Abstract
Background
The prevalence of hypertension is high and increasing worldwide while the proportion of controlled hypertension is low.
Purpose
To assess the comparative effectiveness of 8 implementation strategies for blood pressure (BP) control in adults with hypertension.
Data Sources
Systematic searches of MEDLINE and Embase from inception to September 2017 with no language restriction supplemented with manual reference searches.
Study Selection
Randomized controlled trials lasting at least 6 months comparing implementation strategies versus usual care on BP reduction in adults with hypertension.
Data Extraction
Two investigators independently extracted trial data. Trials were grouped by implementation strategy, and BP reduction effects were compared using multivariate-adjusted generalized estimating equations. A modified Cochrane Risk of Bias tool was used for trial quality assessment.
Data Synthesis
A total of 121 comparisons from 100 articles with 55,920 hypertensive patients were included. Multilevel, multicomponent strategies, such as team-based care with medication titration by non-physician [−7.1 mmHg (95% CI: −8.9, −5.2)], team-based care with medication titration by physician [−6.2 mmHg (−8.1, −4.2)], and multilevel strategies without team-based care [−5.0 mmHg (−8.0, −2.0)] were most effective for systolic BP reduction. Patient-level strategies also resulted in significant systolic BP reductions of −3.9 mmHg (−5.4, −2.3) for health coaching and −2.7 mmHg (−3.6, −1.7) for home BP monitoring. Similar trends were observed for diastolic BP reduction. Provider training was tested in few trials and resulted in non-significant BP reduction.
Limitations
Sparse data from low- and middle-income countries, few trials of some implementation strategies, and possible publication bias.
Conclusions
Multilevel, multicomponent strategies, followed by patient-level strategies, are most effective for BP control in patients with hypertension and ought to be used to improve hypertension control.
Primary Funding Source
US National Institutes of Health
Introduction
Hypertension is a major public health challenge due to its high prevalence and associated cardiovascular disease and premature death (1,2). Pharmaceutical treatment and lifestyle modifications have been shown to reduce blood pressure (BP) and cardiovascular disease risk in randomized clinical trials (3,4). Despite these proven-effective BP interventions, only 13.8% of adults with hypertension and 37.1% of patients with treated hypertension had their BP controlled worldwide in 2010 (1). Barriers to hypertension control at health care system, health care provider, and patient levels have been identified (5). For example, limited health care resources, lack of performance standards, and limited reimbursement for health coaching are major barriers to BP control at the health care system level; lack of adherence to clinical guidelines is a major barrier to BP control at the health care provider level; and lack of adherence to prescribed medications and lifestyle modifications are major barriers at the patient level (5).
Implementation strategies to overcome the barriers to BP control, such as home BP monitoring, health coaching, provider training, and team-based care have been tested in randomized trials (6,7). Most trials, however, have relatively small sample sizes and limited statistical power to provide a reliable estimate of intervention effects. Two previous reviews of implementation strategies for BP reduction included studies published up to 2003 and 2008, respectively (6,7). They showed that compared to usual care, a number of implementation strategies, including team change and home BP monitoring, significantly improved BP control (6,7). However, the effects of various implementation strategies on BP control have not been directly compared in these meta-analyses. In addition, many implementation strategy trials have been published in the recent years. In the current meta-analysis, we aim to assess the comparative effectiveness of various implementation strategies on BP reduction in patients with hypertension by direct comparison. This information could be used by government and non-government organizations to select the most effective implementation strategies for hypertension control in communities.
Methods
We developed and followed a protocol for all steps of the review and meta-analysis (Supplement 1).
Data Sources and Searches
MEDLINE and Embase were searched from inception to September 11, 2017 with search terms “hypertension” or “blood pressure” and an extensive list of terms related to provider education, team-based care, patient education, provider feedback and guideline adherence, and home BP monitoring (Appendix Table 1 and Appendix Table 2) (6–9). The search was restricted to clinical trials in human adults and had no language restrictions. Additional studies were identified by manual review of references cited in reviews, meta-analyses, and original articles. An extensive search of clinicaltrials.gov was also conducted to find additional trials and to assess publication bias by identifying completed trials without published results.
Study Selection
A study was eligible for inclusion if 1) it was a randomized controlled trial; 2) study participants were adults with hypertension defined as average systolic BP ≥ 140 mmHg, average diastolic BP ≥ 90 mmHg, and/or use of antihypertensive medication; 3) a main trial outcome was the net change in systolic BP or diastolic BP; 4) the trial intervention targets barriers to hypertension control at one or more of the patient, provider, and healthcare system levels; 5) the control group received usual care or minimal education ; 6) the trial duration was at least six months; 7) variance of BP changes (or data to calculate it) was reported; and, 8) if a trial was cluster-randomized, clustering must be accounted for in the analysis. No language restrictions were made. Two investigators independently screened all abstracts to determine initial eligibility. They further reviewed full texts for potentially eligible studies. Disagreements were resolved by consensus.
Data Extraction and Quality Assessment
Two investigators independently extracted data from each included trial using a standardized data collection form. Extracted data included study design, participant characteristics, intervention descriptions and study results. Data from the two investigators were compared, and discrepancies were resolved by consensus. For trials reporting results at more than one time point, the report closest to the end of the intervention was selected.
