Abstract
Background:
Phase of Illness describes stages of advanced illness according to care needs of the individual, family and suitability of care plan. There is limited evidence on its association with other measures of symptoms, and health-related needs, in palliative care.
Aims:
The aims of the study are as follows. (1) Describe function, pain, other physical problems, psycho-spiritual problems and family and carer support needs by Phase of Illness. (2) Consider strength of associations between these measures and Phase of Illness.
Design and setting:
Secondary analysis of patient-level data; a total of 1317 patients in three settings. Function measured using Australia-modified Karnofsky Performance Scale. Pain, other physical problems, psycho-spiritual problems and family and carer support needs measured using items on Palliative Care Problem Severity Scale.
Results:
Australia-modified Karnofsky Performance Scale and Palliative Care Problem Severity Scale items varied significantly by Phase of Illness. Mean function was highest in stable phase (65.9, 95% confidence interval = 63.4–68.3) and lowest in dying phase (16.6, 95% confidence interval = 15.3–17.8). Mean pain was highest in unstable phase (1.43, 95% confidence interval = 1.36–1.51). Multinomial regression: psycho-spiritual problems were not associated with Phase of Illness (χ2 = 2.940, df = 3, p = 0.401). Family and carer support needs were greater in deteriorating phase than unstable phase (odds ratio (deteriorating vs unstable) = 1.23, 95% confidence interval = 1.01–1.49). Forty-nine percent of the variance in Phase of Illness is explained by Australia-modified Karnofsky Performance Scale and Palliative Care Problem Severity Scale.
Conclusion:
Phase of Illness has value as a clinical measure of overall palliative need, capturing additional information beyond Australia-modified Karnofsky Performance Scale and Palliative Care Problem Severity Scale. Lack of significant association between psycho-spiritual problems and Phase of Illness warrants further investigation.
Keywords: Palliative care, Phase of Illness, casemix, patient-centred outcome measures, care planning
What is already known about the topic?
Phase of Illness, used for casemix classification for palliative care in Australia and now the United Kingdom, predicts resource use and has fair inter-rater reliability.
Its relationship to validated measures of clinical need has not been demonstrated.
What this paper adds?
The different phases of illness are characterised by differences in function, pain, other physical problems, psycho-spiritual problems and family and carer support needs.
Pain is worst in the unstable phase, family and carer support needs are greatest in the deteriorating phase and function is worst in the dying phase.
Phase of Illness has been used as a measure to reflect changing care needs and captures additional clinical information beyond the combination of Australia-modified Karnofsky Performance Scale (AKPS) and Palliative Care Problem Severity Scale (PCPSS).
Implications for practice, theory or policy
Phase of Illness can be used to inform clinical interventions, casemix-adjust quality measures and potentially to underpin value-based reimbursement mechanisms in palliative care.
Palliative care teams should be trained in using Phase of Illness and be incentivized to record it routinely across all settings.
Introduction
‘Phase of Illness’ is used in advanced illness to describe the distinct stages of an individual’s illness according to the care needs of the individual, the family and the suitability of the current care plan to address these needs.1 It was originally derived from work on casemix classification within palliative care in Australia and is a major determinant of resource use.2
Patients are classified into one of five phases, outlined in Table 1, with a new phase assigned whenever a clinical change requires patient and family re-assessment and modification of the existing care plan. Phase of Illness is (except for the dying and deceased phases) not reliant on stage of disease and prognosis, and patients may move between phases in any sequence.3 Distinct characteristics of Phase of Illness are (1) its relationship with needs and resource use and (2) the individual and family as the unit of care. The term ‘episode of care’ is used to define the period of contact between a patient and a provider or team of providers that occurs in one setting, beginning with the first contact with the patient and ending at discharge or when the patient dies. One episode of care may comprise one, or multiple phases; mean number of phases per episode has been reported as 1.68 for inpatient care and 1.31 for community care.4
Table 1.
