Abstract
Background
Little is known about ethnic differences in post-stroke depression in the United States. This study aims to estimate the prevalence of post-stroke depression (PSD) at 90 days after first-ever stroke, and to examine ethnic differences in the prevalence between Mexican Americans (MAs) and non-Hispanic Whites (NHWs).
Methods and Results
Stroke cases from 2011 to 2015 were identified from the Brain Attack Surveillance in Corpus Christi Project, a population-based stroke surveillance study in south Texas. Participants were interviewed at the onset of stroke (baseline interview) and roughly 90 days post-stroke (outcome interview). PSD was assessed by the Patient Health Questionnaire-8. Inverse probability weights were generated to account for differential attrition, and weighted logistic regression was used to investigate the association between ethnicity and PSD. The study sample consisted of 586 first-ever stroke patients who completed non-proxy baseline and outcome interviews and had depression assessment. Approximately 60% of them were MAs and 40% were NHWs. After accounting for attrition, the prevalence of depression at 90 days post-stroke was 30.4% for MAs (95% Confidence Interval [CI]=25.0%-35.9%), and 20.7% for NHWs (95% CI=15.7%-25.7%). The crude odds of PSD in MAs was 1.69 times greater than that in NHWs (95% CI=1.13-2.51). The odds ratio (OR) decreased by 23.6% after adjustment for education (OR=1.29; 95% CI=0.82-2.02), and was further attenuated with additional adjustment for other covariates.
Conclusions
MAs had a higher prevalence of PSD at 90 days than NHWs. The ethnic difference was explained by sociodemographic and health factors, especially low educational attainment.
Keywords: depression, disparities, ethnic, stroke, epidemiology
INTRODUCTION
Post-stroke depression (PSD) is prevalent but undertreated among stroke survivors.1, 2 It was estimated by a recent meta-analysis that approximately one-third of stroke patients manifest depressive symptoms during the five-year interval after stroke.3 Depression following stroke is associated with additional morbidity and mortality risks, and may interfere with stroke recovery.2
Notably, minority populations, including Hispanics, bear higher risks of stroke and more unfavorable stroke outcomes compared with whites,4–6 however, few studies have investigated ethnic differences in the burden of PSD, and existing studies have varied in terms of measurement of PSD, study design and data sources.7–10 A study using health services utilization data from a national cohort of veteran patients showed that Hispanics had lower PSD diagnosis rates based on the International Classification of Diseases-9th Revision codes for depressive disorders and prescription records for antidepressants.9 It is unclear whether this difference reflected a differential rate of diagnosis, which may be influenced by factors such as access to healthcare and health-seeking behaviors11 or represented disparities in the true disease burden of PSD, given the nature of administrative data.9, 12 In addition, disease severity and some demographic characteristics, such as education and marital status, are not available in administrative data, which limits the ability to control for these potential confounders in analyses using this data source.9
More recent community-based clinical trials overcome some of the above noted limitations using self-report data for ascertainment of PSD. A post hoc analysis of the Prevent Recurrent All-Inner city Stroke through Education (PRAISE) study found that the prevalence of depression on average two years after stroke was significantly higher among Hispanic stroke survivors than that of non-Hispanic survivors adjusted for social-demographic and clinical risks.7 In contrast, results of the Stroke Warning Information and Faster Treatment (SWIFT) study showed that Hispanic stroke patients were less likely to have PSD at one month after stroke than non-Hispanic Whites adjusted for social-demographic factors, stroke severity and history of stroke; however, the difference diminished after one year.8 Both of these studies were conducted in New York City, where the Hispanic population may not be representative of that in other regions of the country. As shown in the PRAISE study, more than half of the Hispanic participants were from Puerto Rico and almost two thirds were not English speaking,7 which limits the generalizability of the results to other Hispanic sub-groups. Additionally, clinical trial participants are usually highly selective due to the inclusion and exclusion criteria for the trial and individuals with depression may be less likely to enroll in trials resulting in an underestimation of PSD.
Selection bias and confounding are particular concerns for studies focused on the estimation of ethnic differences in PSD. Patients with severe post-stroke cognitive impairment and/or aphasia are at higher risk of depression,13 however, this sub-group has typically been excluded from studies due to barriers communicating and lack of appropriate instruments for assessing PSD in these individuals.7, 14, 15 Because minorities have a higher prevalence of such stroke sequelae,5 excluding this sub-group may lead to an underestimation of ethnic differences in the prevalence of PSD. Moreover, because stroke patients self-select to participate and drop out of research studies, using complete case analysis may yield biased estimates when the selection process is influenced by both ethnicity and unmeasured determinants of PSD.16 Additionally, limited data availability can result in unmeasured confounding from key variables, such as pre-stroke depression and functional and cognitive impairment, as these covariates are risk factors for PSD1, 2, 17 and have significant ethnic differences.5 Population-based studies, which aim to reduce selection bias and confounding, are needed to more thoroughly evaluate the presence and extent of ethnic differences in PSD.
The objectives of the present study were to (1) estimate the prevalence of PSD at 90 days after first-ever stroke, (2) examine ethnic differences in the prevalence of PSD, and (3) investigate factors that may explain the potential ethnic difference. We extend existing literature on the association of ethnicity and PSD in several critical ways including: (1) the use of a large, population-based stroke study conducted in a bi-ethnic population, focusing on Mexican Americans - a subgroup with worse stroke outcomes that has been understudied; (2) accounting for potential differential attrition due to aphasia and cognitive deficits; and (3) adjusting for important confounders of the ethnicity-PSD association.
METHODS
Study Setting
Data for the present study were obtained from the Brain Attack Surveillance in Corpus Christi (BASIC) Project, January 5th 2011 through December 31st 2015. BASIC is a population-based stroke surveillance study that captures stroke cases in the bi-ethnic community of Nueces County, Texas.18 Mexican Americans composed approximately three fifths of the population in 2000, followed by non-Hispanic Whites and other races. The Mexican American community is non-immigrant and representative of the broader Mexican American population in the state.18 Details of the study have been described elsewhere.18, 19 The data will not be made available to the public due to its restricted nature.