Trials were divided into eight implementation strategy categories based on intervention descriptions (Table 1). Categories were created based on prior literature and availability of trials meeting our inclusion criteria (6,7). Two categories address patient-level barriers to BP control only: (1) health coaching and (2) home BP monitoring; three categories target provider-level barriers only: (3) provider training, (4) audit and feedback, and (5) electronic decision support systems; and three categories are multilevel strategies: (6) multilevel strategies without team-based care, (7) team-based care with physicians titrating medications, and (8) team-based care with non-physician providers titrating medications.
Table 1.
Implementation Strategy Category Descriptions
| Implementation Strategy Category | Description |
|---|---|
| Patient-level | |
| Health Coaching (10) | Multiple sessions for patient-centered health education and motivation delivered with the goal of facilitating lifestyle modification and/or medication adherence. |
| Home Blood Pressure Monitoring | Self-monitoring of patient blood pressure and recording of measurements either manually or by automatic electronic transmission; blood pressure readings provided to providers. |
| Provider-level | |
| Provider Training | Education or training targeting providers on hypertension management, including guideline adherence (treatment goals, lifestyle intervention, and medication titrations), and/or patient communication |
| Audit and Feedback (11) | Repeated, periodic summaries of patient outcomes given to providers, such as blood pressure values, so they can evaluate and improve patient care; could also include provider training |
| Electronic Decision Support System (11) | Computerized alerts, reminders, or order sets intended to aid providers in point of care decision making; could also include provider training |
| Multilevel | |
| Multilevel Strategy without Team-based Care | Interventions that target barriers to hypertension control at multiple levels but do not include team-based care, such as a combination of provider training and patient health coaching |
| Team-based Care with Physicians Titrating Medications (12) | Collaborative provision of care for hypertension by at least two providers, including a primary care physician who titrates medications, working collaboratively with patients to accomplish shared treatment goals. |
| Team-based Care with Non-Physician Providers Titrating Medications (12) | Collaborative provision of care for hypertension by at least two providers, including a non-physician team member who titrates medications, working collaboratively with patients to accomplish shared treatment goals. |
Health coaching strategies could be delivered in-person or by telephone at multiple individual or group sessions over the intervention period. The strategies are patient-centered with a component of behavioral self-monitoring. A health coach (case manager, nurse, medical assistant or community health worker) and patients worked together using self-discovery or active learning processes to improve medication adherence and lifestyle modification (10). Provider-level strategies aim to improve BP management performance of healthcare professionals primarily responsible for patient hypertensive care. Multilevel implementation strategies are aimed at overcoming barriers to hypertension control at two or more levels of patients, providers, health care systems, and communities. Team-based care is characterized by inter-professional collaboration, a patient-centered approach, and an integrated care process (12). In this meta-analysis, team-based care implementation strategies involve task-shifting or task-sharing from primary care physicians in hypertension patient care to nurses, pharmacists, or community health workers. Team-based care is divided into two categories depending on whether or not the non-physician provider can titrate medications. The multilevel strategies without team-based care category includes any intervention that targets more than one level of barriers to BP control, but does not include team-based care, such as patient health coaching combined with provider training. Multicomponent strategies are those that combine more than one approach regardless of barrier level.
Trials were included if their control groups were either usual care or minimal education. Usual care is defined as hypertension management conducted by patients’ normal care providers with no trial intervention. Minimal education includes the provision of educational materials or a brief educational session to either patients or providers.
The Cochrane Risk of Bias tool was modified to make it applicable to cluster trials in implementation research and used for assessing trial quality (13). In this meta-analysis, we have focused on the following domains for study quality assessment: random sequence generation, objective outcome assessment (blinding of BP observers or use of automatic BP cuffs), incomplete outcome data, and selective outcome reporting. Participant recruitment bias was also considered for cluster-randomized trials. Funding sources for all trials were also recorded. Risk of bias assessments were done at the trial level.
Data Synthesis and Analysis
For each trial the net change in mean BP and associated standard error was calculated from available data and defined as the difference (intervention minus control) in the changes of mean values (follow-up minus baseline). If BP was measured at multiple time points during follow-up, the measurements taken closest to the end of the intervention were used. In addition, the changes in mean BP and associated standard errors in each randomized group were calculated separately for comparing effects among implementation strategies.
Random effects models using the Sidik-Jonkman residual heterogeneity estimator with the Knapp-Hartung small sample adjustment were used to calculate pooled mean differences within implementation categories using inverse variance weighting (14–16). In some trials, multiple intervention arms were compared to the same reference group. In these cases, robust variance estimation was used to account for non-independent estimates (17). Heterogeneity was evaluated using the Q test and quantified with the I2 index and 95% confidence interval calculated using the test-based method (18). Publication bias was assessed using Begg’s rank correlation test and Egger’s weighted linear regression test for implementation strategies with at least ten studies due to low statistical power with small sample sizes. When possible publication bias was observed, the trim-and-fill method was used to estimate the number of missing studies not published, augment the data to make the funnel plot more symmetrical, and calculate a summary estimate based on the augmented data (19).
Generalized estimating equations (with an exchangeable correlation matrix between estimates within a study) were used to compare BP reductions associated with each implementation strategy after important covariate adjustment and for pairwise comparisons between implementation strategies. Indicator variables were used for each implementation strategy category with the common control group as the reference. Weights for these models were exported from a random effects meta-analysis including all changes in mean BP and associated standard errors from all treatment groups. As such, these weights take into account within and between trial variance. Trial was treated as a cluster to maintain randomized comparisons, and trial-level baseline characteristics were adjusted: logit-transformed proportion male, centered mean age, centered mean systolic BP, centered trial duration, and whether the control group was usual care or minimal education. A sensitivity analysis was conducted including only trials where all participants had uncontrolled hypertension at baseline.
