Abstract
Context
Influenza vaccination rates remain below Healthy People 2020 goals. This project sought to systematically review economic evaluations of healthcare-based quality improvement interventions for improving influenza vaccination uptake among general populations and healthcare workers.
Evidence acquisition
The databases MEDLINE, Econlit, Centre for Reviews & Dissemination, Greylit, and Worldcat were searched in July 2016 for papers published from January 2004 to July 2016. Eligible studies evaluated efforts by bodies within the healthcare system to encourage influenza vaccination by means of an organizational or structural change. For each study, program costs per enrollee and per additional enrollee vaccinated were derived (excluding vaccine costs, standardized to 2017 U.S. dollars). Complete economic evaluations were examined when available.
Evidence synthesis
Of 2,350 records, 18 articles were eligible and described 29 unique interventions. Most interventions improved vaccine uptake. Among 23 interventions in general populations, the median program cost was $3.27 (interquartile range, $0.82–$11.53) per enrollee and $50.78 (interquartile range, $27.85–$124.84) per additional enrollee vaccinated. Among ten complete economic evaluations in general populations, three studies reported net cost savings, four reported costs <$50,000 per quality-adjusted life year, and three reported costs <$60,000 per life saved. Among six interventions in healthcare workers, the median program cost was $8.09 (interquartile range, $5.03–$10.31) per worker enrolled and $125.24 (interquartile range, $96.06–$171.38) per additional worker vaccinated (there were no complete economic analyses).
Conclusions
Quality improvement interventions for influenza vaccination involve per-enrollee costs that are similar to the cost of the vaccine itself ($11.78–$36.08/dose). Based on limited available evidence in general populations, quality improvement interventions may be cost saving to cost effective for the health system.
CONTEXT
Seasonal influenza causes substantial morbidity and mortality and imposes a large economic burden. In recent years, influenza infection-related hospitalizations in the U.S. ranged from a low of 140,000 (2011–2012) to a high of 710,000 (2014–2015) and influenza-associated deaths were as high as 56,000 per influenza season (2012–2013).1 The annual cost of influenza, including direct medical care and lost earnings, has been estimated at $26.7 billion (2003 U.S. dollars).2
Accordingly, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices recommends annual influenza vaccination for all individuals aged 6 months and older, noting that vaccination is particularly important for children aged 6 months to 5 years, people aged 50 years and older, pregnant women, and individuals with clinical risk factors.3 Vaccination is also strongly advised for healthcare workers to prevent transmission to patients.4 Despite these recommendations, influenza vaccination rates are often suboptimal, with fewer than half of Americans receiving the vaccine annually.5,6 In the U.S., major healthcare-based initiatives have established influenza vaccination rates as two measures of the quality of healthcare. For example, the Centers for Medicare and Medicaid Services publicly reports adherence to these measures for hospitals, physicians, home health agencies, and long-term care facilities and in some cases, links adherence to payment.7–9 Influenza vaccination rates among general populations are also included as a measure in The National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set, which compares the performance of private health plans.10
To increase vaccine coverage, hospitals, physician groups, and public and private payers can implement diverse healthcare-based quality improvement (QI) interventions. A QI intervention has been defined as “an effort to change/improve the clinical structure, process, or outcomes of care by means of an organizational or structural change.”11 QI interventions focus on improving care in routine clinical practice, rather than supplementing such care through vaccination initiatives in nonclinical settings.
Health authorities have recommended certain QI interventions for general populations and others for healthcare workers. For general populations, the Community Preventive Services Task Force recommends multicomponent QI interventions that enhance access to vaccination services (such as reduced out-of-pocket spending or expanded access in healthcare settings) and that increase vaccination prescribing and uptake (such as standing orders, audit, and feedback systems that notify practitioners of their patients’ vaccination rates, patient reminders, and patient education).12 In addition, the National Vaccine Advisory Committee has called for improved accessibility to vaccinations in both clinical and nonclinical locations, such as medical homes, workplaces, and community sites.13 To increase uptake among healthcare workers, these two bodies along with the Joint Commission recommend active promotion and provision of free vaccinations at the worksite.14–16 The National Vaccine Advisory Committee goes further, acknowledging that influenza vaccination as a condition of employment can be effective but recommending it only after other measures have been exhausted.14 Several published systematic reviews examining the effectiveness of QI interventions to promote influenza vaccination in both the general population and healthcare workers have found them to be generally successful.17–19
Prior reviews assessing the economic impact of influenza vaccination have found that vaccination itself is cost effective to cost saving in a variety of populations and settings.20–24 A 2017 review reported that influenza vaccination is usually cost saving in children and costs less than $50,000 per quality-adjusted life year (QALY) among the elderly and pregnant women.24 However, it is important to consider not only the cost of vaccination itself but also costs associated with QI interventions designed to increase vaccine uptake, such as start-up and maintenance costs.
Accordingly, this project sought to systematically review evaluations of the cost and cost effectiveness of QI interventions for improving systems of care, such that influenza vaccination is delivered more consistently in routine practice. Two target groups are included: general populations and healthcare workers. Peer-reviewed and non-peer-reviewed literature were searched to identify partial or complete economic evaluations that also reported clinical effectiveness. The nature of the interventions, their clinical effectiveness, the associated costs, and the quality of complete economic evaluations are examined.
EVIDENCE ACQUISITION
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.25 The study protocol is posted on the Prospero registry (CRD42015014950).26 An eight-member technical expert panel provided input during key phases of the project.
Data Sources and Searches
A reference librarian developed search terms related to influenza, and expanded on previously published terms related to economic evaluation (Appendix Text 1).27 Databases included MEDLINE, Econlit, and the Centre for Reviews & Dissemination Economic Evaluations. To identify grey literature, Greylit and Worldcat were searched and expert panelists were invited to suggest non-peer-reviewed analyses. The search was last performed in July 2016 and was limited to English-language publications or unpublished analyses from January 1, 2004 to July 26, 2016; the research team relied on a hand search of prior systematic reviews to identify earlier articles.
Study Selection
Studies were eligible if they represented original investigations, compared clinical effectiveness between alternatives (e.g., vaccine uptake in intervention versus status quo), and involved either partial or complete economic evaluations of one or more QI interventions. Partial economic evaluations included analyses that reported only program costs of interventions; complete economic evaluation analyses considered both program and influenza infection-related costs. Program costs were defined as costs associated with implementing the intervention, such as startup and maintenance costs. Influenza infection-related costs incorporated downstream costs associated with influenza infection, such as clinic visits, medications, and hospitalizations. Studies from low- to middle-income countries were excluded because of differences in care practices and cost structures.28 All types of clinical evaluation study designs, economic evaluation approaches, analytic perspectives, and time horizons were included.
Interventions had to be implemented by bodies within the healthcare system, including public or private healthcare payers, hospitals, physician practices, pharmacies, and nursing homes. This analysis also included government-initiated interventions that targeted publicly insured populations. Vaccination programs at worksites or schools were not considered to be QI interventions, unless they were implemented by a healthcare body.
Two reviewers independently examined titles, abstracts, and full-text publications to determine eligibility; discrepancies were resolved by consensus, or when necessary, through discussion with the research team.
Data Extraction and Quality Assessment
A second pair of investigators with training in economic evaluation extracted data. Discrepancies were resolved as described above.
For each study, reviewers extracted data related to the target populations (general populations and healthcare workers), nature of the intervention, context, study design, reporting of the clinical evaluation, funding source, and findings. Reviewers placed interventions that targeted general populations into two categories: 1) efforts by provider entities to promote vaccination among the patients they treat and 2) efforts by healthcare payers to promote vaccination among beneficiaries.
Contextual variables included the sponsoring institution’s academic status and location. Clinical study designs included RCT, non-RCT, controlled before–after analysis, uncontrolled before–after analysis, interrupted time series, and decision analytic models. Reviewers extracted vaccination rates in intervention and comparison groups. The reporting of the clinical evaluation was assessed using elements from the Minimum Quality Criteria Set (items 3–7, 10–11, 13), a tool for critically appraising the reporting of QI interventions.29 Funding sources included government, nonprofit, commercial, and none. Where applicable, potential bias in primary studies evaluating clinical effectiveness was assessed using the Cochrane Collaboration Tool for randomized controlled trials and the Newcastle-Ottawa Scale for nonrandomized studies.30,31 Bias assessments were not applied to decision model-based studies that were not based on a single primary study.