Study Participants
Stroke cases were identified among residents aged 45 and over during the study period using active and passive surveillance methods. Active ascertainment includes the daily screening of admission logs for stroke-related symptoms at all hospitals, and passive ascertainment includes the review of all hospital discharges for International Classification of Diseases, Ninth Revision (ICD-9) diagnoses in the range between 430 and 438.19 Possible cases were validated by stroke fellowship trained physicians blinded to race-ethnicity. Stroke patients were invited to participate in in-person interviews shortly after stroke occurrence (baseline interview) and approximately 90 days after the index stroke (outcome interview). Proxy interviews were conducted if participants were unable to communicate.
In the present study, the analytic sample consisted of 586 first-ever stroke patients who (1) were either Mexican American or non-Hispanic White; (2) survived 90 days after stroke; (3) completed non-proxy baseline and outcome interviews, from which data on pre-stroke depression history and PSD data were collected respectively; and (4) had assessment for depressive symptoms at the outcome interview. Figure 1 presents the sample construction process and sample attrition at different stages. Patients who survived 90 days after stroke but did not participate in the study (n=326), participated but did not have non-proxy baseline interview (n=233), or had non-proxy baseline interview but did not participate in the outcome interview (n=185) were excluded from the analytic sample, but included in the analytic process to account for potential differential attrition (Figure 1). Patient who died before 90 days post-stroke did not contribute to the point prevalence estimates at 90 days, and were therefore excluded from the study.
Figure 1.
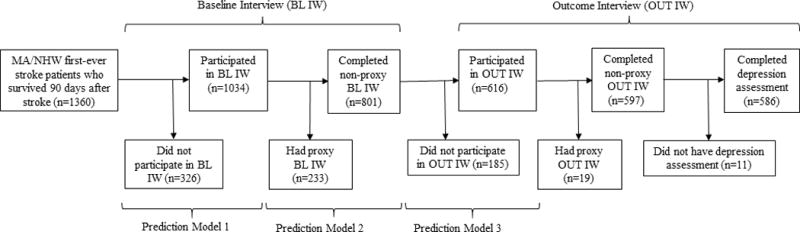
Study flow diagram, the Brain Attack Surveillance in Corpus Christi study, United States, 2011-2015. Abbreviations: BL IW, baseline interview; MA, Mexican Americans; NHW, non-Hispanic Whites; OUT IW, outcome interview.
Measures
Outcome Measure: Depression at 90 days after stroke
The depression assessment was conducted among participants who completed the outcome interview in person. Depression at 90 days after stroke was assessed by the Patient Health Questionnaire (PHQ). The nine-item PHQ (PHQ-9) was used for participants interviewed between 2011 and 2013, and the eight item PHQ (PHQ-8) was used for participants interviewed in 2014 and 2015 due to a protocol change. The PHQ-9 is a validated instrument based on the nine diagnostic criteria for depressive disorder from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), including depressed mood, anhedonia, sleep problems, fatigue, appetite or weight changes, self-esteem, concentration, psychomotor retardation or agitation and suicide ideation or self-injurious thoughts.20 Participants were asked to rate the frequency of having each symptom in the past two weeks on a four-point scale from “not at all” to “nearly every day”.21 A summative score was generated by calculating the total of the nine questions. The score ranges from 0 to 27, with higher scores indicating more depressive symptoms. The PHQ-8, also a commonly used depression scale,21 differs from PHQ-9 in that it does not include the item on thoughts about death and self-harm (item 9), which was used to assess suicide risk. The score therefore ranges from 0 to 24. Both PHQ-9 and PHQ-8 have been validated in English and Spanish.22, 23
To make the depression measure consistent, we converted the summative scores of PHQ-9 for participants interviewed between 2011 and 2013 to PHQ-8 scores by subtracting the endorsed score of the item 9 from the total score. We dichotomized the continuous measure by classifying patients scoring ten or greater as having current depression versus no depression. This scoring threshold, shared by PHQ-9 and PHQ-8,21, 24 demonstrates high sensitivity and specificity for detecting depression among stroke survivors.22
Primary Independent Variable: Ethnicity
Information on race and ethnicity was ascertained from the baseline interview and medical records. Self-report data were considered as the primary data source, and medical records data were used only when self-report was not available. Among 1,034 participants who completed the baseline interview, 99.1% self-reported race and ethnicity. Only Mexican Americans and non-Hispanic Whites were included in the present analysis. Other race-ethnic groups were excluded due to the small sample size.
Covariates
Three sets of variables were evaluated as potential confounders, including demographic characteristics, pre-stroke factors and stroke characteristics. All covariates were assessed or ascertained at baseline.
Demographic characteristics included age, sex (male, female), educational attainment (less than high school, high school and above), marital status (married or living together, others including single, widow and divorced/separated) and insurance status (insured, uninsured).
Pre-stroke factors at baseline included pre-stroke depression (none, past depression history, current antidepressant use), cognitive function (normal, cognitive impairment no dementia [CIND], dementia), functional disability (none, mild/moderate, severe), number of medical conditions, health-risk behaviors (current smoking [Yes, No] and excessive alcohol use [Yes, No]) and obesity (Yes, No). A participant was classified as having a past depression history if he or she reported having ever been diagnosed with depression or taken antidepressants but not taking antidepressants at stroke onset. Cognition was measured by the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) which was completed by someone who knew the patient well.25 Participants were classified as having normal cognitive function (IQCODE≤3), CIND (3<IQCODE<3.44) or dementia (IQCODE≥3.44).26 Pre-stroke disability was assessed by the Modified Rankin Scale (MRS),27 which ranges from 0 to 5. We generated a three-level ordinal variable by collapsing adjacent categories of the MRS: (1) no symptoms or disability (0-1), (2) slight or moderate disability (2-3), and (3) moderately severe or severe disability (4-5). Information on medical conditions and excessive alcohol use was ascertained from medical records (Supplemental material). Number of medical conditions was generated a summative variable indicating the total number of comorbidities a patient had at the time of stroke occurrence (range, 0-12). Obesity was identified based on body mass index (BMI) calculated using participants’ weights and heights. Participants with BMI equal to or greater than 30 were classified as obese.