Analyses were conducted using packages metafor, robumeta, and forestplot in R version 3.3.2 (R Project for Statistical Computing) and PROC GENMOD in SAS version 9.4 (SAS Institute, Cary, NC).
Role of the Funding Source
This work was supported in part by the National Institute of General Medical Sciences under award number P20GM109036 and by the National Heart, Lung, and Blood Institute under award number U01HL114197. The funding sources had no role in the design, conduct, or reporting of the study or the decision to publish the manuscript.
Results
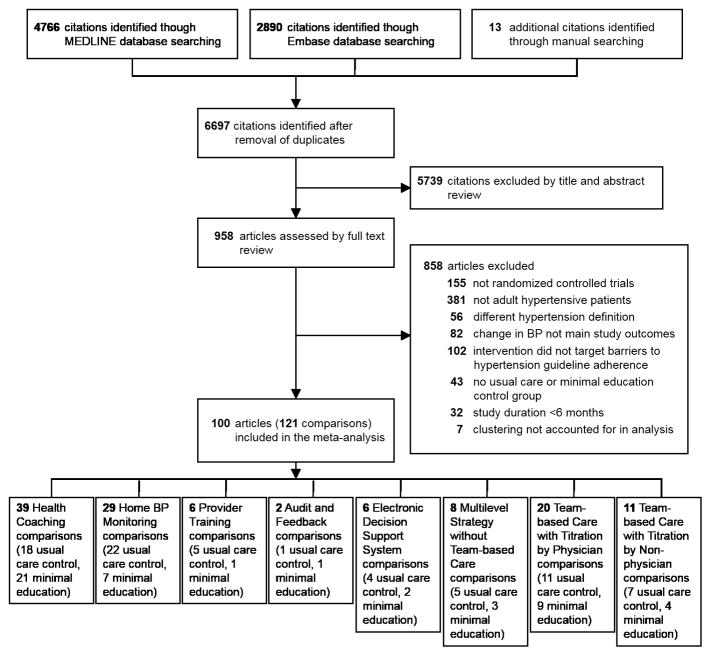
After excluding duplicates, our search strategy identified 6,697 references, of which 958 underwent full text review (Figure 1). In total, 100 articles reporting 121 comparisons with 55,920 participants were included (Appendix Table 3). The median of the study-specific mean ages was 60 years (range: 33–77), and the median of the study-specific mean baseline systolic and diastolic BP were 148 mmHg (range: 124–190) and 86 mmHg (range: 70–105). Trials ranged in length from 6 months to 5 years (median: 6 months). The number of comparisons per implementation category ranged from 38 for health coaching to 2 for audit and feedback (Table 2). Among all trials, none were identified at high risk of bias for random sequence generation, three for objective outcome assessment, 13 for incomplete outcome data, two for selective reporting, and one for recruitment bias (Appendix Tables 4 and 5). Of the 88 studies reporting funding information, 17% reported receiving full or partial funding from pharmaceutical firms. The rest were funded from federal, state, and local governments, foundations, and universities.
Figure 1.
Flowchart of Study Selection
Table 2.
Summary Characteristics of Trials by Implementation Strategy
| Implementation Strategy | No. of Studies* | No. of Participants | Mean Age Range, years | Sex Range, % of Male | Mean Baseline SBP Range, mmHg | Mean Baseline DBP Range, mmHg | Duration | Study Design | BP Measurement Methods | Control Categories | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. of Measurements | Type of Device | ||||||||||
| Patient-level | |||||||||||
|
| |||||||||||
| Health Coaching | 39 | 10,656 | 33–74 | 0–100 | 124–181 | 70–105 | 6 months – 2 years | 97% parallel RCTs (30% cluster-randomized); 3% Factorial RCTs | one visit, 2–6 measurements (n=19); two visits, 2 measurements each (n=1); unknown (n=18) | automated (n=15), standard mercury (n=8), automated or standard mercury (n=1), random zero (n=2), unknown (n=12) | 46% usual care, 54% minimal education |
| Home Blood Pressure Monitoring | 29 | 7,966 | 47–77 | 21–92 | 126–170 | 72–104 | 6 months – 2 years | 100% parallel RCTs (8% cluster-randomized) | one visit, 2–6 measurements (n=15); two visits, 3 measurements each (n=1); nine visits, 1 measurement each (n=1); daytime ambulatory (n=1); routine clinic measurements (n=1); unknown (n=7) | automated (n=13), standard mercury (n=2), random zero (n=1), aneroid (n=1), ambulatory (n=1), device used in clinic (n=3), unknown (n=5) | 76% usual care, 24% minimal education |
|
| |||||||||||
| Provider-level | |||||||||||
|
| |||||||||||
| Provider Training | 6 | 17,642 | 53–67 | 35–47 | 127–153 | 74–96 | 6 months – 2 years | 100% parallel RCTs (100% cluster-randomized) | one visit, 3 measurements (n=2); 24-hour ambulatory (n=1); routine clinic measurements (n=1); unknown (n=2) | automated (n=2), ambulatory (n=1), device used in clinic (n=1), unknown (n=2) | 83% usual care, 17% minimal education |
| Audit and Feedback | 2 | 2,121 | 61–62 | 54–64 | 133–146 | 73–89 | 6 months – 2 years | 100% parallel RCTs (100% cluster-randomized) | two visits, 2 measurements each (n=1); unknown (n=1) | automated (n=1), unknown (n=1) | 50% usual care, 50% minimal education |
| Electronic Decision Support System | 6 | 8,229 | 54–69 | 22–97 | 136–158 | 75–89 | 6 – 18 months | 100% parallel RCTs (100% cluster-randomized) | one visit, 2 measurements (n=1); routine clinic measurements (n=4); unknown (n=1) | automated (n=3), device used in clinic (n=2), unknown (n=1) | 67% usual care, 33% minimal education |