Investigators with training in economic evaluations extracted the economic evaluation approach (cost analyses including cost-consequences and business-case analyses versus cost-effectiveness, cost-benefit, and related analyses), entity bearing cost (i.e., perspective), time horizon, discount rate, year and currency of cost data, and program costs. For complete economic evaluations, reviewers also extracted the following: types of infection-related costs included, costs related to influenza infections, total incremental net costs, incremental cost-effectiveness ratios, and assumed vaccine efficacy.
The quality of economic evaluations for articles with complete economic analyses was assessed by applying a modified version of the Quality of Health Economics Studies Checklist (mQHES), which has demonstrated validity and reliability (Appendix Table 1).32–34 Questions address issues such as whether the study objective is clear, cost and effectiveness estimates are from the best sources, and effects of uncertainty and variability are described. Each question was divided into subparts for easier scoring; two questions related to the adequacy of competing alternatives and credibility of the analysis overall were added. To calculate the total mQHES score (scale 0–115) for each study the percentage of “yes” responses to subparts of each question was determined, multiplied by each question’s assigned weight, and then summed.
Data Standardization
To facilitate comparisons of costs across studies, this analysis examined the effectiveness and costs separately (i.e., a cost-consequences analysis was performed) for each study. The key clinical outcome was the difference in vaccination rates between control and intervention groups. When studies did not report the difference, it was calculated using rates in intervention and control groups. Because several studies had more than one intervention arm, the unit of analysis was the intervention rather than the individual study.
Key economic outcomes included the incremental program cost per enrollee and the incremental program cost per additional enrollee vaccinated. Program costs and when reported, infection-related and net costs were standardized per influenza season in 2017 U.S. dollars. This involved applying currency conversion and inflation factors. When standardizing program costs, costs related to efforts to increase uptake and deliver the vaccine were included. However, the cost of the vaccine itself was excluded because this varied across studies. When the cost of the vaccine was included in the program cost but the exact amount was not reported in the article, the CDC Influenza Price List35 was referenced.
Because of the number and diversity of eligible studies, a formal meta-analysis was not performed. To summarize overall effects, the median and interquartile range (IQR, the difference between the third and first quartiles in the distribution of values) were calculated for key outcome measures.
Subanalyses for key outcome measures were also performed, stratified on whether the authors of the studies had measured or modeled the outcomes because the former may be more accurate. Measured outcomes included those resulting from primary data collection methods, such as internal databases, accounting records, surveys, and chart reviews. Modeled outcomes reflected those produced by models built on assumptions from prior studies and outside literature.
Role of the Funding Source
The study was funded by the Agency for Healthcare Research and Quality (R01 HS22644-01), which did not participate in the study design, literature search, assessment of eligibility, data extraction and analysis, or interpretation of results.
EVIDENCE SYNTHESIS
Study Selection
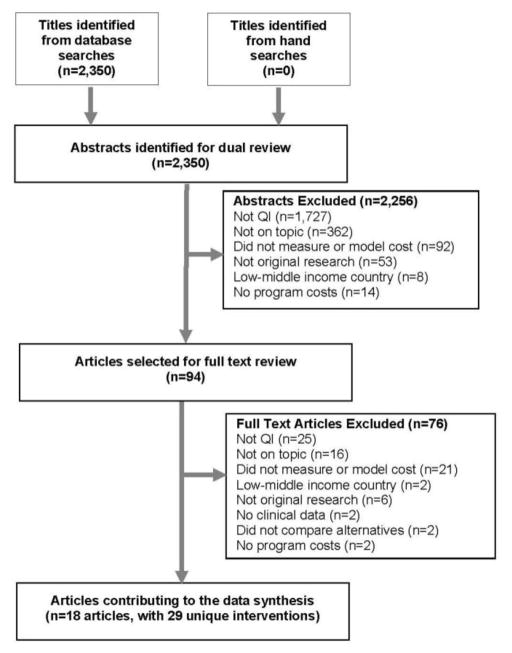
The search identified 2,350 records, selecting 94 for full-text review. Eighteen articles met all eligibility criteria and together reported 29 unique interventions.36–53 Twelve articles reported on one intervention,36–42,46,49–51,53 three articles reported on two interventions,43,52 one article reported on three interventions,47 and two articles reported on four interventions.44,45 Twenty-three interventions focused on general populations36–44,46–48,50–52 and six on healthcare workers.45,49,53 Searches of grey literature did not identify any eligible articles. Figure 1 represents the PRISMA Flow Diagram.
Figure 1.
PRIMSA flow diagram.
QI, quality improvement
Study Characteristics and Quality Assessment
Table 1 lists the interventions. Eighteen of the 23 interventions for general populations involved efforts by provider entities to promote vaccination among the patients they treat.36,37,40,41,43,44,46,47,50–52 These included pre-printed orders, physician reminders, standing order programs,44,47 patient reminders,36,44,52 telephone appointments offered by receptionists in general practitioners’ clinics,37 pharmacist-led vaccine programs,40,51 feedback to practitioners regarding vaccine rates,44 patient risk assessment and counseling services in physician practices,50 and routine vaccination of patients receiving care at hospitals.41,43 Five interventions represented efforts by public or private healthcare payers to promote vaccination among beneficiaries.38,39,42,48 These included promotional mailings42,48 and reduction of out-of-pocket costs in the form of federal subsidies38 and reduction of copayments.39 A majority of the interventions included strategies recommended by major health authorities.44,52
Table 1.