Stroke characteristics included stroke type (ischemic stroke, intracerebral hemorrhage stroke) and stroke severity. Stroke severity measured using the National Institutes of Health Stroke Scale (NIHSS), which ranges from 0 to 42 (higher scores indicating more severe impairment), was ascertained from medical records or calculated using previously validated methods.28
Statistical Analysis
We examined differences in baseline sample characteristics by ethnicity using Chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables, and compared participants who remained in the study with those who did not. We explored missing data patterns, and dealt with missing data using an approach combining multiple imputation and inverse probability weighting methods.29 We used multiple imputation, specifically the fully conditional specification method, to fill in missing data from the baseline interview (Supplemental material). In the study sample, the percentage imputed was 13.1% for the IQCODE score (n=77), 4.4% for current smoking (n=26), 1.5% for pre-stroke depression (n=9), 1.4% for MRS (n=8) and less than 1.0% for NIHSS scores (n=3) and stroke type (n=1). For missing data due to loss to follow-up or inability to complete non-proxy interviews, we used an inverse probability weighting approach with bootstrapping to account for potential differential attrition. To reflect the entire stroke sample, we up-weighted participants in the analytic sample who were similar to those not included in this sample for the various reasons outlined in Figure 1.16 Weights were constructed as the reciprocal of the product of three probabilities: 1) probability of participating in the baseline interview, 2) probability of having a non-proxy baseline interview and 3) probability of completing the 90-day outcome interview (Supplemental material). Stabilized weights were constructed by multiplying the non-stabilized weights by probabilities generated from a nested model with a subset of covariates (age, sex or ethnicity, depending on the prediction model).16 Weights were trimmed at the 1st and 99th percentiles, resulting in overall weights ranging from 0.61 to 3.65.
We estimated the overall and ethnicity-specific 90-day prevalence of depression after first-ever stroke using cross-tabulations. To examine the ethnic difference in the prevalence of PSD, we first fit a weighted logistic regression model to examine the crude association. We then added each potential confounder to the crude model in separate models. The impact of each confounder on the ethnic difference was examined using the percent change in the crude and adjusted odds ratios (calculated by subtracting the adjusted odds ratio from the crude odds ratio and then dividing by the crude odds ratio). We compared the magnitude of the impact of the confounders on the association between ethnicity and PSD, and selected variables that had more than 5% change and/or were associated with the outcome to fit a full model. Covariates selected for the full model included age, education, marital status, pre-stroke disability, pre-stroke depression, number of medical conditions, current smoking and obesity at baseline. PROC SURVEYSELECT was used to perform 1000 bootstrap replications, and the bootstrap standard errors were used to compute 95% confidence intervals. PROC LOGISTIC was used to examine the racial-ethnic difference in prevalence of PSD, with a WEIGHT statement to apply the overall inverse probability weights.
To compare approaches with and without adjustment for attrition, we repeated the above steps in a complete case analysis without application of multiple imputation and the inverse probability weighting. To examine the potential difference in using PHQ-9 and PHQ-8, we conducted a sensitivity analysis by repeating the main analysis among 2011–2013 participants using PHQ-9 and PHQ-8 respectively.
All statistical analyses were completed with SAS version 9.4 (SAS Institute) and Stata version 14.2 (StataCorp). The BASIC study was approved by the institutional review boards at the University of Michigan and the two local hospital systems. Written informed consent was obtained from all patients.
RESULTS
Among 1,360 Mexican American or non-Hispanic white first-ever stroke patients who survived 90 days after stroke, 1,034 (76.0%) agreed to participate in the baseline interview, and 801 (77.5%) had a non-proxy interview. A total of 616 out of 801 participants (76.9%) completed the outcome interview three months after stroke occurrence, with 597 (96.9%) interviewed in person. Eleven out of the 597 participants (1.8%) did not have depression assessment, which yielded a sample of 586 participants who completed both baseline and outcome interviews in person (Figure 1).
Characteristics of eligible patients who refused to participate, participants who had proxy interview at baseline and participants who did not complete the outcome interview at follow-up are presented in Supplemental Tables S1-S3 respectively. Compared to patients who agreed to participate in the BASIC study, eligible patients who refused to participate were more likely to be non-Hispanic Whites, have lower NIHSS scores and have congestive heart failure (all P<0.05). Compared to participants who had non-proxy baseline interviews, those with proxy interviews were more likely to be older, Mexican Americans and insured, have lower educational attainment, have more severe stroke (intracerebral hemorrhage stroke, higher NIHSS scores and higher scores of the Modified Rankin Scale), have poor cognitive function (higher scores of the IQCODE, history of Alzheimer’s disease and dementia), and have more medical conditions (congestive heart failure, hypertension) (all P<0.05). Compared with participants who completed the outcome interview, those lost to follow-up were more likely to be younger, male and uninsured (all P<0.05).
Baseline characteristics of the study sample are presented in Table 1. The mean age was 65.9 years (Standard Deviation [SD] = 11.1 years). Approximately 48.6% were females, 29.2% had educational attainment below high school, 51.2% were either married or living with a partner, 11.4% were uninsured. In terms of stroke characteristics, 92.0% had ischemic stroke, and the mean NIHSS was 4.0 (SD =4.6). Approximately half of the sample was classified as no pre-stroke disability by the MRS (51.6%), and half was classified as having normal cognitive function by the IQCODE (51.9%). Compared with non-Hispanic Whites, Mexican Americans were younger (P<0.001), had lower levels of education (P<0.001), more medical conditions (P=0.068), and were more likely to have intracerebral hemorrhage (P=0.033), be uninsured (P=0.069) and obese (P=0.002). When asked about pre-stroke depression, 15.9% reported a past depression history before onset of stroke but not on medication at the time of the baseline interview, and 17.5% were on antidepressants at stroke onset. There was no statistically significant ethnic difference in pre-stroke depression, in terms of self-reported diagnosis and medication use (P=0.297).
Table 1.