|
| |||||||||||
| Multilevel | |||||||||||
|
| |||||||||||
| Multilevel Strategy without Team-based Care | 8 | 3,436 | 53–67 | 30–100 | 133–169 | 73–95 | 6 months – 2 years | 100% parallel RCTs (75% cluster-randomized) | one visit, 2–3 measurements (n=5); two visits, 2 measurements each (n=1); unknown (n=2) | automated (n=6), standard mercury (n=1), unknown (n=1) | 63% usual care, 37% minimal education |
| Team-based Care with Physicians Titrating Medications | 20 | 6,680 | 47–68 | 21–99 | 127–162 | 76–93 | 6 – 18 months | 100% parallel RCTs (30% cluster-randomized) | one visit, 2–4 measurements (n=8); two visits, 3 measurements each (n=1); 24-hour ambulatory (n=1); routine clinic measurements (n=2); unknown (n=5) | automated (n=7), standard mercury (n=5), random zero (n=1), aneroid (n=1), device used in clinic (n=2), unknown (n=1) | 55% usual care, 45% minimal education |
| Team-based Care with Non-Physician Providers Titrating Medications | 11 | 3,417 | 41–68 | 31–100 | 136–174 | 76–99 | 6 months – 5 years | 100% parallel RCTs (18% cluster-randomized) | one visit, 1 measurement (n=1); one visit, 2–3 measurements (n=8); two visits, 3 measurements each (n=1); routine clinic measurements (n=1) | automated (n=6), standard mercury (n=2), random zero (n=1), device used in clinic (n=1), unknown (n=1) | 64% usual care, 36% minimal education |
A total of 121 comparisons from 100 publications and 55,920 participants are included.
Sixteen publications contribute more than one comparison because they have multiple treatment arms.
Effects of implementation strategies
All five patient-level and multilevel implementation strategies were associated with significant reductions in systolic BP (Appendix Figure 1). Health coaching and home BP monitoring significantly reduced systolic BP by −4.3 mmHg (95% CI: −5.9, −2.6; p<0.001) and −2.2 mmHg (95% CI: −3.5, −1.0; p=0.001), respectively. The multilevel strategies without team-based care reduced systolic BP by −3.9 mmHg (95% CI: −6.5, −1.3; p=0.003). Team-based care with physicians and non-physician providers titrating medications had the largest pooled mean systolic BP reductions of −5.7 mmHg (95% CI: −7.9, −3.6; p<0.001) and −6.6 mmHg (95% CI: −9.0, −4.2; p<0.001), respectively. Strategies targeting provider-level barriers to BP control did not significantly reduce BP compared to the control group. Some evidence of publication bias was observed for health coaching (Egger p=0.27; Begg p=0.051) and team-based care with physicians titrating medications (Egger p=0.146; Begg p=0.020). However, trim-and-fill analysis showed that publication bias did not account of the observed associations for health coaching [−4.3 mmHg (95% CI: −6.1, −2.6; p<0.001)] or for team-based care with physicians titrating medications [−4.2 mmHg (95% CI: −6.5, −1.8; p<0.001)]. In addition, a search of clinicaltrials.gov identified 191 trials potentially eligible for inclusion in these analyses. Of these, only three (one home BP monitoring and two health coaching) met our inclusion criteria, completed primary outcome collection more than two years ago, and had not reported results. This suggests reported results are not attributed to publication bias.
Similar results were observed for diastolic BP (Appendix Figure 2). Health coaching [−1.9 mmHg (95% CI −2.8, −1.0; p<0.001)], home BP monitoring [−1.5 mmHg (−2.0, −1.0; p<0.001)], team-based care with titration by a physician [−2.5 mmHg (−3.9, −1.1; p=0.002)], and team-based care with medication titration by a non-physician provider [−3.5 mmHg (−4.6, −2.5; p<0.001)] were all associated with a significant reduction in diastolic BP compared to the control group. Multilevel strategies without team-based care [−2.7 mmHg (−6.0, 0.6; p=0.114)] was not significantly associated with significant diastolic BP reduction. Provider training, audit and feedback, and electronic decision support systems were also not associated with a significant decline in diastolic BP.