Definitions of Phase of Illness, for use in palliative care/advanced illness.1
| Phase of Illness | Patient in this phase when | Phase ends when |
|---|---|---|
| Stable | Patient’s problems and symptoms are adequately controlled by the established plan of care and further interventions to maintain symptom control and quality of life have been planned and family/carer situation is relatively stable and no new issues are apparent. | The needs of the patient and/or family/carer increase, requiring changes to the existing plan of care. |
| Unstable | An urgent change in the plan of care or emergency treatment is required because the patient experiences a new problem that was not anticipated in the existing plan of care and/or the patient experiences a rapid increase in the severity of a current problem and/or family/carer circumstances change suddenly impacting patient care. | The new plan of care is in place, it has been reviewed and no further changes to the care plan are required. This does not necessarily mean that the symptom/crises has fully resolved but there is a clear diagnosis and plan of care (i.e. patient is stable or deteriorating) and/or death is likely within days (i.e. patient is now dying). |
| Deteriorating | The care plan addresses anticipated needs, but requires periodic review, because the patient’s overall functional status declines and the patient experiences a gradual worsening of existing problem(s) and/or the patient experiences a new, but anticipated, problem and/or the family/carer experiences gradual worsening distress that impacts the patient care. | Patient condition plateaus (i.e. patient is now stable) or an urgent change in the care plan or emergency treatment and/or family/carers experience a sudden change in their situation that impacts patient care, and urgent intervention is required (i.e. patient is now unstable) or death is likely within days (i.e. patient is now dying). |
| Dying | Dying: death is likely within days. | Patient dies or patient condition changes and death is no longer likely within days (i.e. patient is now stable and/or deteriorating). |
| Deceased | The patient has died; bereavement support provided to family/carers is documented in the deceased patient’s clinical record. | Case is closed. |
Phase of Illness was originally developed, through clinical consensus, as a key component of a casemix classification for palliative care.4 Casemix classifications categorise patients into homogeneous groups of need and resource use on the basis of key predictor variables and, in addition to underpinning reimbursement systems within healthcare, provide a means of capturing and communicating patient care needs. In other areas of healthcare, especially within acute hospital-based specialties such as surgery and orthopaedics, diagnosis and interventions often form the basis of casemix classifications, as in the healthcare resource group system in England.5 However, in palliative care, diagnosis is a poor predictor of need and resource use, a challenge common to some other areas of healthcare where improvements in quality of life and function are the goals of care (such as psychiatry, psycho-geriatrics and rehabilitation medicine).6 Phase of Illness was thus developed in response to this challenge, as the key clinical variable within a casemix classification for palliative care in Australia.7 The original definitions of the different phases have been reviewed in recent years with little clinical call for change.1 Phase of Illness remains the basis of the most recent iteration of a casemix classification for palliative care in Australia and has recently been introduced in England in this capacity.8,9
It is important to know how Phase of Illness behaves alongside other validated measures of symptoms and needs in palliative care. A recent study across 10 palliative care services in Australia reported fair inter-rater reliability and acceptability.1 But there remains little published evidence on the association of Phase of Illness with other validated measures of clinical needs. In order to address this knowledge-gap, this study aims to
Describe the distribution of (a) function, (b) pain, (c) other physical problems, (d) psycho-spiritual problems and (e) family and carer support needs, by Phase of Illness;
Examine, using multinomial logistic regression, associations between these five domains and Phase of Illness.
Methods
Setting and participants
A secondary analysis was undertaken of individual patient-level data collected as part of routine clinical care between March 2012 and December 2013 from patients attending three palliative care services within the South East of England. These data were collected as part of a pilot data collection following recommendations by the independent Palliative Care Funding Review in England.10 The hospital support service (‘hospital’) provides a specialist consultative and advisory palliative care service within one hospital site with approximately 1000 beds. The inpatient palliative care unit (‘hospice’) provides on-site, 24-h, multi-disciplinary specialist palliative care to individuals with life-limiting illness within a 48-bed facility. The community palliative care service (‘community’) provides specialist palliative care services to individuals within their primary place of residence, including advice and support to general palliative care providers in the community. All adult patients attending the services over the time period of data collection were eligible for inclusion in this analysis.