Baseline sample characteristics by ethnicity of 586 participants from the Brain Attack Surveillance in Corpus Christi study, United States, 2011-2015.
| Total (n=586) |
MA (n=351) |
NHW (n=235) |
P Value | |
|---|---|---|---|---|
| Age, mean (SD) | 65.9 (11.1) | 64.6 (10.8) | 67.9 (11.1) | <0.001 |
| Sex | 0.289 | |||
| Male | 301 (51.4) | 174 (49.6) | 127 (54.0) | |
| Female | 285 (48.6) | 177 (50.4) | 108 (46.0) | |
| Education, N (%) | <0.001 | |||
| Below high school | 171 (29.2) | 155 (44.2) | 16 (6.8) | |
| High school | 163 (27.8) | 86 (24.5) | 77 (32.8) | |
| Vocational/some college | 167 (28.5) | 87 (24.8) | 80 (34.0) | |
| College or more | 85 (14.5) | 23 (6.6) | 62 (26.4) | |
| Marital status, N (%) | 0.697 | |||
| Married or living together | 300 (51.2) | 182 (51.9) | 118 (50.2) | |
| Single/separated/divorced | 286 (48.8) | 169 (48.1) | 117 (49.8) | |
| Health insurance, N (%) | 0.069 | |||
| Insured | 519 (88.6) | 304 (86.6) | 215 (91.5) | |
| Uninsured | 67 (11.4) | 47 (13.4) | 20 (8.5) | |
| Stroke type*, N (%) | 0.033 | |||
| Ischemic stroke | 538 (92.0) | 315 (90.0) | 223 (94.9) | |
| Intracerebral hemorrhage stroke | 47 (8.0) | 35 (10.0) | 12 (5.1) | |
| Stroke severity (NIHSS)*, mean (SD) | 4.0 (4.6) | 4.0 (4.3) | 3.9 (5.1) | 0.297 |
| Pre-stroke disability (MRS)*, N (%) | 0.159 | |||
| No symptoms or disability (0-1) | 298 (51.6) | 172 (50.0) | 126 (53.9) | |
| Slight/moderate disability (2-3) | 254 (43.9) | 152 (44.2) | 102 (43.6) | |
| Moderately severe/severe disability (4-5) | 26 (4.5) | 20 (5.8) | 6 (2.6) | |
| Cognitive function (IQCODE)*, N (%) | 0.104 | |||
| Normal (0-3) | 304 (51.9) | 196 (55.8) | 108 (46.0) | |
| CIND (3.01-3.43) | 156 (26.6) | 86 (24.5) | 70 (29.8) | |
| Dementia (≥3.44) | 49 (8.4) | 29 (8.3) | 20 (8.5) | |
| Missing | 77 (13.1) | 40 (11.4) | 37 (15.7) | |
| Pre-stroke depression*, N (%) | 0.297 | |||
| None | 384 (66.6) | 231 (66.6) | 153 (66.5) | |
| Past depression history† | 92 (15.9) | 50 (14.4) | 42 (18.3) | |
| Antidepressant use at stroke onset | 101 (17.5) | 66 (19.0) | 35 (15.2) | |
| Number of medical conditions, mean (SD) | 2.4 (1.5) | 2.5 (1.5) | 2.3 (1.5) | 0.068 |
| Health-risk behaviors, N (%) | ||||
| Current smoking* | 143 (25.5) | 85 (25.0) | 58 (26.4) | 0.718 |
| Excessive alcohol use | 49 (8.4) | 26 (7.4) | 23 (9.8) | 0.308 |
| Obesity | 0.002 | |||
| Yes | 243 (41.5) | 164 (46.7) | 79 (33.6) | |
| No | 343 (58.5) | 187 (53.3) | 156 (66.4) |
Abbreviations: CIND, cognitive impairment no dementia; IQCODE, the Informant Questionnaire on Cognitive Decline in the Elderly; MA, Mexican Americans; MRS, Modified Rankin Scale; NHW, non-Hispanic Whites; NIHSS, the National Institutes of Health Stroke Scale; SD, standard deviation.
Variables with missing data. The numbers of missing values are 1 for stroke type, 3 for NIHSS, 8 for Modified Rankin Scale, 77 for IQCODE, 9 for history of depression and antidepressant use at baseline, and 26 for current smoking.
A participant was classified as having a past depression history if he or she reported having ever been diagnosed with depression or taken antidepressants, but not taking antidepressants at stroke onset.
The mean score of PHQ-8 at 90 days was 7.16 for Mexican Americans and 5.54 for non-Hispanic Whites (P=0.009). Without adjustment for attrition, the prevalence of depression (PHQ-8 ≥10) at 90 days after first-ever stroke was 26.5% in the bi-ethnic sample (95% Confidence Interval [CI], 22.9%-30.2%), 21.3% among non-Hispanic Whites (95% CI, 16.2%-27.1%), and 29.9% among Mexican Americans (95% CI, 25.2%-35.0%). The crude odds of PSD in Mexican Americans was 1.58 times greater than that in non-Hispanic Whites (95% CI, 1.07-2.33). After adjustment for attrition, the ethnic difference in the prevalence estimates was widened slightly (non-Hispanic Whites: prevalence rate, 20.7%; 95% CI, 15.7%-25.7%; Mexican Americans: prevalence rate, 30.4%; 95% CI, 25.0%-35.9%), and the crude odds ratio of PSD increased to 1.69 (95% CI, 1.13-2.51).
In the models adjusted for each covariate individually, the odds ratio of PSD for Mexican Americans compared to non-Hispanic Whites decreased by 23.6%, 8.9% and 5.3% after adjustment for education (odds ratio [OR] for ethnicity, 1.29; 95% CI, 0.82, 2.02), age (OR for ethnicity, 1.54; 95% CI, 1.02-2.31) and obesity (OR for ethnicity, 1.60; 95% CI, 1.07-2.39) respectively (Table 2, Figure 2). There was marginal change with adjustment for current smoking, pre-stroke disability, number of medical conditions, pre-stroke cognitive function, stroke type, marital status and insurance status, and almost no change after adjustment for excessive alcohol use, sex, stroke severity and pre-stroke depression respectively.
Table 2.