Comparative effectiveness of implementation strategies
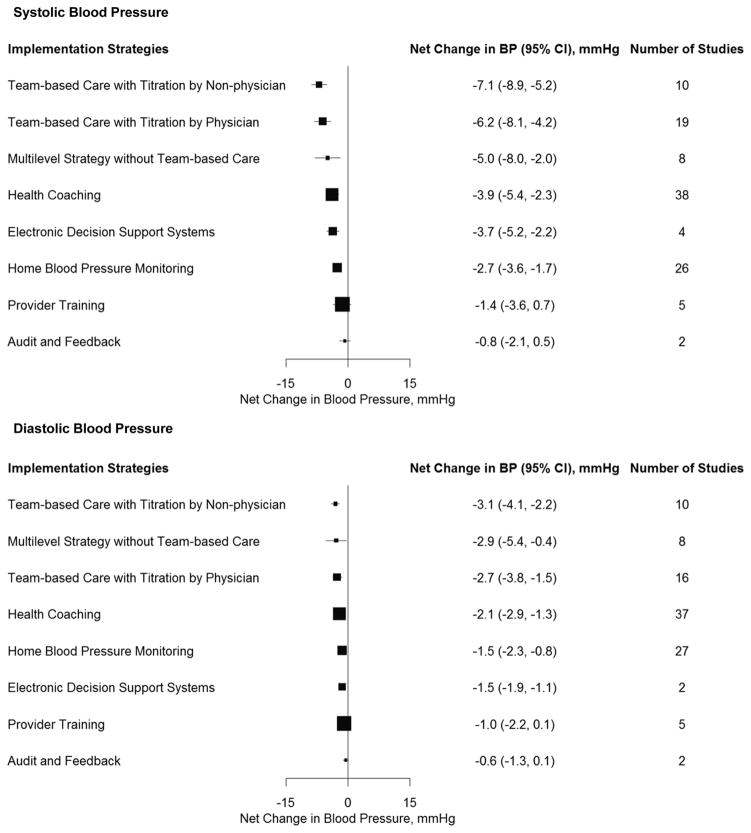
After adjustment for important covariates and all implementation strategies simultaneously using generalized estimating equations, the three multilevel strategies were the most effective for systolic BP reduction (Figure 2). Team-based care with medication titration by a non-physician [−7.1 mmHg (95% CI: −8.9, −5.2; p<0.001)] had the greatest reduction in systolic BP, followed by team-based care with medication titration by a physician [−6.2 mmHg (95% CI: −8.1, −4.2; p<0.001)], and multilevel strategies without team-based care [−5.0 mmHg (95% CI: −8.0, −2.0; p=0.001)]. The patient-level strategies of health coaching [−3.9 mmHg (95% CI: −5.4, −2.3; p<0.001)] and home BP monitoring [−2.7 mmHg (95% CI: −3.6, −1.7; p<0.001)] were also significantly associated with reductions in systolic BP. After multivariate adjustment, electronic decision support systems [−3.7 mmHg (95% CI: −5.2, −2.2; p<0.001)] was associated with a significant systolic BP reduction, but provider training, and audit and feedback were not. Likewise, team-based care with medication titration by a non-physician [−3.1 mmHg (95% CI: −4.1, −2.2; p<0.001)] had the greatest reduction in diastolic BP, followed by multilevel strategies without team-based care [−2.9 mmHg (95% CI: −5.4, −0.4; p=0.025)] and team-based care with medication titration by a physician [−2.7 mmHg (95% CI: −3.8, −1.5; p<0.001)]. The patient-level strategies of health coaching [−2.1 mmHg (95% CI: −2.9, −1.3; p<0.001)] and home BP monitoring [−1.5 mmHg (95% CI: −2.3, −0.8; p<0.001)] were also significantly associated with reductions in diastolic BP. Electronic decision support systems [−1.5 mmHg (95% CI: −1.9, −1.1; p<0.001)] was the only provider-level strategy associated with a significant reduction in diastolic BP. Similar results were observed for patient-level and multilevel interventions when analyses included only trials where all participants had uncontrolled BP at baseline (Appendix Figure 3). There were insufficient studies meeting this criteria to estimate summary effects for audit and feedback and provider training.
Figure 2. Adjusted Mean Net Reduction in Blood Pressure Associated with Implementation Strategies.
Mean net reduction in systolic blood pressure (upper panel) and diastolic blood pressure (lower panel). Mean net reductions estimated using generalized estimating equations and adjusted for sex, age, baseline systolic (or diastolic) blood pressure, trial duration, type of control group, and all other intervention strategies. Boxes weighted by sample size.
Pairwise comparison of implementation strategies
Table 3 provides a pairwise comparison of the intervention strategies ordered by effect sizes of systolic BP reduction and adjusted for covariates. Team-based care with titration by a non-physician resulted in significantly greater systolic BP reductions than any of the patient-level and provider-level strategies ranging from −3.22 to −6.29 mmHg for systolic BP and significantly greater diastolic BP reductions than home BP monitoring and all the provider-level strategies ranging from −1.60 to −2.52 mmHg for diastolic BP. Team-based care with titration by a physician also resulted in a significantly greater reduction in systolic BP compared to all patient-level and provider-level strategies except health coaching.
Table 3.
