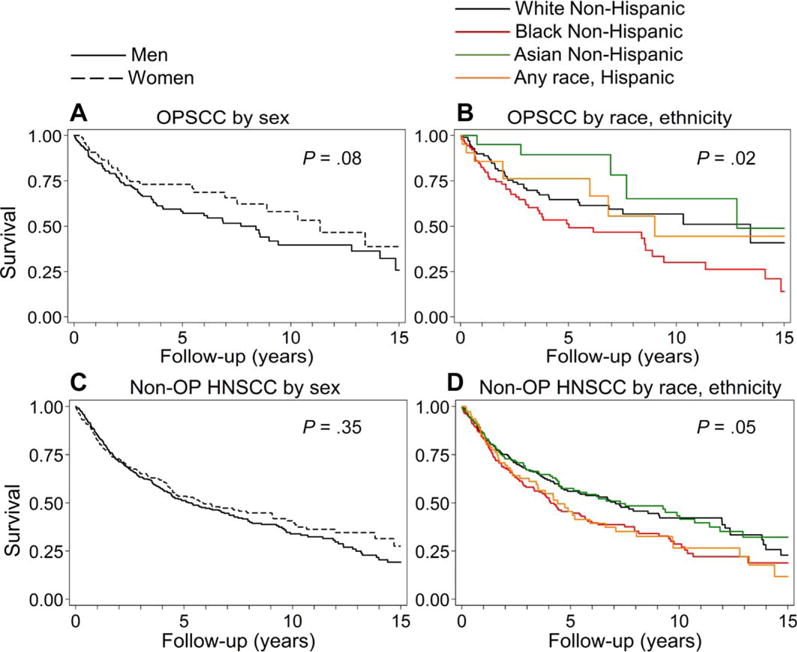
Survival by sex and race/ethnicity among OPSCC and non-OP HNSCC patients. (A) Overall survival for men (n = 160) and women (n = 79) with OPSCC was 58.3% and 73.1%, respectively, at 5 years and 39.6% and 58.1%, respectively, at 10 years. (B) Overall survival for white (n = 103), black (n = 94), Asian (n = 21), and Hispanic patients (n = 21) with OPSCC was 64.7%, 51.3%, 89.4%, and 76.2%, respectively, at 5 years and 56.8%, 30.0%, 65.2%, and 44.4%, respectively, at 10 years. (C) Overall survival for men (n = 389) and women (n = 232) with non-OP HNSCCs was 51.1% and 53.5%, respectively, at 5 years and 33.9% and 40.7%, respectively, at 10 years. (D) Overall survival for white (n = 212), black (n = 182), Asian (n = 149), and Hispanic patients (n = 78) with non-OP HNSCCs was 56.0%, 45.5%, 57.5%, and 45.5%, respectively, at 5 years and 42.2%, 28.6%, 43.3%, and 26.6%, respectively, at 10 years. HNSCC indicates head and neck squamous cell cancer; non-OP, nonoropharyngeal; OPSCC, oropharyngeal squamous cell cancer.
In the multivariate analysis, female sex (adjusted hazard ratio [aHR], 0.48; 95% CI, 0.26-0.88; P = .02) and HPV positivity (aHR, 0.34; 95% CI, 0.18-0.64; P = .001) were each associated with improved survival among OPSCC patients (Table 3). Asian patients had a large (88%) but marginally significant reduction in the risk of death (aHR, 0.12; 95% CI, 0.02-0.92; P = .04). After we controlled for other factors, there was no survival difference between black and white OPSCC patients (aHR, 0.99; 95% CI, 0.54-1.81; P = .97). Estimates were similar when p16 was considered (Supporting Table 3 [see online supporting information]).
To understand whether the prognostic effect of HPV differed by sex or race, statistical interactions between HPV and sex and race were explored. There was no interaction between HPV and sex (P for interaction = .23) or race (P for interaction for blacks vs whites = .97; P for interaction for Asians vs whites = .94; P for interaction for Hispanics vs whites = .50); this suggests similar prognostic utility of the tumor HPV status for men and women and for OPSCC patients of different races.
