Dear Editor
Diabetic retinopathy (DR) is characterized by retinal vascular damage, visual impairment and blindness, but the involvement of retinal neurons in characteristics of DR is now gaining attention. Investigators have reported conflicting results with respect to the effects of diabetes on survival of neuronal cell-types in the retina. There is general agreement that long-standing diabetes leads to a reduction in the number of retinal ganglion cells in diabetes, but the effect of diabetes on retinal photoreceptor cells is less clear. Some investigators have presented evidence that there is some or substantial photoreceptor cell degeneration in diabetic rodents within only months after the induction of diabetes (Énzsöly et al., 2014; Tang et al., 2011; Bogdanov et al., 2014), whereas others have found little or no evidence of generalized loss of photoreceptors in diabetic mice or patients after even longer durations of disease (Liu et al., 2016; Hombrebueno et al., 2014; Tan et al., 2015; Gerhardinger et al., 1998).
We previously conducted 5 year studies of diabetic dogs to investigate the roles of glycemic control (Howell et al., 2013; Engerman and Kern, 1987) or experimental therapies (Kern and Engerman, 2001; Engerman and Kern, 1993) on the development of diabetic retinopathy, and showed that PC or MC diabetes in dogs resulted in characteristic vascular lesions of DR, which were alleviated when insulin was administered in amounts adequate to achieve good glycemic control (Engerman and Kern, 1987; Engerman et al.,1977). Likewise, axonal processes in the optic nerve (which was interpreted as indicating a reduction in the retinal ganglion cells) was subnormal in PC diabetics, but not in GC diabetics (Howell et al., 2013). We now report the effect of long-term diabetes and glycemic control in some of these animals on thickness of the retina and photoreceptor layers.
Alloxan-diabetic dogs were randomly assigned to poor glycemic control (PC), moderate glycemic control (MC), or good glycemic control (GC), and the appropriate level of glycemia was achieved by adjusting insulin and food amounts (Howell et al., 2013). All animals were maintained at their designated levels of glycemic control for the full 5 year period. Using archived retinal tissue (collected from the mid-retinal region of the nontapetal (inferior) nasal retina and equidistant between the optic disc and the peripheral edge of the retina) that had been fixed and embedded in epon from these prior studies, we cut 2µm thick sections from 2 to 3 blocks of retina from representative animals. Sections were stained with toluidine blue, and thickness of the total retina as well as individual cell layers was measured by light-microscopy using computer-assisted methods. All experiments had been conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research and with approval from the IACUC. Because of the small number of animals, data were analyzed using Mann Whitney (non-parametric test), but for the convenience of readers, data are graphically provided as mean ± standard deviation. Differences were considered statistically significant at *P < 0.05.
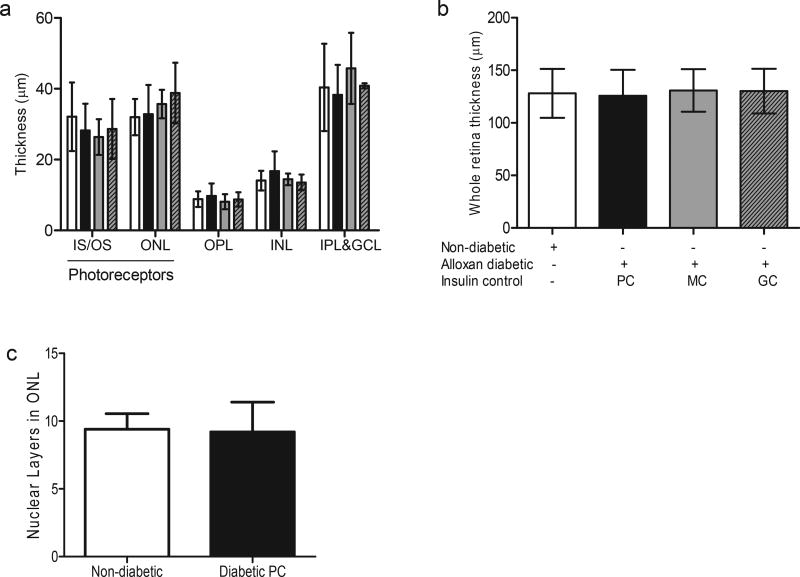
Even in the poorest glycemic control, dogs diabetic for 5 years did not exhibit any evidence of a reduction in thickness of photoreceptor layers (i.e. inner and outer segments and outer nuclear layer of photoreceptor cells) compared to age-matched non-diabetic dogs (Fig. 1A), and in fact failed to show any evidence of a reduction in those cells (Fig. 1C). Consistent with this, diabetic dogs under moderate (MC) or good (GC) glycemic control for 5 years likewise did not show evidence of reduced thickness of photoreceptor layers (Fig. 1A), in contrast to the marked beneficial effect of GC to inhibit retinal vascular pathology (Engerman et al., 1977). The thickness of other retinal layers (i.e. outer plexiform layer, inner nuclear layer, and inner plexiform & ganglion cell layers) and total retina likewise did not change as a result of 5 years of insulin-deficient diabetes (Fig. 1A and B).
Fig. 1. Diabetes of 5 years duration does not result in photoreceptor cell loss in dogs.