Results from logistic regression models of the association between ethnicity and prevalence of post-stroke depression adjusted for each covariate individually (n=586), the Brain Attack Surveillance in Corpus Christi study, United States, 2011-2015.
| Adjusted for attrition | Not adjusted for attrition | |||
|---|---|---|---|---|
| Ethnicity OR (95% CI) |
Individual covariate OR (95% CI) |
Ethnicity OR (95% CI) |
Individual covariate OR (95% CI) |
|
| Not adjusted for covariates | ||||
| Ethnicity (crude) | 1.69 (1.13, 2.51) | – | 1.58 (1.07, 2.33) | – |
| Ethnicity adjusted for each covariate individually | ||||
| Age | 1.54 (1.02, 2.31) | 0.97 (0.95, 0.98) | 1.44 (0.97, 2.14) | 0.97 (0.95, 0.99) |
| Sex | 1.68 (1.13, 2.51) | 1.57 (1.06, 2.31) | ||
| Male | Reference | Reference | ||
| Female | 1.19 (0.82, 1.74) | 1.24 (0.86, 1.80) | ||
| Education | 1.29 (0.82, 2.02) | 1.20 (0.78, 1.85) | ||
| Below high school | 1.60 (0.96, 2.68) | 1.50 (0.91, 2.47) | ||
| High school | Reference | Reference | ||
| Vocational/some college | 0.99 (0.59, 1.67) | 1.00 (0.61, 1.65) | ||
| College or more | 0.60 (0.29, 1.27) | 0.51 (0.25, 1.05) | ||
| Insurance status | 1.67 (1.12, 2.49) | 1.56 (1.06, 2.29) | ||
| Insured | Reference | Reference | ||
| Uninsured | 1.24 (0.71, 2.14) | 1.35 (0.78, 2.35) | ||
| Marital status | 1.70 (1.14, 2.54) | 1.59 (1.08, 2.34) | ||
| Married or living together | Reference | Reference | ||
| Single/separated/divorced | 1.50 (1.02, 2.19) | 1.22 (0.84, 1.76) | ||
| Stroke type | 1.71 (1.14, 2.56) | 1.59 (1.08, 2.34) | ||
| Ischemic stroke | Reference | Reference | ||
| Intracerebral hemorrhage stroke | 0.81 (0.42, 1.55) | 0.99 (0.51, 1.95) | ||
| Stroke severity (log-transformed NIHSS)* | 1.69 (1.13, 2.52) | 1.13 (0.91, 1.39) | 1.53 (1.04, 2.26) | 1.25 (0.99, 1.57) |
| Pre-stroke disability (MRS)* | 1.63 (1.09, 2.45) | 1.51 (1.02, 2.25) | ||
| No symptoms or disability (0-1) | Reference | Reference | ||
| Slight/moderate disability (2-3) | 1.86 (1.24, 2.79) | 1.90 (1.28, 2.80) | ||
| Moderately severe/severe disability (4-5) | 2.77 (1.27, 6.07) | 3.31 (1.45, 7.58) | ||
| Cognitive function (IQCODE)* | 1.72 (1.15, 2.58) | 1.85 (1.20, 2.85) | ||
| Normal (0-3) | Reference | Reference | ||
| CIND (3.01-3.43) | 1.17 (0.74, 1.85) | 1.15 (0.74, 1.80) | ||
| Dementia (≥3.44) | 1.52 (0.81, 2.86) | 1.54 (0.80, 2.97) | ||
| Pre-stroke depression* | 1.69 (1.11, 2.58) | 1.57 (1.04, 2.37) | ||
| None | Reference | Reference | ||
| Past depression history | 2.64 (1.57, 4.44) | 2.53 (1.52, 4.22) | ||
| Antidepressant use at stroke onset | 5.56 (3.40, 9.10) | 5.73 (3.56, 9.21) | ||
| Number of medical conditions | 1.64 (1.10, 2.45) | 1.15 (1.02, 1.29) | 1.54 (1.04, 2.27) | 1.17 (1.04, 1.32) |
| Current smoking* | 1.75 (1.16, 2.64) | 1.57 (1.04, 2.35) | ||
| No | Reference | Reference | ||
| Yes | 2.72 (1.80, 4.13) | 2.71 (1.80, 4.08) | ||
| Excessive alcohol use | 1.69 (1.13, 2.52) | 1.59 (1.08, 2.34) | ||
| No | Reference | Reference | ||
| Yes | 1.22 (0.64, 2.35) | 1.16 (0.61, 2.24) | ||
| Obesity | 1.60 (1.07, 2.39) | 1.49 (1.01, 2.21) | ||
| No | Reference | Reference | ||
| Yes | 1.57 (1.07, 2.31) | 1.56 (1.07, 2.26) | ||
Abbreviations: 95% CI, 95% confidence interval; CIND, cognitive impairment no dementia; IQCODE, the Informant Questionnaire on Cognitive Decline in the Elderly; MRS, Modified Rankin Scale; NIHSS, the National Institutes of Health Stroke Scale; OR, Odds Ratio.
Variables with missing data. The sample sizes of the models not adjusted for attrition are 585 for stroke type, 583 for stroke severity, 578 for pre-stroke disability, 509 for cognitive function, 577 for pre-stroke depression, and 560 for current smoking.
Figure 2.
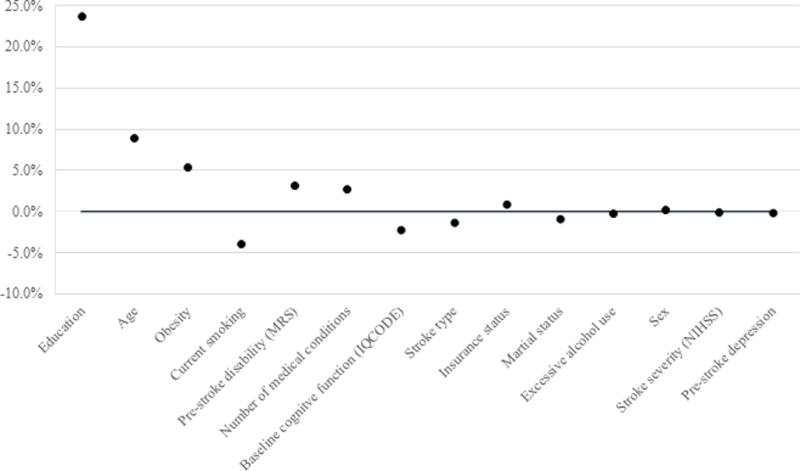
Effect of individual covariates on odds ratio of post-stroke depression for Mexican Americans compared to non-Hispanic Whites among 586 participants of the Brain Attack Surveillance in Corpus Christi study, United States, 2011-2015. Percent change was calculated by subtracting the adjusted odds ratio from the crude odds ratio and then dividing by the crude odds ratio. Abbreviations: IQCODE, the Informant Questionnaire on Cognitive Decline in the Elderly; MRS, Modified Rankin Scale; NIHSS, the National Institutes of Health Stroke Scale.