Factors Associated With OS for Patients With Non-OP HNSCCs
In contrast to the strong prognostic role of the tumor HPV status in OPSCC, in non-OP HNSCCs, the tumor HPV status (P = .77) and p16 (P = .26) had no impact on OS (Table 2). Indeed, the tumor HPV status and p16 were not of prognostic significance in HNSCCs of the oral cavity (n = 253; P = .22), larynx (n = 243; P = .72), or nasopharynx (n = 125; P = .23).
The prognostic role of race and sex for non-OP HNSCCs was evaluated by anatomic site. There were no differences in OS by race for oral cavity, nasopharyngeal, or laryngeal cancers. Women had survival similar to that of men with nasopharyngeal cancer (HR, 1.31; 95% CI, 0.78-2.20) and laryngeal cancer (HR, 0.96; 95% CI, 0.67-1.36) but had lower survival with oral cavity cancer (HR, 0.70; 95% CI, 0.50-0.98; P = .04). There was an interaction between sex and tumor site for OS in the univariate analysis (P for interaction for nasopharyngeal cancer vs oral cavity cancer = .04) but not in the multivariate analysis (P for interaction for nasopharyngeal cancer vs oral cavity cancer = .34).
Risk factors were explored separately for oral cavity, laryngeal, and nasopharyngeal HNSCCs. Because the risk factors were similar (including a lack of prognostic significance for HPV), they were combined into non-OP HNSCCs for further analysis. Survival with non-OP HNSCC was similar for Asian (P = .67) and Hispanic patients (P = .13) versus white patients; however, black patients (HR, 1.33; 95% CI, 1.02-1.73; P = .04) with non-OP HNSCCs appeared to have worse survival than whites (Fig. 2D). As expected, there were no survival differences between men and women (P = .35; Fig. 2C). Other univariate risk factors for worse survival included increasing age, a higher tumor and nodal stage, and tobacco and alcohol use (Table 4).
TABLE 4.
Univariate and Multivariate Risk Factors for Death Among 621 Patients with Nonoropharyngeal Head and Neck Squamous Cell Cancers
| Characteristic at Diagnosis | Univariate Analysis (n = 621)a
|
Multivariate Analysis (n = 397)a,b
|
||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Age (per 10-y increase) | 1.30 (1.20-1.42) | <.001 | 1.45 (1.28- 1.63) | <.001 |
| Tumor stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2 | 1.61 (1.15-2.27) | .006 | 1.48 (0.94-2.33) | .09 |
| T3 | 2.65 (1.88-3.75) | <.001 | 2.33 (1.44-3.76) | <.001 |
| T4 | 2.81 (2.04-3.87) | <.001 | 2.82 (1.82-4.35) | <.001 |
| P for trend | <.001 | <.001 | ||
| Nodal stage | ||||
| N0 | 1.00 | 1.00 | ||
| N1, N2a, N2b | 1.35 (1.06-1.73) | .02 | 1.33 (0.96-1.85) | .08 |
| N2c, N3 | 1.73 (1.27-2.36) | <.001 | 1.83 (1.22-2.75) | .003 |
| P for trend | <.001 | .001 | ||
| Sex | ||||
| Men | 1.00 | 1.00 | ||
| Women | 0.90 (0.72-1.12) | .35 | 0.97 (0.72- 1.30) | .83 |
| Race and ethnicity | ||||
| White Non-Hispanic | 1.00 | 1.00 | ||
| Black Non-Hispanic | 1.33 (1.02-1.73) | .04 | 0.94 (0.67-1.31) | .71 |
| Asian Non-Hispanic | 0.94 (0.70-1.26) | .67 | 0.90 (0.57-1.43) | .66 |
| Any race, Hispanic | 1.30 (0.93-1.82) | .13 | 0.88 (0.53-1.44) | .61 |
| Current tobacco use | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.35 (1.04-1.75) | .02 | 1.16 (0.83-1.62) | .37 |
| Current alcohol use | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.33 (1.03-1.72) | .03 | 1.40 (1.03-1.90) | .03 |
| Tumor p16 status | ||||
| Negative | 1.00 | – | ||
| Positive | 0.80 (0.54-1.18) | .26 | ||
| Tumor HPV status | ||||
| Negative | 1.00 | – | ||
| Positive | 0.93 (0.55-1.55) | .77 | ||
| Anatomic site | ||||
| Oral cavity | 1.00 | 1.00 | ||
| Larynx | 0.69 (0.51-0.93) | .02 | 0.96 (0.59-1.54) | .85 |
| Nasopharynx | 0.93 (0.74-1.18) | .56 | 0.86 (0.62-1.19) | .36 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HPV, human papillomavirus; HR, hazard ratio.