Study Characteristics of Healthcare-based Quality Improvement Interventions to Increase Seasonal Influenza Vaccination (n=29)
| Author | Description of intervention (Matches recommendations by health authorities) | Setting | Population | Clinical study design, comparator | Vaccination rate in comparison group | Difference in vaccination rates (unadjusted) |
|---|---|---|---|---|---|---|
| Interventions focused on general populations | ||||||
| Efforts by provider entities to promote vaccination among the patients they treat | ||||||
| Hull 2002 | Receptionists at practices called intervention households and offered appointments at a nurse-run immunization clinic (N/A) | 3 general practice clinics in England | 1,318 low-risk adults aged >65 years | RCT, status quo | 44% | +6.0% |
| Honeycutt 2007A | Standing orders: Non-physician personnel screened for contraindications and then delivered vaccines without patient-specific orders (N/A) | 3 rural and 1 urban hospital in U.S. | Adult inpatients at high risk | Compare d site and published data, status quo | 1.8% (literature) | +7.1% (range 3.2%–11.7%) |
| Honeycutt 2007B | Pre-printed orders: Staff placed unsigned orders for vaccination in locations where physicians could sign them (CPSTF) | 2 rural and 1 urban hospital in U.S. | Adult inpatients at high risk | Compare d site and published data, status quo | 1.8% (literature) | +1.4% (range 1.0%–1.9%) |
| Honeycutt 2007C | Physician reminders: Staff placed notes in patient records to prompt physicians to assess eligibility and order vaccination (CPSTF) | 3 rural hospitals in U.S. | Adult inpatients at high risk | Compared site and published data, status quo | 1.8% (literature) | +6.1% (range 1.0%–16.0%) |
| Lam 2008 | Pharmacist-conducted vaccine service: Pharmacists and nurses reviewed charts to screen for contraindications and administered vaccines (NVAC) | Assisted living facility in Seattle | 70 indigent, older Asian adults | UCBA, status quo | 64.2% | +18.7% |
| Teufel 2008 | Hospital vaccinations: Registered nurse screened patients for eligibility, communicated with families, and administered vaccine (NVAC) | Hypothetical acute care hospitals in the U.S. | Hospitalized children with asthma | Model based on published data, status quo | 29% (literature) | +30% (with 100% screening) |
| Prosser 2008 | Pharmacy vaccinations: Pharmacists delivered vaccines in an outpatient pharmacy (NVAC) | 5 pharmacies in U.S. | U.S. adults | Model based on site and published data, no vaccination | Not reported | NR |
| Patterson 2012A | Emergency department staff vaccinated older adults presenting for emergent care (NVAC) | Hypothetical emergency departments in the U.S. | U.S. adults aged >50 years | Model based on published data, status quo | 66% (literature) | 80.7% receive vaccine in emergency department |
| Patterson 2012B | Emergency department staff vaccinated older adults presenting for emergent care (NVAC) | Hypothetical emergency departments in the U.S. | U.S. adults aged >65 years | Model based on published data, status quo | 35% (literature) | 80.7% receive vaccine in emergency department |
| Herrett 2016 | General practices sent tailored text message to patients reminding them to get vaccinated; included practice name and phone number (N/A) | 156 general practices in the United Kingdom | Patients aged 18–64 years with chronic conditions | Cluster RCT, status quo | 50.7% | +2.62% |
| Michaelidis 2013A | Outpatient clinics sent autodialed telephone reminders (N/A) | Hypothetical outpatient clinics in the U.S. | African-American and Hispanic birth cohort aged 65 years in 2009 | Model based on published data, status quo | 61% | +8% by year 3 |
| Michaelidis 2013B | Outpatient clinics sent autodialed telephone reminders and established standing orders (N/A) | Hypothetical outpatient clinics in the U.S. | African-American and Hispanic birth cohort aged 65 years in 2009 | Model based on published data, status quo | 61% | +16% by year 3 |
| Michaelidis 2013C | Outpatient clinics sent autodialed telephone reminders, established standing orders, and preformed audit and feedback strategies (N/A) | Hypothetical outpatient clinics in the U.S. | African-American and Hispanic birth cohort aged 65 years in 2009 | Model based on published data, status quo | 61% | +20% by year 3 |
| Michaelidis 2013D | Outpatient clinics sent autodialed telephone reminders, established standing orders, performed audit and feedback and assigned vaccination champion (N/A) | Hypothetical outpatient clinics in the U.S. | African-American and Hispanic birth cohort aged 65 years in 2009 | Model based on published data, status quo | 61% | +20% by year 3 |
| Shoup 2015A | Interactive voice response: automated telephone service reminded patients to obtain vaccine for free and without appointment, and offered more information if desired (N/A) | Integrated healthcare organization in the U.S. | 12,428 adults aged 19–64 years with asthma or COPD | 3-arm RCT, status quo plus postcard reminder | 29.5% (postcard reminder only) | +1.6% |
| Shoup 2015B | Interactive voice response and postcard: automated telephone service reminded patients to obtain vaccine for free and without appointment, and offered more information if desired. Postcard reminders were also sent (N/A) | Integrated healthcare organization in the U.S. | 12,428 adults aged 19–64 years with asthma or COPD | 3-arm RCT, status quo plus postcard reminder | 29.5% (postcard reminder only) | +1.1% |
| Stuck 2015 | Health risk assessment, computer generated feedback to patients and primary care providers, and counseling to promote multiple primary prevention and screening behaviors (N/A) | 19 primary care clinics in Switzerland | 2,284 community-dwelling adults aged >65 years | RCT, status quo | 59.2% | +6.6% |
| Atkins 2015 | City-wide vaccination program administered by pharmacists (NVAC) | 1,230 pharmacies in London | Adults | UCBA, status quo | 60.4% (year prior) | +0.01% |
| Efforts by public or private payers to promote vaccination among beneficiaries | ||||||
| Berg 2008 | Two waves of mailings sent by payer that described the benefits of vaccination particularly for high risk groups, the timing of vaccination, and the benefits of hand-washing (CPSTF) | Preferred provider organization in 5 U.S. states | 107,927 households of federal employee s aged >65 years | RCT, status quo | 20.76% | −0.02% |
| Terrell-Perica 2001A | Influenza mailer: State send mailings encouraging beneficiaries to take advantage of Medicare coverage for influenza vaccination (CPSTF) | Department of Health, Hawaii | 6,528 newly eligible Medicare beneficiaries aged >65 years | 3-arm RCT with 2 intervention groups, status quo | 17.1% | +2.7% |
| Terrell-Perica 2001B | Influenza/pneumococcal mailer: State send mailings encouraging beneficiaries to take advantage of Medicare coverage for both influenza and pneumococcal vaccination (CPSTF) | Department of Health, Hawaii | 6,528 newly eligible Medicare beneficiaries aged >65 years | 3-arm RCT with 2 intervention groups, status quo | 17.1% | +3.8% |
| Ohkusa 2005 | A national immunization program reduced patient copayments by subsidizing a portion of the cost of vaccination; copayments vary across cities (CPSTF) | 13 large cities in Japan | Elderly adults | Cross-sectional analysis of variation in vaccination rate with size of copayment, status quo | 29.7% | +7.0% per $8 increase in subsidy |
| Hoshi 2007 | A national immunization program reduced patient copayment by subsidizing 71% of the cost of vaccination (hypothetical intervention) (CPSTF) | Japan | Adults aged >65 years | Model based on country-specific data, status quo (zero subsidy) | Normal risk individual 7.6%, High risk individual 9.5% | +38.7% among both normal and high risk individuals |
| Interventions focused on healthcare workers | ||||||
| Leitmeyer 2006 | Nationwide campaign that engaged stakeholders and sent promotional and training materials to hospitals (CPSTF, NVAC) | 2,000 hospitals in Germany | German healthcare workers | UCBA, status quo | 21% (based on 20 hospitals) | +5% (based 20 hospitals) |
| Lin 2012A | Education and publicity: promotional materials, free mass vaccination clinics, feedback to hospital leaders (no incentives or carts) (CPSTF, NVAC, JC) | 2 of 11 hospitals in a health system in Pennsylvania | 2,016 personnel other than physicians and trainees | UCBA, status quo | 34.3% (derived) | +3% (37.3% vaccinated in follow-up group) |
| Lin 2012B | Mobile vaccine carts: In addition to education and publicity, an emergency medical technician visited all clinical units during all shifts over 2 months (CPSTF, NVAC, JC) | 2 of 11 hospitals in a health system in Pennsylvania | 3,961 personnel other than physicians and trainees | UCBA, status quo | 31.5% (derived) | +7% (38.5% vaccinated in follow-up group) |
| Lin 2012C | Incentives: In addition to education and publicity, vaccinated personnel received incentives ($10 gift card, lottery for paid time off, or party) (CPSTF, NVAC, JC) | 4 of 11 hospitals in a health system in Pennsylvania | 7,029 personnel other than physician s and trainees | UCBA, status quo | 32.2% (derived) | +9% (41.2% vaccinate d in follow-up group) |
| Lin 2012D | Incentives and mobile vaccine carts: In addition to education and publicity, both incentives and vaccine carts were used (CPSTF, NVAC, JC) | 3 of 11 hospitals in a health system in Pennsylvania | 14,227 personnel other than physicians and trainees | UCBA, status quo | 33.4% (derived) | +6% (39.4% vaccinate d in follow-up group) |
| LaVela 2015 | Influenza declination form program for healthcare workers (NVAC) | 2 spinal cord injury centers in Veterans Affairs hospitals | 173 healthcare workers | UCBA, status quo | 53.5% | +23.9% (worker self-report) |
Note: Boldface indicates statistical significance (p<0.05).
NR, not reported; N/A, not applicable; UCBA, uncontrolled before-after analysis; CPSTF, Community Preventative Services Task Force; NVAC, National Vaccine Advisory Committee, U.S. DHHS; JC, The Joint Commission; COPD, chronic obstructive pulmonary disease
Six interventions described in three articles represented efforts to increase vaccination rates among healthcare workers.45,49,53 One intervention included a multicomponent nationwide campaign to promote vaccination in all German hospitals.49 The intervention incorporated newsletters/bulletins, promotional materials, and information packages sent to hospitals. Another article tested four different interventions that included combinations of promotional materials, free vaccination clinics, feedback to hospital leaders, financial incentives for vaccinated healthcare workers, and mobile vaccine carts.45 In the final intervention, practitioners were required to be vaccinated or complete a vaccination declination form.53 All six interventions incorporated strategies recommended by either the Community Preventive Services Task Force or the CDC.45,49,53 None of the interventions included mandatory vaccination as a condition of employment.