Results of the multivariable model adjusting for selected covariates and all covariates simultaneously are presented in Table 3 and Supplemental Table S5 respectively. The odds ratio of PSD for Mexican Americans compared to non-Hispanic Whites was attenuated and became non-significant after adjustment (Selected covariates: OR, 1.14; 95% CI, 0.68-1.90. All covariates: OR, 1.15; 95% CI, 0.68-1.95). Factors associated with greater odds of PSD were younger age, pre-stroke depression and current smoking at baseline (all P<0.05). The above results were in concordance with that from the complete case analysis (Tables 2 and 3).
Table 3.
Results from multivariable logistic regression of the association between ethnicity and prevalence of post-stroke depression adjusted for selected covariates, the Brain Attack Surveillance in Corpus Christi study, United States, 2011-2015.
| Adjusted for attrition (n=586) |
Not adjusted for attrition (n=551) |
|
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Ethnicity | ||
| Mexican Americans | 1.14 (0.68, 1.90) | 1.12 (0.68, 1.84) |
| Non-Hispanic Whites | Reference | Reference |
| Age | 0.97 (0.95, 0.99) | 0.98 (0.96, 1.00) |
| Education | ||
| Below high school | 1.61 (0.89, 2.89) | 1.31 (0.74, 2.32) |
| High school | Reference | Reference |
| Vocational/some college | 0.94 (0.53, 1.67) | 0.97 (0.55, 1.70) |
| College or more | 0.62 (0.27, 1.41) | 0.54 (0.24, 1.20) |
| Marital status | ||
| Married or living together | Reference | Reference |
| Single/separated/divorced | 1.23 (0.79, 1.90) | 0.99 (0.64, 1.52) |
| Pre-stroke disability (MRS) | ||
| No symptoms or disability (0-1) | Reference | Reference |
| Slight/moderate disability (2-3) | 1.36 (0.84, 2.19) | 1.37 (0.87, 2.17) |
| Moderately severe/severe disability (4-5) | 1.97 (0.78, 4.97) | 2.04 (0.77, 5.42) |
| Pre-stroke depression | ||
| None | Reference | Reference |
| Past depression history | 2.25 (1.28, 3.97) | 2.03 (1.16, 3.56) |
| Antidepressant use at stroke onset | 4.61 (2.66, 7.98) | 4.54 (2.67, 7.72) |
| Number of medical conditions | 1.14 (0.99, 1.31) | 1.13 (0.98, 1.31) |
| Current smoking | ||
| No | Reference | Reference |
| Yes | 2.32 (1.43, 3.76) | 2.51 (1.57, 4.01) |
| Obesity | ||
| No | Reference | Reference |
| Yes | 1.35 (0.86, 2.12) | 1.21 (0.77, 1.89) |
Abbreviations: 95% CI, 95% confidence interval; CIND, cognitive impairment no dementia; IQCODE, the Informant Questionnaire on Cognitive Decline in the Elderly; OR, Odds Ratio.
Among 301 participants interviewed with PHQ-9 between 2011 and 2013, Mexican Americans were more likely to have suicidal thoughts than non-Hispanic Whites, but the difference was not statistically significant (P=0.24). In the sensitivity analysis comparing PHQ-9 and PHQ-8, after adjustment for attrition the prevalence estimates were 30.7% for Mexican Americans (95% CI, 22.8%-38.5%) and 22.4% for non-Hispanic Whites (95% CI, 15.6%-29.2%) using PHQ-9, and 29.2% for Mexican Americans (95% CI, 21.6%-36.8%) and 20.6% for non-Hispanic Whites (95% CI, 13.9%-27.3%) using PHQ-8. The odds ratios of PSD comparing Mexican Americans with non-Hispanic Whites were 1.55 using PHQ-9 (95% CI, 0.89-2.70), and 1.61 using PHQ-8 (95% CI, 0.91-2.84).
DISCUSSION
This study examined prevalence of depression 90 days after first-ever stroke, and the association of ethnicity with the prevalence in a bi-ethnic, population-based sample in Nueces County, Texas, with adjustment for differential attrition. Among stroke survivors, Mexican Americans had a significantly higher prevalence of PSD at 90 days than non-Hispanic Whites, with nearly a third reporting PSD. The ethnic difference was widened after accounting for attrition, and attenuated when controlling for covariates, especially educational attainment.