Thickness of retinal regions (a), and the total retina (b) after 5 years of diabetes in dogs. Nuclear layers in the photoreceptor ONL were also compared between the non-diabetic and diabetic groups (c). All measurements were taken in the mid-retinal region of the nontapetal (inferior) nasal retina. There were no statistically significant differences noted between the diabetic and the non-diabetic group in all the data presented. n = 6 animals in the non-diabetic and diabetic PC groups, n = 3 for other groups. IS/OS, inner segment/outer segment of photoreceptors; ONL, outer nuclear layer (photoreceptors); OPL, outer plexiform layer; INL, inner nuclear layer; IPL&GCL, inner plexiform and ganglion cell layers. White bars are non-diabetic animals, black bars are alloxan diabetic animals with poor glycemic control, grey bars are alloxan diabetic animals with moderate insulin control, and grey bars with hatch lines are alloxan diabetic animals with good insulin control.
The previously reported loss of axons in the optic nerve (which was interpreted as indicating a reduction in the retinal ganglion cells) (Howell et al., 2013), seems not to be associated with a reduction in retinal thickness based on the present data. We acknowledge that a limitation of the present study is that only a single area of retina was evaluated (due to limited archived retinal tissue) for our evaluation of retinal and photoreceptor thickness.
We demonstrate that in contrast to the retinal microangiopathy and degeneration of retinal ganglion axons that developed in this same group of PC diabetic dogs, the severe and chronic hyperglycemia did not result in any evidence of photoreceptor cell loss or shortening of the photoreceptor outer segments. Thus, this evidence suggests that retinal vascular lesions that developed in dogs diabetic for 5 years developed despite the absence of significant degeneration of photoreceptor cells. Our previous studies in mice likewise showed no loss of photoreceptor cells after 8 months of diabetes, a time at which early retinal capillary degeneration and vascular permeability are demonstrable (Liu et al., 2016). Even though diabetes induces stress in photoreceptor cells (e.g. increased superoxide production and inflammation) (Du et al., 2013; Tonade et al., 2016) which contributes to retinal vascular damage in diabetes (Liu et al., 2016; Du et al., 2013; Tonade et al., 2016; de Gooyer et al., 2006), degeneration of the photoreceptor cells seems not necessary to induce the retinal vascular damage characteristic of DR.
Acknowledgments
Funding
The studies reported in this manuscript were supported by grants from the National Eye Institute of the National Institutes of Health under awards R01EY00300, R01EY022938, and R24EY021126; and a Merit grant from the Department of Veterans Affairs.
Tissue used in these studies are from archived samples generated originally with R.L. Engerman (University of Wisconsin e Madison).
Footnotes
Duality of interest
No conflict of interest.
Contributing statement
D.T. performed the experiment, D.T. and T.S.K. wrote and revised the manuscript.
Contributor Information
Deoye Tonade, Department of Pharmacology, Case Western Reserve University, Cleveland OH 44106, United States.
Timothy S. Kern, Department of Pharmacology, Case Western Reserve University, Cleveland OH 44106, United States; Department of Medicine, Case Western Reserve University, Cleveland OH 44106, United States; Veterans Administration Medical Center Research Service, Cleveland, OH 44106, United States.
References
- Bogdanov P, et al. The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One. 2014;9(5):e97302. doi: 10.1371/journal.pone.0097302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooyer TE, et al. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47(12):5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. U.S.A. 2013;110(41):16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Aldose reductase inhibition fails to prevent retinopathy in diabetic and galactosemic dogs. Diabetes. 1993;42(6):820–825. doi: 10.2337/diab.42.6.820. [DOI] [PubMed] [Google Scholar]
- Engerman R, Bloodworth JM, Nelson S. Relationship of microvascular disease in diabetes to metabolic control. Diabetes. 1977;26(8):760–769. doi: 10.2337/diab.26.8.760. [DOI] [PubMed] [Google Scholar]
- Énzsöly A, et al. Pathologic alterations of the outer retina in streptozotocin-induced diabetes. Investig. Opthalmology Vis. Sci. 2014;55(6):3686. doi: 10.1167/iovs.13-13562. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, et al. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am. J. Pathol. 1998;152(6):1453–1462. [PMC free article] [PubMed] [Google Scholar]
- Hombrebueno JR, Chen M, Penalva RG, Xu H. Loss of synaptic connectivity, particularly in second order neurons is a key feature of diabetic retinal neuropathy in the Ins2Akita mouse. PLoS One. 2014;9(5):e97970. doi: 10.1371/journal.pone.0097970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SJ, Mekhail MN, Azem R, Ward NL, Kern TS. Degeneration of retinal ganglion cells in diabetic dogs and mice: relationship to glycemic control and retinal capillary degeneration. Mol. Vis. 2013;19:1413–1421. [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes. 2001;50(7):1636–1642. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. Photoreceptor cells influence retinal vascular degeneration in mouse models of retinal degeneration and diabetes. Invest. Ophthalmol. Vis. Sci. 2016;57(10):4272–4281. doi: 10.1167/iovs.16-19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, et al. Cone-photoreceptor density in adolescents with type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 2015;56(11):6339–6343. doi: 10.1167/iovs.15-16817. [DOI] [PubMed] [Google Scholar]
- Tang L, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. (Maywood) 2011;236(9):1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonade D, Liu H, Kern TS. Photoreceptor cells produce inflammatory mediators that contribute to endothelial cell death in diabetes. Invest. Ophthalmol. Vis. Sci. 2016;57(10):4264–4271. doi: 10.1167/iovs.16-19859. [DOI] [PMC free article] [PubMed] [Google Scholar]