Twenty-two of the 29 interventions were based in the U.S.,40–48,52,53 three in the United Kingdom,36,37,51 two in Japan,38,39 one in Switzerland,50 and one in Germany.49
Twenty-four of the 29 interventions were implemented in a healthcare provider setting (such as hospitals, physician practices, and pharmacies)36,37,40,41,43–47,49–53 whereas five were implemented in a payer setting.38,39,42,48 Fourteen interventions targeted older adults,37–39,42–44,46,48,50 eight focused on adults of any age,36,40,47,51,52 six on healthcare workers,45,49,53 and one on children.41 The comparator group for clinical and cost evaluations in 26 of 29 interventions was the status quo36–39,41–51,53; other comparator groups included status quo plus postcard reminder52 and no vaccination.40
To evaluate clinical effectiveness studies the authors employed diverse designs. Nine interventions were assessed using decision analytic modeling techniques,38,40,41,43,44 eight using RCTs,36,37,42,48,50,52 and eight using uncontrolled before–after designs.45,46,49,51,53 For three interventions, studies compared results to published literature.47 One study employed a cross-sectional design.39
For 25 of the 29 interventions, authors compared vaccination rates between intervention and control groups.36,37,39,41,42,44–53 For interventions that targeted general populations, the median difference in the vaccination rate between intervention and control groups was 6.1% (IQR, 1.6% to 16%).36,37,39,41,42,44,46–48,50–52 Among the ten interventions in the general population for which vaccination rates were measured (versus modeled or assumed by authors) the median difference in vaccination rate was 2.2% (IQR, 0.8% to 6.1%).36,37,42,46,48,50–52 For interventions that focused on healthcare workers, the median difference in the vaccination rate between intervention and control groups was 6.5% (IQR, 4.5% to 12.7%; Table 1).45,49,53 Additional outcome measures used in studies included influenza episodes averted,43 QALYs,44 years of life saved,38 and lives saved.39,43
Responses to Minimum Quality Criteria Set items, funding source, and bias assessments are given in the Appendix (Appendix Tables 2–4). Many studies were at risk of bias because of incomplete outcome data and uncontrolled confounding.
Table 2 describes program costs as reported by authors, standardized program costs, and economic evaluation methods for all 29 interventions.36–53 Fifteen interventions were subjected to cost analyses,36,45,47,48,50–53 three to business-case analyses,37,42,46 ten to cost-effectiveness analyses,38,40,41,43,44,49 and one to a cost-benefit analysis.39 The entity bearing program costs varied between interventions: the healthcare system bore the cost in 11 interventions,36,40,44,45,51 hospitals in seven interventions,41,43,47,53 payers or the government in six interventions,38,39,42,48,49 clinics or physician practices in two interventions,37,50 integrated healthcare systems in two interventions,52 and an assisted living facility in one intervention.46 For 26 interventions, authors evaluated costs during one influenza season,36–39,41–48,51–53 two studies adopted a two-year time horizon,49,50 and one study used a 1-year timeframe.40
Table 2.
Program Costs of Healthcare-based Quality Improvement Interventions to Increase Season Influenza Vaccination (n=29)
| Author | Time horizon | Setting | Program cost as reported by authors | Program cost reported by authors includes vaccine? | Year of costs | Standardized program costs per enrollee / per additional enrollee vaccinated (excluding vaccine cost, 2017 USD) | Economic evaluation method | Entity bearing program cost |
|---|---|---|---|---|---|---|---|---|
| Interventions focused on general populations | ||||||||
| Efforts by provider entities to promote vaccination among the patients they treat | ||||||||
| Hull 2002 | 1 season | 3 general practice clinics in England | £98.21 per 16 additional individuals vaccinated (£72.65 receptionist labor, £12.25 telephone calls, £13.31 nurse labor) | No | 2000 | $1.02 / $16.95 | Business case | Physician practice |
| Honeycutt 2007A | 1 season | 3 rural and 1 urban hospital in U.S. | $57.60 per additional individual vaccinated ($6,032–$87,534 per hospital to assess eligibility and administer vaccine) | No | 2004 | $8.52 / $119.64 | Cost analysis | Hospital |
| Honeycutt 2007B | 1 season | 2 rural and 1 urban hospital in U.S. | $411.18 per additional individual vaccinated ($27,510–$84,778 per hospital to assess eligibility and administer vaccine) | No | 2004 | $11.63 / $854.07 | Cost analysis | Hospital |
| Honeycutt 2007C | 1 season | 3 rural hospitals in U.S. | $89.70 per additional individual vaccinated ($1,840–$27,926 per hospital to assess eligibility and administer vaccine) | No | 2004 | $11.42 / $186.32 | Cost analysis | Hospital |
| Lam 2008 | 1 season | Assisted living facility in Seattle | $1,110 per 13 additional individuals vaccinated ($990 pharmacist labor, $90 medical assistant labor, $30 supplies) | No | 2004 | $24.28 / $129.80 | Business case | Assisted living facility |
| Teufel 2008 | 1 season | Hypothetical acute care hospitals in the U.S. | $13.66 per patient screened (registered nurse labor, supplies, vaccine) | Yes, $6.75 per dose | 2006 | $21.52 / $71.72 | CEA | Hospital |
| Prosser 2008 | 1 year | 5 pharmacies in U.S. | +$11.57 per additional individual vaccinated (promotion of vaccine, labor, administration, vaccine, supplies, overhead) | Yes, $7.48 per dose | 2004 | NR / $6.26 | CEA | Healthcare system |
| Patterson 2012A | 1 season | Hypothetical emergency departments in the U.S. | $34.19 per additional individual vaccinated (vaccine administration and vaccine) | Yes, not reporteda | 2008 | NR / $38.81 | CEA | Hospital |
| Patterson 2012B | 1 season | Hypothetical emergency departments in the U.S. | $34.19 per additional individual vaccinated (vaccine administration and vaccine) | Yes, not reporteda | 2008 | NR / $38.81 | CEA | Hospital |
| Herrett 2015 | 1 season | 156 general practices in the U.K. | £98,370 per 51,151 patients enrolled (£15,600 incentive payments to general practices, £3,700 letters, £1,200 software support, £53,770 research, £24,100 data costs) | No | 2013 | $3.27 / $191.48 | Cost analysis | Healthcare system |
| Michaelidis 2013A | 10 years | Hypothetical outpatient clinics in the U.S. | $2.00 per targeted patient per year | No | 2011 | $2.38 / $29.69 | CEA | Healthcare system |
| Michaelidis 2013B | 10 years | Hypothetical outpatient clinics in the U.S. | $7.62 per targeted patient per year ($2.00 patient reminders, $5.62 standing orders) | No | 2011 | $9.05 / $56.56 | CEA | Healthcare system |
| Michaelidis 2013C | 10 years | Hypothetical outpatient clinics in the U.S. | $9.61 per targeted patient per year ($2.00 patient reminders, $5.62 standing orders, $1.99 audit and feedback) | No | 2011 | $11.41 / $57.07 | CEA | Healthcare system |
| Michaelidis 2013D | 10 years | Hypothetical outpatient clinics in the U.S. | $17.84 per targeted patient per year ($2.00 patient reminders, $5.62 standing orders, $1.99 audit and feedback, and $8.23 vaccination champion) | No | 2011 | $21.19 / $105.94 | CEA | Healthcare system |
| Shoup 2015A | October of 1 season | Integrated healthcare organization in the U.S. | $0.78 per enrolled patient with postcard-only, $1.23 per enrolled patient with interactive voice response system | No | 2011 | $0.53 / $33.66 | Cost analysis | Integrated healthcare system |