Bolding indicates statistical significance.
The multivariate model was restricted to patients for whom the current tobacco-use status, the current alcohol-use status, tumor and nodal stage.
In the multivariate analysis, neither sex nor race was a predictor of survival for non-OP HNSCC patients. Indeed, only older age (aHR, 1.45; 95% CI, 1.28-1.63; P < .001), a higher tumor and nodal stage (P for trend < .001 for each), and current alcohol use (P = .03) were significant predictors of OS among patients with non-OP HNSCC (Table 4).
Recurrence-Free Survival
Patients with HPV-positive OPSCC had modestly improved recurrence-free survival in comparison with patients with HPV-negative OPSCC, although this difference was not statistically significant (5-year survival, 86.0% vs 76.7%; 10-year survival, 86.0% vs 76.7%; HR, 0.58; 95% CI, 0.28-1.21; P = .14). Among non-OP HNSCC patients, recurrence-free survival was similar when a comparison was made by the tumor HPV status (P = .12) or p16 status (P = .07).
DISCUSSION
This is one of the first large studies to demonstrate that women with HPV-positive OPSCC have a survival advantage in comparison with men and that the tumor HPV status has no prognostic significance for non-OPSCCs. Although the incidence of HPV-positive OPSCC is lower among women than men, the vast majority of OPSCCs among women in the United States are now HPV-positive.13 To date, the largest analyses evaluating prognostic factors for OPSCC have not included sex, although an Institute of Medicine report and the National Institutes of Health have called for sex to be included in the design and analysis of all studies (from womb to tomb).23,24 This report highlights that female sex is indeed an important prognostic consideration for patients with OPSCC, even after we account for the tumor HPV status.
Population-based studies have shown that for cancers of anatomic sites outside the head and neck, survival is worse for men than women.25 The reasons for the observed survival differences by sex among patients with OPSCC are unknown. It is possible that women have less tobacco exposure than men with OPSCC, and residual confounding for the amount of lifetime tobacco exposure may contribute to the observed differences. Although a lower proportion of women have ever been tobacco users, there are no sex-related differences in current tobacco use (data not shown). In non–squamous cell lung cancers, improved outcomes among women versus men are not explained by smoking and instead appear to be a reflection of distinct phenotypes of disease by sex.26 Differences in comorbidities and death rates for men and women may also contribute to the observed survival differences. Without accounting for the tumor HPV status, a prior US population–based study observed a 3-fold higher cancer-specific mortality rate for men versus women after adjustments for age.25 Our retrospective study focused on OS, not cancer-specific mortality, but deaths due to head and neck–related causes overall were similar for men and women (P > .05; Supporting Table 1 [see online supporting information]). This finding is intriguing and warrants further investigation.