Comparison of Systolic and Diastolic Blood Pressure Reduction among Implementation Strategies
| Adjusted Difference (95% Confidence Interval) in Mean Systolic Blood Pressure Reduction, mm Hg | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Difference (95% Confidence Interval) in Mean Diastolic Blood Pressure Reduction, mm Hg | Team-based care with titration by non-physician | −0.88 (−3.58, 1.80) | −2.05 (−5.53, 1.43) | −3.22 (−5.72, −0.72)* | −3.35 (−5.75, −0.96)† | −4.41 (−6.50, −2.32)‡ | −5.63 (−8.57, −2.69)‡ | −6.29 (−8.52, −4.05)‡ |
| −0.48 (−1.95, 0.99) | Team-based care with titration by physician | −1.16 (−4.73, 2.41) | −2.34 (−4.86, 0.18) | −2.47 (−4.42, −0.52)* | −3.52 (−5.56, −1.49)‡ | −4.74 (−7.66, −1.83)† | −5.40 (−7.71, −3.09)‡ | |
| −0.29 (−2.84, 2.26) | 0.19 (−2.55, 2.93) | Multilevel strategy without team-based care | −1.17 (−4.47, 2.13) | −1.30 (−4.60, 1.99) | −2.36 (−5.48, 0.76) | −3.58 (−8.02, 0.86) | −4.24 (−6.26, −2.22)‡ | |
| −1.08 (−2.29, 0.14) | −0.60 (−2.03, 0.84) | −0.79 (−3.26, 1.69) | Health coaching | −0.13 (−2.28, 2.02) | −1.19 (−3.00, 0.63) | −2.41 (−4.71, −0.11)* | −3.06 (−5.04, −1.09)† | |
| −1.68 (−2.64, −0.72)‡ | −1.19 (−2.09, −0.30)† | −1.39 (−3.85, 1.08) | −0.60 (−1.48, 0.28) | Electronic decision support system | −1.06 (−2.78, 0.66) | −2.28 (−4.93, 0.38) | −2.93 (−4.81, −1.06)† | |
| −1.60 (−2.71, −0.48)† | −1.12 (−2.50, 0.27) | −1.31 (−3.87, 1.25) | −0.52 (−1.62, 0.58) | 0.08 (−0.76, 0.91) | Home blood pressure monitoring | −1.22 (−3.61, 1.18) | −1.88 (−3.48, −0.28)* | |
| −2.12 (−3.57, −0.68)† | −1.64 (−3.28, 0.00) | −1.83 (−4.61, 0.95) | −1.04 (−2.35, 0.27) | −0.44 (−1.65, 0.77) | −0.52 (−1.90, 0.85) | Provider training | −0.66 (−3.60, 2.28) | |
| −2.52 (−3.54, −1.51)‡ | −2.04 (−3.40, −0.67)† | −2.23 (−4.21, −0.25)* | −1.44 (−2.36, −0.53)† | −0.84 (−1.57, −0.12)* | −0.92 (−1.92, 0.07) | −0.40 (−1.73, 0.93) | Audit and feedback | |
Adjusted for sex, age, baseline systolic blood pressure (or diastolic blood pressure), trial duration, and type of control group. Strategies are ordered by rankings of net reduction in mean systolic blood pressure. Blood pressure reduction differences are in the cell in common between the row-defining and column-defining implementation strategy. For the mean difference in systolic blood pressure, the difference is the row strategy blood pressure reduction minus the column strategy blood pressure reduction. For the mean difference in diastolic blood pressure, the difference is the column strategy minus the row strategy. For example, the differences in mean systolic and diastolic blood pressure reduction between team-based care with titration by a non-physician and health coaching are −3.22 (95% CI: −5.72 to −0.72) and −1.08 (−2.29 to 0.14), respectively.
P <0.05;
P<0.01; and
P<0.001
Conclusions
Our findings indicate that implementation strategies targeting multiple-level or patient-level barriers are effective for BP reduction. Specifically, team-based care with and without a non-physician team member titrating medications and multilevel strategies without team-based care were the most effective implementation strategies for hypertension control. In addition, patient health coaching and home BP monitoring were also effective.
These findings have important public health implications. Despite strong evidence that antihypertensive medications and lifestyle modifications reduce BP and subsequent cardiovascular disease morbidity and mortality, hypertension control rates are low worldwide (5). The US Institute of Medicine and the National Heart Lung and Blood Institute have both called for research focusing on integrating evidence-based strategies into routine health care for hypertension control (20,21). Our findings provide evidence that multilevel, multicomponent implementation strategies are most useful and should be recommended in clinical practice and public health policy for hypertension control in communities.
Two previous meta-analyses of intervention strategies for BP reduction reviewed studies published up to 2003 and 2008 and showed that a number of implementation strategies, including team-based care and home BP monitoring, compared to the control group improved hypertension control (6,7). Since 2008 through September 2017 (MEDLINE search), meta-analyses for some individual implementation strategies have been published (8,22–25), but none that included all implementation strategies for BP control. Our study expanded on the previous meta-analyses by including many trials published since 2008. Moreover, our meta-analysis is the first study to directly compare the effectiveness of various implementation strategies on BP control after adjusting for key trial and participant characteristics. Generalized estimating equations using studies as clusters allowed for comparisons of intervention strategies while still preserving individual study randomization.
Team-based care strategies, where hypertension management responsibilities are shared among team members (i.e., nurses, pharmacists, medical assistants, or community health workers) in addition to primary care physicians, were found to be most effective for BP control in our analyses. Santschi and colleagues reported that compared with usual care, pharmacist-led interventions showed greater reductions in systolic (7.6 mmHg, 95% CI: 6.3 to 9.0) and diastolic BP (3.9 mmHg, 95% CI: 2.8 to 5.1) in a meta-analysis of randomized controlled trials (22). In addition, Clark and colleagues reported that compared with usual care, nurse-led interventions with a nurse prescribing medications showed greater reductions in systolic (8.9 mmHg, 95% CI: 5.3 to 12.5) and diastolic BP (4.0 mmHg, 95% CI: 2.7 to 5.3) in a meta-analysis of four trials (23). Team-based care is particularly effective because it frees physicians’ time to focus on urgent and complex cases, while allowing for patient-centered care that is tailored, frequent, and collaborative (28). Taken together, our findings and those from previous research provide strong evidence that team-based care is an effective approach for BP control among hypertensive patients (24,27,28).
Among the included trials reporting positive findings, pharmacist-led team-based care often includes provider training, health coaching, and/or home BP monitoring, in addition to task-sharing by pharmacists (29–34). Likewise, nurse-led team-based care usually includes health coaching and/or home BP monitoring (35,36). Community health worker-led team-based strategies typically include health coaching, home BP monitoring, and provider training (37). Multilevel implementation strategies without team-base care commonly consist of health coaching, home BP monitoring, and/or provider training (38,39). In some multilevel intervention trials, pharmacists conducted medication titration, health coaching, and/or home BP monitoring independent of the primary care team (40). Clearly, multilevel, multicomponent strategies, combining team-based care, health coaching, home BP monitoring, and provider training, have been proven to be the most effective strategy for BP control among patients with hypertension.