The overall prevalence of PSD at 90 days in the present study was in concordance with the pooled prevalence estimates of PSD at 1–6 months in a meta-analysis.1 In terms of the ethnic difference in prevalence of PSD, we found that both the magnitude and significance of the association between ethnicity and PSD increased after adjustment for attrition. This result highlights the importance of accounting for selection bias, and supported our hypothesis that using complete cases underestimates ethnic differences in PSD. Mexican Americans have poorer post-stroke functional and cognitive outcomes than non-Hispanic Whites, therefore were less likely to complete an in-person interview and have depression assessment.5 As there is an ethnic difference in remaining in the study sample, restricting the sample to complete cases may result in bias due to “conditioning on continuation”.16 That is, when unmeasured factors associated with both continuation in the study and PSD exist, using a complete case analysis may induce a spurious association between ethnicity and the unmeasured factors and result in a downward bias in association between ethnicity and PSD.16 Although attrition due to death is common among stroke patients, it is unlikely a threat to validity in the present study, as findings from the same stroke population indicated that there were no significant differences in 30-day and 1-year mortality after ischemic stroke between Mexican Americans and non-Hispanic Whites in 2011.30
Our results showed that the observed ethnic difference in prevalence of PSD at 90 days was largely explained by education. Education levels differed significantly between Mexican Americans and non-Hispanic Whites in the study sample. The percentage of participants without high school education among Mexican Americans was approximately six times greater than that among non-Hispanic Whites. Evidence on the association between education and PSD is very limited.1 To the best of our knowledge, only two studies on ethnic differences in PSD or mental distress adjusted for educational attainment.8, 14 Our finding on the confounding influence of education is consistent with that from the National Health Interview Survey, which indicated that low levels of education partially accounted for the difference in prevalence of post-stroke mental distress between Hispanics and non-Hispanic Whites.14
Mechanisms of the association between education and PSD are unknown. We present three hypotheses. First, education is a proxy for socioeconomic status, which is associated with access to and quality of stroke treatment and post-stroke rehabilitation. Patients with low socioeconomic status are more likely to have unmet health needs due to lack of economic resources and adequate insurance, which may lead to brain deficits or functional impairments and increase risks for PSD.31 Second, education is also a proxy for cognitive reserve, which represents individuals’ capacity to resist brain pathology.32 Post-stroke cognitive impairment is common among stroke survivors,33 and has been recognized as a predictor of PSD.1 Patients with low educational attainment have less reserve to maintain cognitive function, and therefore bear higher risk for depression. Third, as low education is associated with depression history in Mexican Americans but not in non-Hispanic Whites,34 future studies may investigate the interaction between ethnicity and education on PSD.
We also found that the ethnic difference in prevalence of PSD at 90 days between Mexican Americans and non-Hispanic Whites was independent of pre-stroke depression history, a well-established risk factor for PSD.2 Findings from the National Health and Nutrition Examination Survey III showed that among participants aged 15 to 40 years, Mexican Americans had a lower lifetime prevalence rate of major depressive disorder assessed by the Diagnostic Interview Schedule than Whites.34 In our study, the self-reported lifetime prevalence of pre-stroke depression did not differ significantly between Mexican Americans and non-Hispanic Whites. When holding history of pre-stroke depression constant, Mexican Americans still had significantly higher odds of PSD than non-Hispanic Whites. However, the results should be interpreted with caution, as prevalence of depression history based on self-report can be influenced by recall bias, social disability bias and ethnic differences in depression awareness and help-seeking behaviors.
Our finding of an ethnic difference in prevalence of PSD may not be comparable to the few existing studies for the following reasons. First, depressive symptoms were assessed at different time points after index stroke. Research on natural history of PSD has showed that the prevalence rate changes over time, and it is not yet known if the natural history varies by ethnicity. In the PRAISE study, time since last stroke varied across study participants, and was approximately two years on average.7 In the SWIFT study, depressive symptoms were assessed at one month and 12 months after stroke respectively.8 Second, because the prevalence of depression after first-ever stroke may differ from that after recurrent stroke, we focused on first-ever stroke patients in order to exclude the possibility that preexisting depression was due to previous stroke events, which is different from the existing studies. Third, our study sample is from a population-based surveillance study, which may differ from highly selected participants in clinical trials.
The study has several limitations. First, depressive symptoms of participants with stroke onset in later years were assessed using PHQ-8, which does not include the item on thoughts of death and self-harm from PHQ-9. However, endorsement of this item largely represent passive thoughts about mortality as a consequence of stroke events, instead of suicidal ideation.22 Use of PHQ-8 is also supported by findings from the Heart and Soul Study that this item was not an accurate measure for suicide screening among patients with coronary artery disease.35 Second, our measure of pre-stroke depression may classify subjects with undiagnosed pre-stroke depression as not having depression, and thus is subject to measurement error. Third, there might be eligible patients not in the study sample that cannot be represented by the study participants, such as stroke patients with severe aphasia, and therefore cannot be accounted for by the inverse probability weighting approach. Fourth, because the Mexican Americans in the present study are non-immigrants, the results may not be generalized to recent Mexican immigrants in the US.
Despite the limitations, the study advances the literature by providing more valid estimates on the ethnic difference in prevalence of PSD using validated depression scales, applying the inverse probability weighting approach, and exploring the role of related factors in the ethnic difference. Our study suggests that low educational attainment accounts for a significant amount of the ethnic differences in PSD. In clinical settings, screening for PSD among stroke survivors should be a priority given the high prevalence in general, and should particularly target patients with low education levels or low socioeconomic status, who bear a disproportionate burden of PSD. Research should further understand the mechanism through which education influences PSD. Future research with longitudinal data may also examine potential ethnic differences in the natural history of PSD, which could be helpful for planning depression prevention, allocating healthcare resources, and tailoring treatment for both stroke and depression.
Supplementary Material
What is Known.
Depressive symptoms are prevalent among stroke survivors.
Compared with whites, minority populations in the United States bear higher risks of unfavorable stroke outcomes, which might translate into a higher prevalence of post-stroke depression but population-based studies are lacking.
What the Study Adds.
In this bi-ethnic, population-based study, Mexican Americans had higher prevalence of post-stroke depression at 90 days than non-Hispanic Whites.
Lower educational attainment contributed to the ethnic difference in prevalence of post-stroke depression at 90 days.
Selection bias due to differential attrition may lead to an underestimate of the association between ethnicity and post-stroke depression in complete-case analysis.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (grant numbers R01NS38916, R01NS070941).
Footnotes
Disclosures
None.