There has been interest in better understanding racial differences in survival among cancer patients.14,15,27 Several prior analyses have identified differences in survival for OPSCC patients in white and black study populations. The current study broadens our racial understanding of OPSCC to include Asians and His-panics; this has not been previously reported to our knowledge. Moreover, prior analyses used less specific methods of HPV detection (polymerase chain reaction) or small sample sizes for nonwhite study populations.14,15,17 Using the largest sample size of OPSCCs among black patients to date and rigorous methods of tumor HPV detection, we did not observe a survival difference after accounting for HPV. This is the first report of improved survival for Asian patients versus white or black patients, although caution is warranted because there were only 21 Asian OPSCC patients.
There is interest in understanding whether the prognostic advantage of HPV positivity observed in OPSCCs applies to non-OP HNSCCs because initial reports have been conflicting. The current study is one of the largest studies to date and includes oral cavity, laryngeal, and nasopharyngeal cancers. No prognostic effect of p16 or HPV was found among non-OP HNSCC patients, and this is consistent with several previous studies.28,29 The finding that HPV and p16 are not prognostic for patients with laryngeal cancer is consistent with a study of 140 laryngeal squamous cell cancers tested with p16 and HPV: only 5% (7 cases) were found to be HPV-positive, and there was no difference in terms of prognosis28 or with a Danish population study.29 However, a cooperative group trial did detect survival differences when p16 was considered for laryngeal and oral cavity cancers, although not when HPV ISH was used.18
The current study is one of the largest series of nasopharyngeal cancers tested to date for the tumor HPV status. A recent study highlighted the prognostic difference between HPV-positive and Epstein-Barr virus (EBV)– positive nasopharyngeal cancer, but the 18 HPV-positive patients and 16 HPV-negative patients had similar survival.30 Other reports have shown a prognostic significance associated with an HPV-positive tumor status31; however, site misclassification has been suggested to explain the observed HPV positivity and survival benefit.32 In the current analysis, site classification was performed by head and neck surgeons. Only 13 of 125 nasopharyngeal cancers were HPV-positive, and there was no survival benefit associated with an HPV-positive tumor status. Although EBV is responsible for nasopharyngeal cancers, its role and interplay with HPV were outside the scope of this analysis. The EBV tumor status for these tumors was unknown.
The results of our study do not support HPV testing of all HNSCCs in clinical practice.33 Rather, the absence of prognostic relevance for HPV positivity outside the oropharynx validates the recommendation endorsed by several practice guideline organizations (eg, the College of American Pathologists, the Royal College of Pathologists, the National Comprehensive Care Network, and Cancer Care Ontario) that routine HPV testing be reserved for HNSCCs of known or presumed oropharyngeal origin.33,34
The current analysis provides a large sample including relatively large numbers of women and nonwhites, and tumor testing was standardized at a centralized laboratory. Its limitations include its retrospective nature, which precluded a more rigorous evaluation of the prognostic impact of tobacco use, and an inability to account for comorbidities and treatment. In addition, this retrospective population received heterogeneous therapies, which may account for prognostic differences.
In summary, OPSCC and non-OP HNSCC have distinct risk factors. The contributions of age, sex, race, and stage differ for each entity. Notably, women with OPSCC appear to have an improved prognosis in comparison with men. Race does not appear to affect the prognosis for OPSCC or non-OP HNSCC patients after we account for other factors. Lastly, the prognostic impact of HPV positivity is reserved for the oropharynx. Given this result, we recommend that patients with non-OP HNSCCs not be routinely tested for p16 or HPV because a positive test result cannot be contextualized.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Funding was provided by the National Institute of Dental and Cra-niofacial Research (grant P50 DE019032) and the Oral Cancer Foundation. The funders had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
William R. Ryan reports personal fees from Medtronic and Ziteo outside the submitted work. Patrick K. Ha reports consultant fees from Bristol-Myers Squibb outside the submitted work. Gypsyam-ber D’Souza reports prior funding from Merck outside the submitted work.