| Shoup 2015B | October of 1 season | Integrated healthcare organization in the U.S. | $0.78 per enrolled patient with postcard-only, $1.93 per enrolled patient with interactive voice response system and reminder postcard | No | 2011 | $1.36 / $126.57 | Cost analysis | Integrated healthcare system |
| Stuck 2015 | 2 years | 19 primary care clinics in Switzerland | $1,017 per participant per 2 years ($961 labor, $56 administering questionnaires and feedback reports) | No | 2014 | NR / NR | Cost analysis | Clinic |
| Atkins 2015 | 1 season | 1,230 pharmacies in London | £17.13 per dose for usual care vs. £14.88 per dose for pharmacy vaccination delivery (program development and vaccine administration) | Yes, £7.37 per dose | 2013 | NR / NR | Cost analysis | Healthcare system |
| Efforts by public or private payers to promote vaccination among beneficiaries | ||||||||
| Berg 2008 | 1 season | Preferred provider organization in 5 U.S. states | +$16,000 per 26,474 household s with 1.3 members each (to produce and mail materials) | No | 2003 | $0.74 / Dominate d by the status quo | Business case | Payer |
| Terrell - Perica 2001A | 1 season | Department of Health, Hawaii | +$0.36 per letter, or +$2,350 across 6,528 study subjects (materials, labor, mailing) for letters that recommend influenza vaccination | No | 1997 | $0.73 / $27.24 | Cost analysis | Governmental body |
| Terrell - Perica 2001B | 1 season | Department of Health, Hawaii | +$0.36 per letter, or +$2,350 across 6,528 study subjects (materials, labor, mailing) for letters that recommend both influenza and pneumococcal vaccination | No | 1997 | $0.73 / $19.20 | Cost analysis | Governmental body |
| Ohkusa 2005 | 1 season | 13 large cities in Japan | +$8 subsidy per individual vaccinated equates to $50.4 million per population of 120 million nationally | Yes, $36 cost to patient | 2003 | $0.90 / $12.82 | CBA | Governmental body |
| Hoshi 2007 | 1 season | Japan | +$27 subsidy per additional individual vaccinated | Yes, $38 cost to patient | 2002 | NR / $45.00 | CEA | Governmental body |
| Interventions focused on healthcare workers | ||||||||
| Leitmeyer 2006 | 2 years | 2,000 hospitals in Germany | $45,000 across 2,000 German hospitals (to produce and distribute materials, plus 2 months labor of Public Health Scientist (cost not described) | No | 2004 | NR / NR | CEA | Governmental body |
| Lin 2012A | 1 season | 2 of 11 hospitals in a health system in Pennsylvania | $13,537–$15,471 across 2 hospitals, including oversight, clinic, vaccine, and supplies | Yes, $14.42 per dose including supplies | 2007 | $4.02 / $134.08 | Cost analysis | Healthcare system |
| Lin 2012B | 1 season | 2 of 11 hospitals in a health system in Pennsylvania | $35,392–$40,448 across 2 hospitals, including oversight, carts, clinic, vaccine, and supplies | Yes, $14.42 per dose including supplies | 2007 | $8.15 / $116.40 | Cost analysis | Healthcare system |
| Lin 2012C | 1 season | 4 of 11 hospitals in a health system in Pennsylvania | $59,869–$74,116 across 4 hospitals, including oversight, incentives, clinic, vaccine, and supplies | Yes, $14.42 per dose including supplies | 2007 | $8.04 / $89.29 | Cost analysis | Healthcare system |
| Lin 2012D | 1 season | 3 of 11 hospitals in a health system in Pennsylvania | $137,691–$170,668 across 3 hospitals, including oversight, incentives, carts, clinic, vaccine, and supplies | Yes, $14.42 per dose including supplies | 2007 | $11.03 / $183.81 | Cost analysis | Healthcare system |
| LaVela 2015 | 1 season | 2 spinal cord injury centers in Veterans Affairs hospitals | $2,098 per site, with 2 sites ($2,093 staff time, <$5.00 printed materials) | No | 2013 | NR / NR | Cost analysis | Hospital |
When calculating standardized program cost per enrollee and per additional enrollee vaccinated excluding vaccine cost, the 2008 Centers for Disease Control (CDC) Influenza Price List was used to estimate a vaccine private sector cost of $13.25/dose (in 2008 USD).
CEA, cost-effectiveness analysis; CBA, cost benefit analysis; NR, not reported
Program costs were standardized for 25 interventions, including 21 for general populations36–44,46–48,52 and four for healthcare workers (Appendix Table 5).45 Four interventions were unable to be standardized because of missing information,49,51 small sample size and low response rates,53 and inclusion of unrelated costs.50
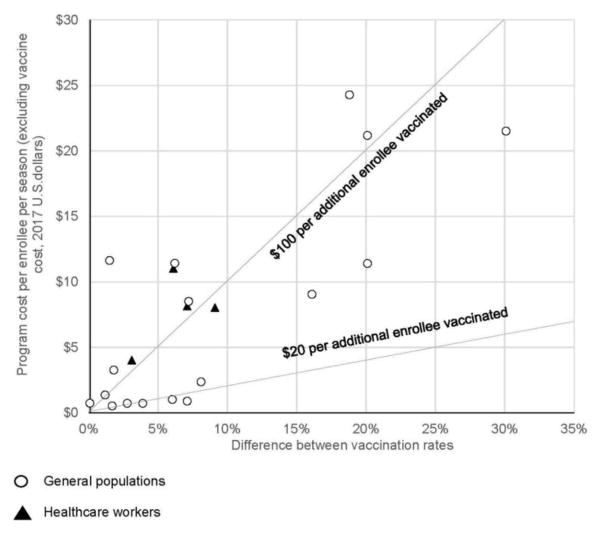
Figure 2 shows differences in vaccination rates and standardized program costs per enrollee per influenza season. Dashed lines represent hypothetical thresholds ($20 and $100) for what the health system might be willing to pay per additional enrollee vaccinated. For general populations (circles), the median program cost was $3.27 (IQR, $0.82–$11.53) per enrollee and $50.78 (IQR, $27.85–$124.84) per additional enrollee vaccinated.36–44,46–48,52 Limited to the eight interventions in general populations for which program costs were measured by authors, the median program cost was $0.88 (IQR, $0.73–$2.79) per enrollee and $33.66 (IQR, $19.20–$129.80) per additional enrollee vaccinated.36,37,42,46,48,52 Interventions with the lowest cost per additional enrollee vaccinated varied widely and included: pharmacist-delivered vaccinations,40 reductions in influenza vaccination copayments for those aged ≥65 years, direct phone calls to patients offering vaccination appointments,37 and mass mailings encouraging vaccination.48
Figure 2.
Standardized program costs per enrollee per season and difference between vaccination rates.
For healthcare workers (triangles), the median program cost was $8.09 (IQR, $5.03–$10.31) per worker and $125.24 per additional worker vaccinated (IQR, $96.06–$171.38).45 The low number of healthcare worker interventions made drawing conclusions regarding their relative value difficult.
For ten interventions that focused on general populations, authors performed complete economic analyses that incorporated both program and influenza infection-related costs (Appendix Tables 6 and 7).38,40–44 The influenza infection-related costs included varied: ten economic evaluations included outpatient visits38,40–44; ten included hospitalizations38,40–44; eight included productivity losses because of illness, disability, or death40,41,44; seven included vaccine adverse events40,43,44; three included medications38,40,41; and one included laboratory and diagnostic testing.40 The assumed vaccine efficacy ranged from 50% to 69%,38,40–44 except for in one study where a more conservative estimate of 29% was used.38 Overall, the quality of the economic evaluations that examined these ten interventions was moderate to high, with mQHES scores ranging from 105 to 115.
Among the ten interventions, three yielded net cost savings,40–42 four yielded costs below $50,000 per QALY,44 and three yielded costs <$60,000 per life saved (Appendix Table 7).38,43 The three interventions that reported net cost savings were diverse in terms of intervention components, context, and target population. The interventions assessed in these studies included promotional mailings sent to older adults by a payer,42 a pharmacist-led vaccine program for the general population,40 and a hospital-based vaccine program for pediatric inpatients with asthma.41
No studies on healthcare workers had complete economic evaluations.