Our findings also showed that health coaching and home BP monitoring alone resulted in significant BP reduction among hypertensive patients. Health coaching is effective for behavioral change, including lifestyle modification and antihypertensive medication adherence (41). Therefore, in settings where multilevel strategies are not feasible due to limited resources, health coaching, especially when combined with home BP monitoring, might be an effective alternative for BP control. Future studies testing whether health coaching plus home BP monitoring provides a cost-effective approach could help to inform BP control strategies in populations with health disparities.
A few trials tested strategies targeting only physician-level barriers to hypertension control (i.e., provider training, audit and feedback, and electronic decision support systems), and only electronic decision support systems was significantly association with BP reduction after multivariate adjustment while contributing only four trials to the analysis. Although provider-level strategies had limited effect on their own, these intervention strategies were commonly a part of multilevel, multicomponent strategies shown to be effective. For example, Veterans Affairs medical centers and Kaiser Permanente have seen improvements in BP control among their patients after adopting multilevel strategies that included audit and feedback and electronic decision support systems (42–44). Due to the limited number of trials available in this category, the positive findings for electronic decision support systems after adjustment, and the effective use of these interventions as part of multicomponent interventions, future clinical trials are needed to test additional physician-targeted implementation strategies, such as physician-patient communication which could improve patient engagement and adherence to hypertension treatment (45,46).
Our analyses have several limitations. First, despite the inclusion of a large number of trials in this meta-analysis, some implementation strategies did not have sufficient numbers of studies. For example, provider training, audit and feedback, electronic decision support systems, and multilevel strategies without team-based care all had less than 10 comparisons. Second, very few multilevel intervention trials addressed system-level barriers (i.e., lack of performance standards, leadership commitment, and reimbursement of physician-to-patient health coaching). These factors could have a substantial impact on BP control among patients with hypertension and should be evaluated in future studies. Third, few clinical trials tested the effect of implementation strategies for free or low cost medications or financial incentives on BP control. They did not meet our inclusion criteria and were not included in this meta-analysis. Fourth, there were insufficient studies conducted in subgroups of interest, such as patients with diabetes or chronic kidney disease, to estimate associations within these groups. Finally, only 20% of included trials were from low- and middle-income countries where uncontrolled hypertension is a serious public health problem. However, many studies included were conducted in low-income, ethnic minority, and other populations with health disparities in the US and other high-income countries. Furthermore, sixteen studies funded by the Global Alliance for Chronic Disease will help to partially fill this knowledge gap (37,47).
In order to translate these findings into routine clinical practice through scale-up and dissemination at the healthcare system level, additional research is needed on cost-effectiveness and sustainability of implementation strategies for BP control (20, 26). While some trials included in this meta-analysis conducted cost-effectiveness analyses (31, 33, 34, 37, 38, 48–52), there were not enough data for a systematic review. In addition, there were no long-term follow-up studies after trial completion to assess intervention sustainability.
In conclusion, multilevel, multicomponent implementation strategies with and without team-based care are most effective for BP control among patients with hypertension. In addition, health coaching and home BP monitoring targeting barriers at the patient level are also effective. These strategies should be disseminated and scaled up in clinical practices and public health programs to improve hypertension control in communities.
Supplementary Material
Acknowledgments
We would like to thank Dr. Lawrence J. Fine for critically reviewing the manuscript and providing insightful comments for improving this work, Dr. Barry Carter for providing additional information for this publication, Dr. Max Gordon for his help with R programming, and Ruisi Zhang for her help with data abstraction. We would also like to thank Alan Clerk, Yoriko Heianza, Arthur Fernandes, and Ayako Suzuki for translation assistance. The research reported here was supported in part by the National Institute of General Medical Sciences under award number P20GM109036 and by the National Heart, Lung, and Blood Institute under award number U01HL114197.
Financial Support: National Institute of General Medical Sciences (P20GM109036) and National Heart, Lung, and Blood Institute (U01HL114197).