Data collection
For each participant, any member of the specialist palliative care team (i.e. doctors, registered nurses, nurse practitioners or social workers) with knowledge of the individual’s clinical status made an assessment of Phase of Illness, along with function, pain, other physical problems, psycho-spiritual problems and family and carer support needs, at entry into the service and at each subsequent change in Phase of Illness (determined by the palliative care team member as defined above). Function was measured on an 11-point scale, the Australia-modified Karnofsky Performance Scale (AKPS), anchored between 0 (deceased) and 100 (best possible function), demonstrated to have good face and construct validity.11 Pain, other physical problems, psycho-spiritual problems and family and carer support needs were all measured using items within the Palliative Care Problem Severity Scale (PCPSS), rated as 0 (absent), 1 (mild), 2 (moderate) and 3 (severe). These items have been demonstrated to have good inter-rater reliability for the measurement of these domains.12 Clinical and demographic data were extracted from the clinical record. Trained administrators entered data into Microsoft Excel, which was then imported into SPSS version 22. The data were anonymised at point of extraction.
Statistical analysis
All analyses were undertaken using SPSS version 22. Analysis was restricted to the first phase within an episode of care. Where an individual had more than one episode of care, the first episode of care within the time period of analysis was used. This approach was adopted to satisfy the assumption of independence of observations necessary for the analyses.
Sample characteristics were described using mean and standard deviation (SD) for continuous variables, and frequencies and percentages for categorical variables. Function, pain, other physical problems, psycho-spiritual problems and family and carer support needs were treated as continuous variables, and described using the mean and 95% confidence intervals (CIs). Phase of Illness was treated as a categorical variable. Comparison of AKPS and items on the PCPSS across Phase of Illness was undertaken using one-way analysis of variance (ANOVA) where assumptions were met (or Welch’s ANOVA in the case of failure to meet the assumption of homogeneity of variance). Post hoc pairwise comparisons were undertaken using Tukey’s honest significant difference (HSD) test or Games Howell test as appropriate, accounting for multiple testing. A multinomial regression was then undertaken with Phase of Illness as the dependent variable. AKPS and items on PCPSS significantly associated with Phase of Illness in the univariate analysis were entered into the model in one block as main effects. We hypothesised that symptom burden would be highest in the unstable phase. We therefore chose to present the analysis using the unstable phase as the reference category, to enable assessment of differences between this category and the other categories of Phase of Illness.
Ethical approval
The study used data that were fully anonymised and therefore did not require approval from a research ethics committee in line with King’s College London’s Research Ethics Committee procedures. The protocol for data extraction and analysis was approved by Caldicott Guardians within the participating organisations before commencement of the project.
Results
Sample characteristics
The initial sample comprised 1317 individuals, who experienced 2354 phases of illness, within 1578 episodes of care. The first phase within the first episode of care for each of these 1317 individuals was used for the analysis; this consisted of 241 stable phases, 618 unstable phases, 336 deteriorating phases and 122 dying phases. Table 2 outlines the sample characteristics. The mean age of the sample was 72.9 (SD ± 14.1 years). Fifty-one percent were female. Seventy-one percent were based in the community, 15.2% in the hospice and 13.7% in the hospital at the time of assessment. Seventy-five percent of the sample had a primary diagnosis of cancer.
Table 2.
Clinical and baseline characteristics (n = 1317).