DISCUSSION
In this systematic review, the cost of implementing 23 diverse QI interventions designed to increase seasonal influenza vaccination rates among the general population and six among healthcare workers was examined. The interventions were generally aligned with recommendations by major health authorities12–16 and effective relative to the status quo. The median program cost per additional individual vaccinated was $50.78 for general populations and $125.24 among healthcare workers. These estimates exclude vaccine costs, which range from $11.78 to $36.08 per dose. Using a cost-effectiveness threshold of $100,000 to $150,000 per QALY (i.e., a year lived in perfect health),54 the ten interventions with complete economic evaluations were cost saving to cost effective relative to the status quo with three reporting net cost savings, four reporting costs below $50,000 per QALY, and three reporting costs under $60,000 per life saved.
Although results were limited to studies that reported costs, and costs may be more likely assessed when studies are effective, the effectiveness estimates of the interventions studied in this review were similar to those reported in previous systematic reviews in both general populations and healthcare workers. In fact, in a systematic review of 57 RCTs focused on older adults, relative changes in vaccination rates ranged from no effect to an 8-fold increase.55 In a prior meta-analysis of interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults, relative changes in vaccination rates ranged from no effect to a 3-fold increase.18 In this analysis, relative changes in vaccination rates ranged from no effect to a 5-fold increase. In a prior systematic review of 12 RCTs focused on healthcare workers, the authors reported absolute changes in vaccination rates ranging from a 13.0% decline to a 26.0% increase.19 The studies in this analysis reported absolute increases in vaccination rates among healthcare workers of 3.0% to 23.9%.
Prior literature has generally reported that influenza vaccination itself is cost effective to cost saving in a variety of populations including older adults, children, and pregnant women.20–24 In particular, a 2017 review assessed the cost effectiveness of influenza vaccination in any setting and found it to be generally cost effective. However, the studies examined only the cost of the vaccination itself and in some cases, the cost of vaccine administration.24 By contrast, this review examined the additional costs associated with implementing QI interventions to increase vaccination rates, including not only the cost of administration but any costs associated with start-up and maintenance of programs, such as costs related to: tracking who has been vaccinated, conducting outreach to target populations, creating standardized order sets, and setting up special situations in which patients and healthcare workers can be vaccinated. This review found that the program costs per additional patient vaccinated were generally higher, sometimes several-fold higher, than the cost of the vaccine itself ($11.78–$36.08/dose), particularly among healthcare workers. Among general populations, intervention strategies with low program costs per additional patient vaccinated included: pharmacist-delivered vaccine programs, reductions in copayments, direct phone calls offering vaccination appointments, and mass mailings to encourage vaccination uptake. Among healthcare worker interventions, hospital-wide vaccination campaigns with incentives were relatively low cost per additional worker vaccinated.45
Although program costs per additional enrollee vaccinated were large relative to vaccine costs, a smaller number of studies with complete economic analyses found that QI interventions in general populations were either cost saving or cost effective relative to the status quo (at a threshold of $100,000–$150,000 per QALY)54 indicating they may be good value to the healthcare system. Influenza infection-related costs can include hospitalizations, outpatient visits, diagnostic tests, medications, and productivity losses. Medical costs can range from $90 to $989 per clinic visit and from $2,428 to $50,620 per hospitalization,38,40–44 depending on age and the risk of severe complications. Costs can be even higher for high-risk infants, young children, and older adults.56–59 However, because the QI interventions are only rarely cost saving to the health system and third-party payers often accrue any savings associated with reduced clinic visits and hospitalizations, the costs of these interventions may be a barrier to implementation.
This review of interventions among healthcare workers was based on a few studies that were not complete evaluations, which made it difficult to make comparisons and draw conclusions. Notably, none of the studies described the cost of mandatory influenza vaccination among healthcare workers, which is an increasingly common, extremely effective, and yet often controversial strategy.60–65 In fact, 18 U.S. states have established influenza vaccination requirements for healthcare workers, eight of which necessitate hospitals ensure their workers are vaccinated; local laws can be even stricter.66 For example, the state of California requires that all acute care hospitals offer free onsite influenza vaccinations to all workers and requires a signed declination if a worker elects not to be vaccinated.66 In 2013, Los Angeles mandated all healthcare personnel in acute care hospitals, long-term care facilities, and intermediate-care facilities be vaccinated against influenza, or wear a protective mask.67 Stricter still, some individual hospitals prohibit non-vaccinated healthcare workers from performing patient-related duties during the influenza season. Although mandatory influenza vaccination programs often achieve near-total compliance, the financial cost and buy-in from hospital leadership may be barriers to implementation. In addition to costs associated with voluntary vaccination programs, such as the hiring of additional nurses for administration of the vaccination and the vaccination dose itself, mandatory programs further require the establishment of administrative processes to ensure worker receipt of vaccination. These commonly include: electronic monitoring systems, engagement with hospital human resources, employee health, and communications departments, and establishment of exemption committees to evaluate exemption requests. Given the well-documented effectiveness of these mandatory programs, understanding and quantifying the additional financial costs of such programs remains an important and unanswered question.
Limitations
First, because of heterogeneity in study design, meta-analyses were not feasible. Second, because the unit of analysis was the individual intervention, studies containing multiple interventions were overrepresented. Next, most of the studies included program costs only and omitted costs related to influenza infection, thereby limiting the ability to examine the complete economic implications of vaccine programs. Further, program costs considered may have varied across studies thereby limiting the comparability between studies. Finally, future QI interventions may have somewhat different costs than observed in this review because of variation in organizational context, intervention scale, and vaccine efficacy, and thus generalizability may be a concern.
CONCLUSIONS
QI interventions for influenza vaccination in general populations involve program costs per enrollee that are similar to the cost of the vaccine itself and program costs per additional enrollee vaccinated that are somewhat higher than the vaccine cost. Based on limited available evidence, such interventions may be cost saving to cost effective to the health system. For interventions targeting healthcare workers, less data on cost is available. The cost and cost effectiveness of mandatory vaccination programs for healthcare workers are a high priority for future study.
Supplementary Material
Acknowledgments
We would like to thank the members of the technical expert panel: Craige Blackmore, MD; Jo Carol Hiatt, MD; Catherine MacLean, MD; Emma Hoo; Paul McGann, MD; Lucy Johns, MPH; Julie Cerese, RN, MSN; David Meltzer, MD, PhD; the research assistants and fellows who contributed to the work: Philip Ros, MPH, Cedars-Sinai; Aziza Afrikhanova, MS, the Rand Corporation; Courtney Coles, MPH, Cedars-Sinai; the individuals who assisted with data collection and analysis: Kanaka Shetty, MD, MS; Daniel Waxman, MD, PhD; Martha Timmer, MA, the Rand Corporation; Aneesa Motala, BA, the Rand Corporation; Margaret Kelley, MS, Cedars-Sinai, who provided logistical and managerial support throughout the project; and Jonathan Grein, MD, Cedars-Sinai, who provided invaluable expert opinion. Funding Source: Agency for Healthcare Research and Quality.
Author contributions are as follows: concept and design: Anderson, Nuckols; acquisition, analysis, or interpretation of data: Anderson, Nuckols, Keeler, Uscher-Pines, Shanman, Morton, Shekelle, Aliyev; drafting of the manuscript: Anderson, Nuckols; critical revision of the manuscript for important intellectual content: Anderson, Nuckols, Keeler, Morton, Uscher-Pines, Shekelle; statistical analysis: Anderson, Nuckols, Morton; administrative, technical, or material support: Anderson, Nuckols, Aliyev; study supervision: Nuckols, Anderson; Other: Shanman. Prospero Registration Number: CRD42015014950.
No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rolfes MA, Foppa IM, Garg S, et al. [Accessed January 5, 2016];Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths Averted by Vaccination in the United States. www.cdc.gov/flu/about/disease/2015-16.htm. Published 2016.