References
- 1.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–82. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 3.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67:733–9. doi: 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 4.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 5.Mills KT, Rubinstein A, Irazola V, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the Hypertension Control Program in Argentina. Am J Med Sci. 2014;348:139–45. doi: 10.1097/MAJ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh JME, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Medical Care. 2006;44:646–57. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 7.Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J of Gen Pract. 2010 doi: 10.3399/bjgp10X544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omboni S, Guarda A. Impact of home blood pressure telemonitoring and blood pressure control: a meta-analysis of randomized controlled studies. Am J Hypertens. 2011;24:989–98. doi: 10.1038/ajh.2011.100. [DOI] [PubMed] [Google Scholar]
- 9.Tao D, Xie L, Wang T, et al. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21:3–13. doi: 10.1177/1357633X14541041. [DOI] [PubMed] [Google Scholar]
- 10.Wolever RQ, Simmons LA, Sforzo GA, et al. A systematic review of the literature on health and wellness coaching: defining a key behavioral intervention in healthcare. Glob Adv Health Med. 2013;2:38–57. doi: 10.7453/gahmj.2013.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan WV, Pearson TA, Bennett GC, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI Implementation Science Work Group. Circulation. 2017;135:e122–37. doi: 10.1161/CIR.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 12.Baik D. Team-based care: a concept analysis. Nurs Forum. doi: 10.1111/nuf.12194. Epub 2016 Dec 9. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidik K, Jonkman JN. A note on variance estimation in random effects meta-regression. J Biopharm Stat. 2005;15:823–38. doi: 10.1081/BIP-200067915. [DOI] [PubMed] [Google Scholar]
- 15.Sidik K, Jonkman JN. Simple heterogeneity variance estimation for meta-analysis. J Royal Stat Soc. 2005;54:367–84. [Google Scholar]
- 16.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 17.Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015;20:375–93. doi: 10.1037/met0000011. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:15–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Whelton PK, Einhorn PT, Muntner P, et al. National Heart, Lung, and Blood Institute Working Group on Research Needs to Improve Hypertension Treatment and Control in African Americans. Research Needs to Improve Hypertension Treatment and Control in African Americans. Hypertension. 2016;68:1066–72. doi: 10.1161/HYPERTENSIONAHA.116.07905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee on Public Health Priorities to Reduce and Control Hypertension in the U.S. Population, Institute of Medicine. A population-based policy and systems change approach to prevent and control hypertension. Washington DC: National Academy Press; 2010. [PubMed] [Google Scholar]
- 22.Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718. doi: 10.1161/JAHA.113.000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748–55. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal R, Bills JE, Hecht TJW, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911. [DOI] [PubMed] [Google Scholar]
- 26.Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse, and teamwork in hypertension therapy. J Clin Hypertens (Greenwich) 2012;14:51–65. doi: 10.1111/j.1751-7176.2011.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50:S34–44. doi: 10.1016/j.amepre.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N, Goedken AM, Chrischilles EA, Carter BL. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178–87. doi: 10.1097/HJH.0000000000001126. [DOI] [PubMed] [Google Scholar]
- 29.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med. 2010;152:689–96. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966–72. doi: 10.1007/s11606-008-0791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235–43. doi: 10.1161/CIRCOUTCOMES.114.001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich) 2008;10:260–71. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudd P, Houston Miller N, Kaufman J, et al. Nurse management for hypertension: a systems approach. Am J Hypertens. 2004;17:921–7. doi: 10.1016/j.amjhyper.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Hebert PL, Sisk JE, Tuzzio L, et al. Nurse-led disease management for hypertension control in a diverse urban community: a randomized trial. J Gen Intern Med. 2011;27:630–9. doi: 10.1007/s11606-011-1924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J, Irazola V, Mills KT, et al. A cluster randomized trial of a comprehensive approach for hypertension control in low-income patients in Argentina. Circulation. 2016;134:e702–e20. [Google Scholar]
- 38.Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country. Ann Intern Med. 2009;151:593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Pladevall M, Brotons C, Gabriel R, et al. Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (The COM99 Study) Circulation. 2010;122:1183–91. doi: 10.1161/CIRCULATIONAHA.109.892778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart K, George J, McNamara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial) J Clin Pharm Ther. 2014;39:527–34. doi: 10.1111/jcpt.12185. [DOI] [PubMed] [Google Scholar]
- 41.Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns. 2014;97:147–57. doi: 10.1016/j.pec.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Sim JJ, Handler J, Jacobsen SJ, et al. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. 2014;30:544–52. doi: 10.1016/j.cjca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher RD, Amdur Rl, Kolodner R, et al. Blood pressure control among veterans: a large multiyear analysis of blood pressure data from the Veterans Administration health data repository. Circulation. 2012;125:2462–8. doi: 10.1161/CIRCULATIONAHA.111.029983. [DOI] [PubMed] [Google Scholar]
- 44.Shaw KM, Handler J, Wall HK, Kanter MH. Improving blood pressure control in a large multiethnic California population through changes in health care delivery, 2004–2012. Prev Chronic Dis. 2015;11:140173. doi: 10.5888/pcd11.140173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011;26:1297–1304. doi: 10.1007/s11606-011-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jolles EP, Clark AM, Braam B. Getting the message across: opportunities and obstacles in effective communication in hypertension care. J Hypertens. 2012;30:1500–10. doi: 10.1097/HJH.0b013e32835476e1. [DOI] [PubMed] [Google Scholar]
- 47.Tobe SW. The Global Alliance for Chronic Diseases supports 15 major studies in hypertension prevention and control in low- and middle-income countries. J Clin Hypertens. 2016;18:600–5. doi: 10.1111/jch.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy. 2001;21:1337–44. doi: 10.1592/phco.21.17.1337.34424. [DOI] [PubMed] [Google Scholar]
- 49.Soghikian K, Casper SM, Fireman BH, et al. Home blood pressure monitoring: effect on use of medical services and medical care costs. Med Care. 1992;30:855–65. [PubMed] [Google Scholar]
- 50.Anchala R, Kaptoge S, Pant H, et al. Evaluation of effectiveness and cost-effectiveness of a clinical decision support system in managing hypertension in resource constrained primary health care settings: results from a cluster randomized trial. J Am Heart Assoc. 2015;4:3001213. doi: 10.1161/JAHA.114.001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parati G, Omboni S, Albini F, et al. Home blood pressure telemonitoring improves hypertension control in general practice. The TeleBPCare study. J Hypertens. 2009;27:198–203. doi: 10.1097/hjh.0b013e3283163caf. [DOI] [PubMed] [Google Scholar]
- 52.McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control hypertension (TASMINH2): a randomized controlled trial. Lancet. 2010;376:163–72. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.