AUTHOR CONTRIBUTIONS
Carole Fakhry: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, writing–review and editing, visualization, supervision project administration, and funding acquisition. William H. Westra: Conceptualization, methodology, validation, and writing–review and editing. Steven J. Wang: Formal analysis, investigation, resources, and writing–review and editing. Annemieke van Zante: Investigation, resources, and writing–review and editing. Yuehan Zhang: Formal analysis, data curation, writing–review and editing, and visualization. Eleni Rettig: Investigation, data curation, and writing–review and editing. Linda X. Yin: Investigation, data curation, and writing–review and editing. William R. Ryan: Resources and writing–review and editing. Patrick K. Ha: Resources and writing–review and editing. Alicia Wentz: Formal analysis, data curation, writing–review and editing, and visualization. Wayne Koch: Resources and writing–review and editing. Jeremy D. Richmon: Resources and writing–review and editing. David W. Eisele: Resources and writing–review and editing. Gypsyamber D’Souza: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration, and funding acquisition.
References
- 1.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: implications for prevention and therapy. Expert Rev Anticancer Ther. 2015;15:35–49. doi: 10.1586/14737140.2015.957189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T, Rettig E, Fakhry C. Understanding the impact of survival and human papillomavirus tumor status on timing of recurrence in oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;52:97–103. doi: 10.1016/j.oraloncology.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Qualliotine JR, Ha PK, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121:1977–1984. doi: 10.1002/cncr.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph AW, Guo T, Hur K, et al. Disease-free survival after salvage therapy for recurrent oropharyngeal squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1501–E1509. doi: 10.1002/hed.24268. [DOI] [PubMed] [Google Scholar]
- 10.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Reply to B. O’Sullivan et al. J Clin Oncol. 2015;33:1708–1709. doi: 10.1200/JCO.2014.60.3555. [DOI] [PubMed] [Google Scholar]
- 11.Fakhry C, Zhang Q, Ngyeun-Tan F, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3067. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worsham MJ, Stephen JK, Chen KM, et al. Improved survival with HPV among African Americans with oropharyngeal cancer. Clin Cancer Res. 2013;19:2486–2492. doi: 10.1158/1078-0432.CCR-12-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papil-lomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandberg DP, Liu S, Goloubeva OG, et al. Emergence of HPV16-positive oropharyngeal cancer in black patients over time: University of Maryland 1992-2007. Cancer Prev Res (Phila) 2015;8:12–19. doi: 10.1158/1940-6207.CAPR-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–16. doi: 10.1016/j.oraloncology.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus–associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 22.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health. Considering sex as a biological variable: in the NIH guide. http://orwh.od.nih.gov/about/director/director_biological_variable.asp. Accessed on July 1, 2016.
- 24.National Academy of Sciences. Exploring the biological contributions to human health: does sex matter? http://www.nationalacade-mies.org/hmd/Reports/2001/Exploring-the-Biological-Contributions-to-Human-Health-Does-Sex-Matter.aspx. Accessed on July 1, 2016.
- 25.Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakelee HA, Wang W, Schiller JH, et al. Survival differences by sex for patients with advanced non–small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006;1:441–446. [PubMed] [Google Scholar]
- 27.Fakhry C, Cohen E. The rise of HPV-positive oropharyngeal cancers in the United States. Cancer Prev Res (Phila) 2015;8:9–11. doi: 10.1158/1940-6207.CAPR-14-0425. [DOI] [PubMed] [Google Scholar]
- 28.Young RJ, Urban D, Angel C, et al. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptional-ly active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112:1098–1104. doi: 10.1038/bjc.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassen P, Primdahl H, Johansen J, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113:310–316. doi: 10.1016/j.radonc.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88:580–588. doi: 10.1016/j.ijrobp.2013.11.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan S, Hedberg ML, Ferris RL, et al. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck. 2014;36:511–516. doi: 10.1002/hed.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 33.Witt BL, Albertson DJ, Coppin MG, et al. Use of in situ hybridization for HPV in head and neck tumors: experience from a national reference laboratory. Head Neck Pathol. 2015;9:60–64. doi: 10.1007/s12105-014-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westra WH. Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014;50:771–779. doi: 10.1016/j.oraloncology.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