- 2.Molinari N, Ortega-Sanchez I, Messonnier M, et al. The annual impact of seasonal influenza in the U.S.: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. https://doi.org/10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016;65(RR-5):1–54. doi: 10.15585/mmwr.rr6505a1. https://doi.org/10.15585/mmwr.rr6505a1. [DOI] [PubMed] [Google Scholar]
- 4.National Vaccine Advisory Committee. Recommendations on Strategies to Achieve the Healthy People 2020 Annual Influenza Vaccine Coverage Goal for Health Care Personnel. Published 2012. [PMC free article] [PubMed] [Google Scholar]
- 5.Black C, Yue X, Ball S, et al. Influenza vaccination coverage among health care personnel--United States, 2013–14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(37):805–811. [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. [Accessed August 16, 2017];National Early-Season Flu Vaccination Coverage, United States. 2016 Nov; www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2016.htm. Published 2016.
- 7.Centers for Medicare and Medicaid Services. [Accessed January 3, 2017];Quality Measures. www.CMS.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures.html. Published 2016.
- 8.Centers for Medicare and Medicaid Services. [Accessed January 3, 2016];Hospital Value-Based Purchasing. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Published 2017.
- 9.Centers for Medicare and Medicaid Services. [Accessed January 4, 2017];Physician Quality Reporting System. www.CMS.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/PQRI. Published 2016.
- 10.The National Committee for Quality Assurance. [Accessed February 24, 2017];HEIDIS 2017 Summary Table of Measures, Product Lines and Changes. www.ncqa.org/Portals/0/HEDISQM/HEDIS2017/HEDIS%202017%20Volume%202%20List%20of%20Measures.pdf?ver=2016-06-27-135433-350. Published 2017.
- 11.Danz MS, Rubenstein LV, Hempel S, et al. Identifying quality improvement intervention evaluations: is consensus achievable? Qual Saf Health Care. 2010;19(4):279–283. doi: 10.1136/qshc.2009.036475. https://doi.org/10.1136/qshc.2009.036475. [DOI] [PubMed] [Google Scholar]
- 12.Task Force on Community Preventive Services. Recommendations to improve targeted vaccination coverage among high-risk adults. Am J Prev Med. 2005;28(5 Suppl):231–237. doi: 10.1016/j.amepre.2005.02.011. https://doi.org/10.1016/j.amepre.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Helms C, Guerra F, Klein J, Schaffner W, Arvin A, Peter G. Strengthening the nation’s influenza vaccination system: a National Vaccine Advisory Committee assessment. Am J Prev Med. 2005;29(3):221–226. doi: 10.1016/j.amepre.2005.05.011. https://doi.org/10.1016/j.amepre.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.The National Vaccine Advisory Committee. Recommendations on Strategies to Achieve the Healthy People 2020 Annual Influenza Vaccine Coverage Goal for Health Care Personnel. U.S. DHHS; Published February 8, 2012. [PMC free article] [PubMed] [Google Scholar]
- 15.Community Preventive Services Task Force. [Accessed February 28, 2017];Worksite: Seasonal Influenza Vaccinations Using Interventions with On-Site, Free, Actively Promoted Vaccinations – Healthcare Workers. www.thecommunityguide.org/findings/worksite-seasonal-influenza-vaccinations-healthcare-on-site. Published 2008.
- 16.The Joint Commission. Joint Commission on Accreditation of Healthcare Organizations. 2009. Providing a Safer Environment for Health Care Personnel and Patients Providing a Safer Environment for Health Care Through Influenza Vaccination: Strategies from Research and Practice. [Google Scholar]
- 17.Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2014;(7):Cd005188. doi: 10.1002/14651858.CD005188.pub3. https://doi.org/10.1002/14651858.CD005188.pub3. [DOI] [PMC free article] [PubMed]
- 18.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: a systematic review and meta-analysis. Ann Fam Med. 2012;10(6):538–546. doi: 10.1370/afm.1405. https://doi.org/10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid H, Yin JK, Ward K, King C, Seale H, Booy R. Assessing Interventions To Improve Influenza Vaccine Uptake Among Health Care Workers. Health Aff (Millwood) 2016;35(2):284–292. doi: 10.1377/hlthaff.2015.1087. https://doi.org/10.1377/hlthaff.2015.1087. [DOI] [PubMed] [Google Scholar]
- 20.Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson MA. Influenza cost and cost-effectiveness studies globally--a review. Vaccine. 2013;31(46):5339–5348. doi: 10.1016/j.vaccine.2013.09.013. https://doi.org/10.1016/j.vaccine.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Salo H, Kilpi T, Sintonen H, Linna M, Peltola V, Heikkinen T. Cost-effectiveness of influenza vaccination of healthy children. Vaccine. 2006;24(23):4934–4941. doi: 10.1016/j.vaccine.2006.03.057. https://doi.org/10.1016/j.vaccine.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Ott JJ, Klein Breteler J, Tam JS, Hutubessy RC, Jit M, de Boer MR. Influenza vaccines in low and middle income countries: a systematic review of economic evaluations. Hum Vaccin Immunother. 2013;9(7):1500–1511. doi: 10.4161/hv.24704. https://doi.org/10.4161/hv.24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waure C, Veneziano MA, Cadeddu C, et al. Economic value of influenza vaccination. Hum Vaccin Immunother. 2012;8(1):119–129. doi: 10.4161/hv.8.1.18420. https://doi.org/10.4161/hv.8.1.18420. [DOI] [PubMed] [Google Scholar]
- 24.Ting EE, Sander B, Ungar WJ. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine. 2017;35(15):1828–1843. doi: 10.1016/j.vaccine.2017.02.044. https://doi.org/10.1016/j.vaccine.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. https://doi.org/10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 26.Nuckols TKMM, Shekelle P, Morton S, Escarce J, Keeler E. Systematic review of cost outcomes of quality improvement. [Accessed January 4, 2017];PROSPERO. 2015 CRD42015014950. www.crd.york.ac.uk/prospero/display_record.php?RecordID=14950.
- 27.Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care. 2009;25(4):522–529. doi: 10.1017/S0266462309990523. https://doi.org/10.1017/S0266462309990523. [DOI] [PubMed] [Google Scholar]
- 28.Goeree R, He J, O’Reilly D, et al. Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. Clinicoecon Outcomes Res. 2011;3:89–104. doi: 10.2147/CEOR.S14404. https://doi.org/10.2147/CEOR.S14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796–804. doi: 10.1136/bmjqs-2014-003151. https://doi.org/10.1136/bmjqs-2014-003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Altman DG, Sterne JAC. Cochrane Statistical Methods Group, Cochrane Bias Methods Group, editors. Chapter 8: Assessing risk of bias in included studies. [Accessed January 5, 2017];Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm. Published 2011.
- 31.Wells GA, Shea B, O’Connell D, et al. [Accessed January 5, 2017];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 32.Chiou CF, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41(1):32–44. doi: 10.1097/00005650-200301000-00007. https://doi.org/10.1097/00005650-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Walker DG, Wilson RF, Sharma R, et al. Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2012. AHRQ Methods for Effective Health Care. [PubMed] [Google Scholar]
- 34.Nuckols TK, Keeler E, Morton SC, et al. Economic Evaluation of Quality Improvement Interventions for Bloodstream Infections Related to Central Catheters: A Systematic Review. JAMA Intern Med. 2016;176(12):1843–1854. doi: 10.1001/jamainternmed.2016.6610. https://doi.org/10.1001/jamainternmed.2016.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC. [Accessed August 31, 2016];VFC CDC Vaccine Price List Archives. www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/archive.html. Published 2017.