| Characteristics | |
|---|---|
| Age, mean ± SD, years | 72.9 ± 14.1 |
| Gender, n (%) | |
| Male | 664 (49.5) |
| Female | 652 (50.5) |
| Missing | 1 (0.0) |
| Care setting, n (%) | |
| Home | 936 (71.1) |
| Hospice | 200 (15.2) |
| Hospital | 181 (13.7) |
| Diagnosis, n (%) | |
| Cancer | |
| Gastrointestinal (including pancreas) | 267 (20.2) |
| Lung | 202 (15.3) |
| Genitourinary | 189 (14.4) |
| Breast | 91 (6.9) |
| Haematological | 68 (5.2) |
| Brain | 41 (3.1) |
| Head and neck | 25 (1.9) |
| Other cancer | 109 (8.3) |
| Non-cancer | |
| Chronic obstructive pulmonary disease | 52 (3.9) |
| Dementia | 45 (3.4) |
| Neurological | 41 (3.1) |
| Cardiac failure | 41 (3.0) |
| Chronic renal failure | 26 (2.0) |
| Liver failure | 8 (0.6) |
| Other non-cancer conditions | 113 (8.7) |
Level of palliative care need by Phase of Illness
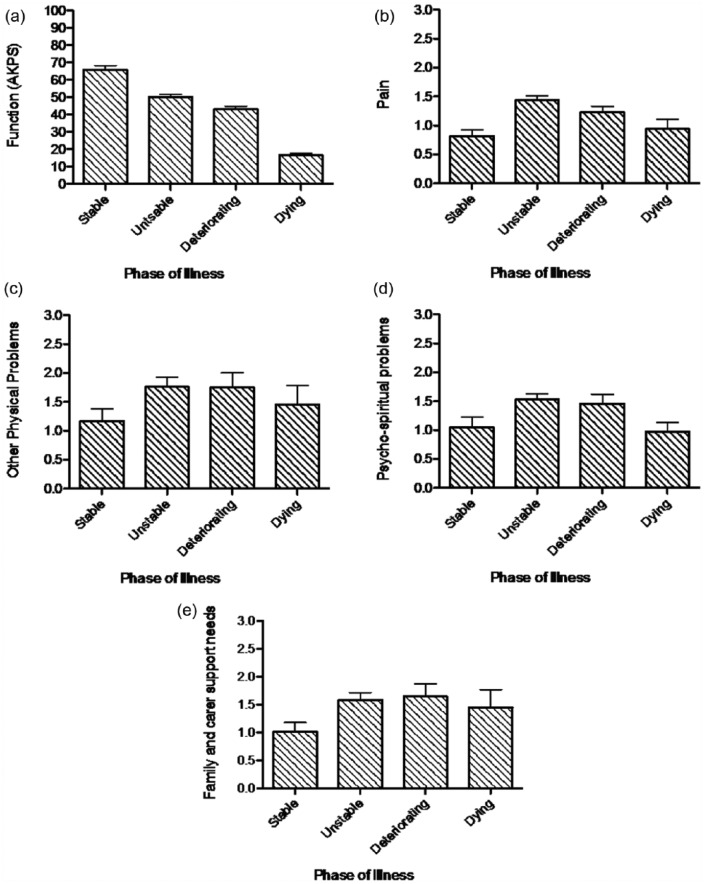
Function, pain, other physical problems, psycho-spiritual problems and family and carer support needs varied significantly by Phase of Illness, as reported in Table 3 and depicted in Figure 1. Function was highest in the stable phase, lowest in the dying phase and higher in the unstable phase than the deteriorating phase; the difference in function was significant across all pairwise comparisons (at p < 0.05). Pain, other physical problems and psycho-spiritual problems were all significantly higher in the unstable and deteriorating phases than the stable and dying phases (at p < 0.05), with higher pain additionally discriminating the unstable from the deteriorating phase (p < 0.05). Family and carer support needs did not differ significantly between the unstable, deteriorating and dying phases, but in all three phases were significantly higher than in the stable phase (at p < 0.05).
Table 3.
Mean level of function, pain, other physical problems, family and carer support needs and psycho-spiritual problems by Phase of Illness.
| Functiona (n = 1305) |
Painb (n = 1261) |
Other physical problemsc (n = 1260) |
Psycho-spiritual problemsd (n = 1259) |
Family and carer support needse (n = 1259) |
|
|---|---|---|---|---|---|
| Possible range: 0 (worst)–100 (best) | Possible range: 0 (best)–3 (worst) | Possible range: 0 (best)–3 (worst) | Possible range: 0 (best)–3 (worst) | Possible range: 0 (best)–3 (worst) | |
| Stable | 65.9 (63.4–68.3) | 0.82 (0.72–0.92) | 1.29 (1.19–1.38) | 1.12 (1.02–1.22) | 1.08 (0.98–1.18) |
| Unstable | 50.2 (48.8–51.6) | 1.43 (1.36–1.51) | 1.87 (1.81–1.93) | 1.56 (1.49–1.63) | 1.64 (1.57–1.71) |
| Deteriorating | 42.9 (41.0–44.9) | 1.23 (1.13–1.33) | 1.92 (1.83–2.00) | 1.53 (1.44–1.62) | 1.78 (1.69–1.87) |
| Dying | 16.6 (15.3–17.8) | 0.94 (0.77–1.11) | 1.62 (1.45–1.79) | 0.97 (0.81–1.14) | 1.61 (1.47–1.77) |
Difference in mean function by Phase of Illness statistically significant by one-way ANOVA (Welch’s F(3, 546.040) = 656.287, p < 0.001). Post hoc pairwise comparisons (Games Howell test) demonstrated statistically significant difference in the level of function for all pairwise comparisons (p < 0.05).