References
- 1.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. The British Journal of Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 2.Robinson RG, Jorge RE. Post-stroke depression: A review. American Journal of Psychiatry. 2015;173:221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 3.Hackett ML, Pickles K. Part i: Frequency of depression after stroke: An updated systematic review and meta‐analysis of observational studies. International Journal of Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 4.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al-Wabil A. Excess stroke in mexican americans compared with non-hispanic whites the brain attack surveillance in corpus christi project. American journal of epidemiology. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisabeth LD, Sánchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, Brown DL, Morgenstern LB. Neurological, functional, and cognitive stroke outcomes in mexican americans. Stroke. 2014;45:1096–1101. doi: 10.1161/STROKEAHA.113.003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisabeth LD, Smith MA, Brown DL, Moyé LA, Risser JM, Morgenstern LB. Ethnic differences in stroke recurrence. Annals of neurology. 2006;60:469–475. doi: 10.1002/ana.20943. [DOI] [PubMed] [Google Scholar]
- 7.Fei K, Benn EK, Negron R, Arniella G, Tuhrim S, Horowitz CR. Prevalence of depression among stroke survivors racial–ethnic differences. Stroke; a journal of cerebral circulation. 2016;47:512–515. doi: 10.1161/STROKEAHA.115.010292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldmann E, Roberts ET, Parikh NS, Lord AS, Boden-Albala B. Race/ethnic differences in post-stroke depression (psd): Findings from the stroke warning information and faster treatment (swift) study. Ethnicity & disease. 2015;26:1–8. doi: 10.18865/ed.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia H, Chumbler NR, Wang X, Chuang HC, Damush TM, Cameon R, Williams LS. Racial and ethnic disparities in post‐stroke depression detection. International journal of geriatric psychiatry. 2010;25:298–304. doi: 10.1002/gps.2339. [DOI] [PubMed] [Google Scholar]
- 10.Husaini B, Levine R, Sharp L, Cain V, Novotny M, Hull P, Orum G, Samad Z, Sampson U, Moonis M. Depression increases stroke hospitalization cost: An analysis of 17,010 stroke patients in 2008 by race and gender. Stroke research and treatment. 2013;2013 doi: 10.1155/2013/846732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care (full printed version) National Academies Press; 2002. [PubMed] [Google Scholar]
- 12.Docherty JP. Barriers to the diagnosis of depression in primary care. Journal of clinical psychiatry. 1997 [PubMed] [Google Scholar]
- 13.Kauhanen M-L, Korpelainen J, Hiltunen P, Määttä R, Mononen H, Brusin E, Sotaniemi K, Myllylä V. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovascular Diseases. 2000;10:455–461. doi: 10.1159/000016107. [DOI] [PubMed] [Google Scholar]
- 14.Skolarus LE, Lisabeth LD, Burke JF, Levine DA, Morgenstern LB, Williams LS, Pfeiffer PN, Brown DL. Racial and ethnic differences in mental distress among stroke survivors. Ethnicity & disease. 2015;25:138. [PMC free article] [PubMed] [Google Scholar]
- 15.van Dijk MJ, de Man-van Ginkel JM, Hafsteinsdóttir TB, Schuurmans MJ. Identifying depression post-stroke in patients with aphasia: A systematic review of the reliability, validity and feasibility of available instruments. Clinical rehabilitation. 2016;30:795–810. doi: 10.1177/0269215515599665. [DOI] [PubMed] [Google Scholar]
- 16.Weuve J, Tchetgen EJT, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, de Leon CFM. Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology (Cambridge, Mass) 2012;23:119. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutlubaev MA, Hackett ML. Part ii: Predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. International Journal of Stroke. 2014;9:1026–1036. doi: 10.1111/ijs.12356. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Risser JM, Moyé LA, Garcia N, Akiwumi O, Uchino K, Morgenstern LB. Designing multi-ethnic stroke studies: The brain attack surveillance in corpus christi (basic) project. Ethnicity and Disease. 2004;14:520–526. [PubMed] [Google Scholar]
- 19.Piriyawat P, Šmajsová M, Smith MA, Pallegar S, Al-Wabil A, Garcia NM, Risser JM, Moyé LA, Morgenstern LB. Comparison of active and passive surveillance for cerebrovascular disease the brain attack surveillance in corpus christi (basic) project. American journal of epidemiology. 2002;156:1062–1069. doi: 10.1093/aje/kwf152. [DOI] [PubMed] [Google Scholar]
- 20.Association AP, Association AP. Diagnostic and statistical manual of mental disorders (dsm) Washington, DC: American psychiatric association; 1994. pp. 143–147. [Google Scholar]
- 21.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The phq-8 as a measure of current depression in the general population. Journal of affective disorders. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Williams LS, Brizendine EJ, Plue L, Bakas T, Tu W, Hendrie H, Kroenke K. Performance of the phq-9 as a screening tool for depression after stroke. Stroke. 2005;36:635–638. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 23.Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital spanish inpatients. Psychosomatic Medicine. 2001;63:679–686. doi: 10.1097/00006842-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the patient health questionnaire (phq-9): A meta-analysis. Canadian Medical Association Journal. 2012;184:E191–E196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorm A, Jacomb P. The informant questionnaire on cognitive decline in the elderly (iqcode): Socio-demographic correlates, reliability, validity and some norms. Psychological medicine. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 26.Saposnik G, Kapral MK, Cote R, Rochon PA, Wang J, Raptis S, Mamdani M, Black SE. Is pre-existing dementia an independent predictor of outcome after stroke? A propensity score-matched analysis. Journal of neurology. 2012;259:2366–2375. doi: 10.1007/s00415-012-6508-4. [DOI] [PubMed] [Google Scholar]
- 27.Rankin J. Cerebral vascular accidents in patients over the age of 60: Ii. Prognosis. Scottish medical journal. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 28.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the nih stroke scale. Stroke; a journal of cerebral circulation. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 29.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical methods in medical research. 2013;22:278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 30.Morgenstern LB, Brown DL, Smith MA, Sánchez BN, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF. Loss of the mexican american survival advantage after ischemic stroke. Stroke. 2014;45:2588–2591. doi: 10.1161/STROKEAHA.114.005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grube MM, Koennecke H-C, Walter G, Thümmler J, Meisel A, Wellwood I, Heuschmann PU. Association between socioeconomic status and functional impairment 3 months after ischemic stroke. Stroke. 2012;43:3325–3330. doi: 10.1161/STROKEAHA.112.669580. [DOI] [PubMed] [Google Scholar]
- 32.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- 33.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment. Stroke; a journal of cerebral circulation. 2013;44:138–145. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- 34.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: Findings from the national health and nutrition examination survey iii. American journal of public health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The phq-9 versus the phq-8—is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the heart and soul study. Journal of psychosomatic research. 2012;73:163–168. doi: 10.1016/j.jpsychores.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