- 36.Herrett E, Williamson E, van Staa T, et al. Text messaging reminders for influenza vaccine in primary care: a cluster randomised controlled trial (TXT4FLUJAB) BMJ Open. 2016;6(2):e010069. doi: 10.1136/bmjopen-2015-010069. https://doi.org/10.1136/bmjopen-2015-010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hull S, Hagdrup N, Hart B, Griffiths C, Hennessy E. Boosting uptake of influenza immunisation: a randomised controlled trial of telephone appointing in general practice. Br J Gen Pract. 2002;52(482):712–716. [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshi SL, Kondo M, Honda Y, Okubo I. Cost-effectiveness analysis of influenza vaccination for people aged 65 and over in Japan. Vaccine. 2007;25(35):6511–6521. doi: 10.1016/j.vaccine.2007.05.067. https://doi.org/10.1016/j.vaccine.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 39.Ohkusa Y. Policy evaluation for the subsidy for influenza vaccination in elderly. Vaccine. 2005;23(17–18):2256–2260. doi: 10.1016/j.vaccine.2005.01.042. https://doi.org/10.1016/j.vaccine.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Prosser LA, O’Brien MA, Molinari NA, et al. Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics. 2008;26(2):163–178. doi: 10.2165/00019053-200826020-00006. https://doi.org/10.2165/00019053-200826020-00006. [DOI] [PubMed] [Google Scholar]
- 41.Teufel RJ, 2nd, Basco WT, Jr, Simpson KN. Cost effectiveness of an inpatient influenza immunization assessment and delivery program for children with asthma. J Hosp Med. 2008;3(2):134–141. doi: 10.1002/jhm.286. https://doi.org/10.1002/jhm.286. [DOI] [PubMed] [Google Scholar]
- 42.Berg GD, Silverstein S, Thomas E, Korn AM. Cost and utilization avoidance with mail prompts: a randomized controlled trial. Am J Manag Care. 2008;14(11):748–754. [PubMed] [Google Scholar]
- 43.Patterson BW, Khare RK, Courtney DM, Lee TA, Kyriacou DN. Cost-effectiveness of influenza vaccination of older adults in the ED setting. Am J Emerg Med. 2012;30(7):1072–1079. doi: 10.1016/j.ajem.2011.07.007. https://doi.org/10.1016/j.ajem.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaelidis CI, Zimmerman RK, Nowalk MP, Smith KJ. Cost-effectiveness of programs to eliminate disparities in elderly vaccination rates in the United States. BMC Public Health. 2014;14:718. doi: 10.1186/1471-2458-14-718. https://doi.org/10.1186/1471-2458-14-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CJ, Nowalk MP, Zimmerman RK. Estimated costs associated with improving influenza vaccination for health care personnel in a multihospital health system. Jt Comm J Qual Patient Saf. 2012;38(2):67–72. doi: 10.1016/s1553-7250(12)38009-4. https://doi.org/10.1016/S1553-7250(12)38009-4. [DOI] [PubMed] [Google Scholar]
- 46.Lam AY, Chung Y. Establishing an on-site influenza vaccination service in an assisted-living facility. J Am Pharm Assoc (2003) 2008;48(6):758–763. doi: 10.1331/JAPhA.2008.07135. https://doi.org/10.1331/JAPhA.2008.07135. [DOI] [PubMed] [Google Scholar]
- 47.Honeycutt AA, Coleman MS, Anderson WL, Wirth KE. Cost-effectiveness of hospital vaccination programs in North Carolina. Vaccine. 2007;25(8):1484–1496. doi: 10.1016/j.vaccine.2006.10.029. https://doi.org/10.1016/j.vaccine.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Terrell-Perica SM, Effler PV, Houck PM, Lee L, Crosthwaite GH. The effect of a combined influenza/pneumococcal immunization reminder letter. Am J Prev Med. 2001;21(4):256–260. doi: 10.1016/s0749-3797(01)00372-5. https://doi.org/10.1016/S0749-3797(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 49.Leitmeyer K, Buchholz U, Kramer M, et al. Influenza vaccination in German health care workers: effects and findings after two rounds of a nationwide awareness campaign. Vaccine. 2006;24(47–48):7003–7008. doi: 10.1016/j.vaccine.2006.04.040. https://doi.org/10.1016/j.vaccine.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 50.Stuck AE, Moser A, Morf U, et al. Effect of health risk assessment and counselling on health behaviour and survival in older people: a pragmatic randomised trial. PLoS Med. 2015;12(10):e1001889. doi: 10.1371/journal.pmed.1001889. https://doi.org/10.1371/journal.pmed.1001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atkins K, van Hoek AJ, Watson C, et al. Seasonal influenza vaccination delivery through community pharmacists in England: evaluation of the London pilot. BMJ Open. 2016;6(2):e009739. doi: 10.1136/bmjopen-2015-009739. https://doi.org/10.1136/bmjopen-2015-009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405–413. [PubMed] [Google Scholar]
- 53.LaVela SL, Hill JN, Smith BM, Evans CT, Goldstein B, Martinello R. Healthcare worker influenza declination form program. Am J Infect Control. 2015;43(6):624–628. doi: 10.1016/j.ajic.2015.02.013. https://doi.org/10.1016/j.ajic.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. https://doi.org/10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 55.Thomas RE, Russell M, Lorenzetti D. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2010;(9):Cd005188. doi: 10.1002/14651858.CD005188.pub2. https://doi.org/10.1002/14651858.CD005188.pub2. [DOI] [PubMed]
- 56.Fairbrother G, Cassedy A, Ortega-Sanchez IR, et al. High costs of influenza: Direct medical costs of influenza disease in young children. Vaccine. 2010;28(31):4913–4919. doi: 10.1016/j.vaccine.2010.05.036. https://doi.org/10.1016/j.vaccine.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 57.Keren R, Zaoutis TE, Saddlemire S, Luan XQ, Coffin SE. Direct medical cost of influenza-related hospitalizations in children. Pediatrics. 2006;118(5):e1321–1327. doi: 10.1542/peds.2006-0598. https://doi.org/10.1542/peds.2006-0598. [DOI] [PubMed] [Google Scholar]
- 58.Lee BY, Tai JH, Bailey RR, Smith KJ. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009;27(50):7110–7115. doi: 10.1016/j.vaccine.2009.09.056. https://doi.org/10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortega-Sanchez IR, Molinari NA, Fairbrother G, et al. Indirect, out-of-pocket and medical costs from influenza-related illness in young children. Vaccine. 2012;30(28):4175–4181. doi: 10.1016/j.vaccine.2012.04.057. https://doi.org/10.1016/j.vaccine.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 60.Karanfil LV, Bahner J, Hovatter J, Thomas WL. Championing patient safety through mandatory influenza vaccination for all healthcare personnel and affiliated physicians. Infect Control Hosp Epidemiol. 2011;32(4):375–379. doi: 10.1086/659155. https://doi.org/10.1086/659155. [DOI] [PubMed] [Google Scholar]
- 61.Ksienski DS. Mandatory seasonal influenza vaccination or masking of British Columbia health care workers: Year 1. Can J Public Health. 2014;105(4):e312–316. doi: 10.17269/cjph.105.4346. https://doi.org/10.17269/cjph.105.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leibu R, Maslow J. Effectiveness and acceptance of a health care-based mandatory vaccination program. J Occup Environ Med. 2015;57(1):58–61. doi: 10.1097/JOM.0000000000000294. https://doi.org/10.1097/JOM.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 63.Miller BL, Ahmed F, Lindley MC, Wortley PM. Increases in vaccination coverage of healthcare personnel following institutional requirements for influenza vaccination: a national survey of U.S. hospitals. Vaccine. 2011;29(50):9398–9403. doi: 10.1016/j.vaccine.2011.09.047. https://doi.org/10.1016/j.vaccine.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 64.Pitts SI, Maruthur NM, Millar KR, Perl TM, Segal J. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47(3):330–340. doi: 10.1016/j.amepre.2014.05.035. https://doi.org/10.1016/j.amepre.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 65.Rakita RM, Hagar BA, Crome P, Lammert JK. Mandatory influenza vaccination of healthcare workers: a 5-year study. Infect Control Hosp Epidemiol. 2010;31(9):881–888. doi: 10.1086/656210. https://doi.org/10.1086/656210. [DOI] [PubMed] [Google Scholar]
- 66.CDC. Menu of State Hospital Influenza Vaccination Laws. Published 2016. [Google Scholar]
- 67.Fielding JE. Health Officer Order for Annual Influenza Vaccination Programs for Healthcare Personnel or Masking of Health Care Workers during the Influenza Season. County of Los Angeles Department of Public Health. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.