Difference in mean pain by Phase of Illness statistically significant by one-way ANOVA (Welch’s F(3, 409.635) = 34.902, p < 0.001). Post hoc comparisons (Games Howell test) demonstrated statistically significant difference in all pairwise comparisons (p < 0.05) except between the stable and the dying phases (p = 0.619).
Difference in mean other physical problems by Phase of Illness statistically significant by one-way ANOVA (Welch’s F(3, 390.481) = 39.930, p < 0.001). Post hoc comparisons (Games Howell test) demonstrated statistically significant difference in all pairwise comparisons (p < 0.05) except between the unstable and the deteriorating phases (p = 0.766).
Difference in mean psycho-spiritual problems by Phase of Illness statistically significant by one-way ANOVA (Welch’s F(3, 403.815) = 28.356, p < 0.001). Post hoc pairwise comparisons (Games Howell test) demonstrated statistically significant difference in the level of psycho-spiritual problems for all pairwise comparisons (p < 0.05) except between the unstable and the deteriorating phases (p = 0.979) and the stable and the dying phases (p = 0.431).
Difference in mean family and carer support needs by Phase of Illness statistically significant by one-way ANOVA (Welch’s F(3, 405.727) = 38.778, p < 0.001). Post hoc pairwise comparisons (Games Howell test) demonstrated statistically significant difference in family and carer support needs between the stable phase and all other phases (p < 0.05), but no other statistically significant differences (p < 0.05).
Figure 1.
Mean level of (a) function, (b) pain, (c) other physical problems, (d) psycho-spiritual problems and (e) family and carer support needs by Phase of Illness.
Reports are based on the analysis of available data: (a) 1305 phases, (b) 1261 phases, (c) 1260 phases, (d) 1259 phases and (e) 1259 phases (n = 1317). Figures within each bar denote mean value for corresponding Phase of Illness. Error bars denote 95% confidence interval around the mean.
Multinomial logistic regression
According to the procedure outlined in the ‘Methods’ section, a multinomial logistic regression model was fitted to the data, with ‘Phase of Illness’ as the dependent variable, and AKPS and the four PCPSS items entered as independent variables (Table 4). A model containing all five variables provided a good overall fit to the data (Pearson’s χ2 = 1516.796, df = 1779, p = 1.000) and was significantly better than a constant-only model (χ2 = 741.034, df = 15, p < 0.005). Function (χ2 = 478.999, df = 3, p < 0.005), pain (χ2 = 47.521, df = 3, p < 0.005), other physical problems (χ2 = 24.369, df = 3, p < 0.005) and family and carer support needs (χ2 = 21.435, df = 3, p < 0.005) all made a significant contribution to the model, with function most strongly associated with Phase of Illness. Only the psycho-spiritual problem score, after controlling for all other variables in the model, was not associated with Phase of Illness (χ2 = 2.940, df = 3, p = 0.401). Forty-nine percent of the variance in Phase of Illness was explained by measures of function, pain, other physical problems and family and carer support needs (Nagelkerke’s pseudo R2 = 0.488).
Table 4.
Multinomial logistic regression of Phase of Illness (dependent variable) using function, pain, other physical problems, psycho-spiritual problems and family and carer support needs as independent variables, based on analysis of 1252 admission phases – unstable phase as the reference phase.
| Function | Pain | Other physical problems | Psycho-spiritual problems | Family and carer support needs | |
|---|---|---|---|---|---|
| Unstable | |||||
| Stable vs | 1.44 (1.30–1.59) | 0.51 (0.41–0.63) | 0.57 (0.45–0.73) | 0.87 (0.68–1.11) | 0.68 (0.54–0.85) |
| Deteriorating vs | 0.81 (0.75–0.88) | 0.77 (0.66–0.90) | 1.03 (0.85–1.26) | 0.94 (0.77–1.14) | 1.23 (1.01–1.49) |
| Dying vs | 0.13 (0.08–0.19) | 0.73 (0.53–0.99) | 1.10 (0.77–1.57) | 0.78 (0.55–1.11) | 1.16 (0.83–1.61) |
Data are based on the analysis of 1252 complete cases due to missing data on 65 cases. Model χ2 = 741.034, df = 15, p < 0.005; Pearson’s χ2 = 1516.796, df = 1779, p = 1.000; Nagelkerke’s pseudo R2 = 0.488. Numbers indicate odds ratios (95% confidence intervals in brackets) for the association between the reference phase (italics) and the corresponding comparison phase.
Table 4 reports the odds ratios (ORs) for the association of AKPS and the four PCPSS items with Phase of Illness, with unstable phase as the reference category. The results are consistent with the univariate analysis, although with some exceptions. In the multivariate analysis, other physical problems were higher in the dying phase than both the deteriorating phase and the unstable phase, and the differences between these three phases did not reach statistical significance in this additional analysis. Family and carer support needs, in addition to being lower in the stable phase as compared to all other phases, consistent with the univariate analysis, were also significantly higher in the deteriorating phase than the unstable phase (OR (deteriorating vs unstable) = 1.23 (95% CI = 1.01−1.49)). In additional analyses, the difference in family and carer support needs between the deteriorating and unstable phases reached significance after adjustment for pain alone, significantly higher in the unstable phase, (OR (deteriorating vs unstable) = 1.25 (1.06–1.48), p = 0.007) and retained significance after adjusting for function, significantly lower in the deteriorating phase (OR (deteriorating vs unstable) = 1.20 (1.01–1.42), p = 0.034).
Discussion
Main findings
In this study, we have described the significant differences in palliative care needs (as represented by measures of function, pain, other physical problems, psycho-spiritual problems and family and carer support needs) according to Phase of Illness. The variance in Phase of Illness was not accounted for entirely by AKPS and the selected items on the PCPSS; however, demonstrating that Phase of Illness captures more than the combination of these measures. These findings underpin the value of Phase of Illness in clinical practice as a measure of overall clinical need, and potentially as a determinant of resource use to meet those needs.
Considering the characteristics of the individual phases, these were consistent with the definitions outlined in Table 1. Thus, the stable phase was differentiated from all other phases by higher function, lower other physical problems and lesser family and carer support needs. Furthermore, a lower pain level distinguished the stable phase from all except the dying phase. The unstable phase was discriminated from all other phases by a higher level of pain. A lower level of function distinguished the deteriorating phase from the stable and unstable phases, and the dying phase from all other phases. These results, while largely anticipated, illuminate the constructs used by healthcare providers when assigning Phase of Illness and serve to illustrate that Phase of Illness has clinically meaningful relationships with validated measures of function and problem severity.
A number of findings deserve further consideration. While psycho-spiritual problems varied significantly with Phase of Illness in the univariate analysis, they were not associated with Phase of Illness in the multivariate analysis. This may reflect the limitation of a 4-point scale to sufficiently discriminate between higher and lower levels of psycho-spiritual problems. The PCPSS has demonstrated good inter-rater reliability but it is yet to undergo further validation.12 An alternate possibility, however, is that psycho-spiritual problems are addressed over longer time periods than a single phase, or may be less likely to trigger a change in care plan, and hence Phase of Illness. Psychological and emotional support has been incorporated within sets of quality indicators for palliative care and the possibility that such symptoms, of themselves, do not trigger a change in the plan of care and Phase of Illness, is of concern and requires further investigation.13,14
The finding that family and carer support needs were significantly greater in the deteriorating phase than the unstable phase in the multivariate analysis was of further interest. Pain (highest in the unstable phase) has been demonstrated to be positively associated with caregiver burden.15 The difference in family and carer support needs between the deteriorating and unstable phases reached significance after adjustment for pain alone. Reduced function, a feature of the deteriorating phase, has been associated with increased caregiver burden.16,17 However, the increase in family and carer support needs in the deteriorating phase compared to the unstable phase retained significance after adjusting for reduced function suggesting there are other factors – in addition to reduced function – driving the higher family and carer support needs associated with the deteriorating phase. Of note, we did not explore the role of place of care as a driver of family and carer support needs. Place of care is conceivably associated with both Phase of Illness and family and carer support needs, and was not adjusted for in the analysis. Future studies should focus on understanding the role of place of care and other potential factors in mediating increased family and carer support needs in the deteriorating phase.
Finally, it is of interest that the combination of PCPSS and AKPS did not fully account for the variation in Phase of Illness (accounting for 49% by Nagelkerke’s pseudo R2). In making the determination of Phase of Illness, this suggests that healthcare providers are taking considerations beyond the combination of AKPS and PCPSS into account. In this sense, Phase of Illness can be considered to capture clinical information in addition to the combination of these instruments. We examined only the relationship between the absolute values on the PCPSS, AKPS and Phase of Illness. It is conceivable that change in these dimensions is a greater determinant of Phase of Illness than absolute values.
Strengths and limitations
Understanding the relationship between Phase of Illness and existing validated measures of need in palliative care is important and valuable. Phase of Illness already forms the basis of the casemix classification in operation in Australia as well as the newly developed casemix classification for specialist palliative care within England, which is currently undergoing evaluation.2,18 A considerable strength of the study was the successful use of routinely collected individual patient-level data to answer the research question, an approach that is both economical and facilitates research on a large sample.19 The analysis of data from a large number of patients, with a range of diagnoses, in a variety of settings, serves furthermore to enhance the generalisability of the study’s findings.
The study’s limitations merit further consideration. First, while the use of routinely collected data has its strengths (described above), there are also a number of limitations, specifically constraints in terms of the range of variables measured, the consistency and quality of data recording, and the instruments used. In particular, pain, other physical problems, psycho-spiritual problems and family and carer support needs were all measured using the PCPSS. While this measure is yet to undergo formal validation, it has good inter-rater reliability and has been used extensively in the Palliative Care Outcomes Collaborative, a national initiative in Australia to collate and compare patient-centred outcomes among palliative care services.3,12 However, the four 4-point subscales may be limited in scope or sensitivity to capture the full range of problems experienced by this population, which could account for some of the unexplained variation in Phase of Illness in the multivariate analysis. Other instruments, such as the Palliative care Outcome Scale or the Edmonton Symptom Assessment Scale, could be considered in the collection of patient-reported outcomes.20 A further limitation of this study is the risk of sampling bias. The study sample is broadly representative of patients receiving palliative care based on gender, age and diagnosis; however, lack of data on individuals not included and reasons for exclusion limits our evaluation of sample bias.21 Future studies relying on the use of routinely collected data would be strengthened by characterisation of all eligible individuals within the service over the time period of analysis, independent of their inclusion in the final sample.
Conclusion
This study, to our knowledge, is the first to examine the association between Phase of Illness and validated measures of palliative care need, including function, pain, other physical problems, psycho-spiritual problems and family and carer support needs. The findings are of great importance in providing an understanding of the metrics used by healthcare providers to inform Phase of Illness and in characterising the nature of the different phases. Furthermore, they provide necessary and timely evidence to support the use of Phase of Illness as a measure of need within palliative care.
Acknowledgments
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, MRC, CCF, NETSCC or the Department of Health. CLAHRC South London is part of the National Institute for Health Research (NIHR) and is a partnership between King’s Health Partners; St George’s, University of London; and St George’s Healthcare NHS Trust.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This paper presents independent research funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research Scheme (RP-PG-1210-12015) and is supported in part by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research & Care (CLAHRC) South London. Fund Ref Funding Sources Programme Grants for Applied Research: Grant/Award Number: ‘RP-PG-1210-12015’.
References
- 1. Masso M, Allingham SF, Banfield M, et al. Palliative care phase: inter-rater reliability and acceptability in a national study. Palliat Med 2015; 29: 22–30. [DOI] [PubMed] [Google Scholar]
- 2. Eagar K, Gordon R, Hodkinson A, et al. The Australian national sub-acute and non-acute casemix classification (AN-SNAP): report of the national sub-acute and non-acute casemix classification study. Report, University of Wollongong, Wollongong, NSW, Australia, August 1997. [Google Scholar]
- 3. Currow DC, Allingham S, Yates P, et al. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 2015; 23: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eagar K, Gordon R, Green J, et al. An Australian casemix classification for palliative care: lessons and policy implications of a national study. Palliat Med 2004; 18: 227–233. [DOI] [PubMed] [Google Scholar]
- 5. Grasic K, Mason AR, Street A. Paying for the quantity and quality of hospital care: the foundations and evolution of payment policy in England. Health Econ Rev 2015; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner-Stokes L, Sutch S, Dredge R, et al. International casemix and funding models: lessons for rehabilitation. Clin Rehabil 2012; 26: 195–208. [DOI] [PubMed] [Google Scholar]
- 7. Eagar K, Green J, Gordon R. An Australian casemix classification for palliative care: technical development and results. Palliat Med 2004; 18: 217–226. [DOI] [PubMed] [Google Scholar]
- 8. Development of ANSNAP Version 4. Final Report, Centre for Health Service Development, University of Wollongong, Wollongong, NSW, Australia, May 2015. [Google Scholar]
- 9. Developing a new approach to palliative care funding. Final Report, NHS England, Leeds, 22 March 2015. [Google Scholar]
- 10. Developing a new approach to palliative care funding. a first draft for discussion. Report, NHS England, Leeds, October 2014. [Google Scholar]
- 11. Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky performance status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care 2005; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masso M, Allingham SF, Johnson CE, et al. Palliative care problem severity score: reliability and acceptability in a national study. Palliat Med 2015; 30: 479–485. [DOI] [PubMed] [Google Scholar]
- 13. Dy SM, Kiley KB, Ast K, et al. Measuring what matters: top-ranked quality indicators for hospice and palliative care from the American academy of hospice and palliative medicine and hospice and palliative nurses association. J Pain Symptom Manage 2015; 49: 773–781. [DOI] [PubMed] [Google Scholar]
- 14. Leemans K, Deliens L, Van den Block L, et al. Systematic quality monitoring for specialized palliative care services: development of a minimal set of quality indicators for palliative care study (QPAC). Am J Hosp Palliat Care 2017; 34: 532–546. [DOI] [PubMed] [Google Scholar]
- 15. Harding R, Higginson IJ, Donaldson N. The relationship between patient characteristics and carer psychological status in home palliative cancer care. Support Care Cancer 2003; 11: 638–643. [DOI] [PubMed] [Google Scholar]
- 16. Rha SY, Park Y, Song SK, et al. Caregiving burden and the quality of life of family caregivers of cancer patients: the relationship and correlates. Eur J Oncol Nurs 2015; 19: 376–382. [DOI] [PubMed] [Google Scholar]
- 17. Guerriere D, Husain A, Zagorski B, et al. Predictors of caregiver burden across the home-based palliative care trajectory in Ontario, Canada. Health Soc Care Community 24: 428–438. [DOI] [PubMed] [Google Scholar]
- 18. Joint statement from NHS England and public health England: update on development of the palliative care clinical data set and palliative care currencies. Report, Public Health England, London, April 2016. [Google Scholar]
- 19. Davies JM, Gao W, Sleeman KE, et al. Using routine data to improve palliative and end of life care. BMJ Support Palliat Care 6: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pidgeon T, Johnson CE, Currow D, et al. A survey of patients’ experience of pain and other symptoms while receiving care from palliative care services. BMJ Support Palliat Care 6: 315–322. [DOI] [PubMed] [Google Scholar]
- 21. National survey of patient activity data for specialist palliative care services. Report, National Council for Palliative Care, London, July 2016. [Google Scholar]