Abstract
Background
Lithium is widely used to treat bipolar disorders and displays mood stabilizing properties. In addition, lithium relieves painful cluster headaches and has a strong analgesic effect in neuropathic pain rat models.
Objectives
To investigate the analgesic effect of lithium on the cuff model of neuropathic pain.
Methods
We used behavioral and pharmacological approaches to study the analgesic effect of a single injection of lithium in wild-type and mu opioid receptor (MOR) null cuffed neuropathic mice. Mass spectrometry and enzyme-linked immunosorbent assay allowed to measure the levels of endogenous MOR agonist beta-endorphin as well as monoamines in brain and plasma samples 4 h after lithium administration.
Results
A single injection of lithium chloride (100 mg/kg, ip) alleviated mechanical allodynia for 24 h, and this effect was absent in MOR null neuropathic mice. Biochemical analyses highlight a significant increase in beta-endorphin levels by 30% in the brain of lithium-treated mice compared to controls. No variation of beta-endorphin was detected in the blood.
Conclusions
Together, our results provide evidence that lithium induces a long-lasting analgesia in neuropathic mice presumably through elevated brain levels of beta-endorphin and the activation of MORs.
Keywords: Lithium, neuropathy, analgesia, mu opioid receptor, beta-endorphin, monoamines
Background
Lithium is a metallic ion displaying chaotropic and denaturating properties.1 This metal is described to treat bipolar disorders,2 to be able to reduce painful cluster headache3 and to decrease inflammation.4 Several research groups reported functional interactions between lithium and the opioid system. In particular, lithium affects morphine-induced analgesia,5,6 and reduces morphine tolerance and dependence.7,8 Lithium attenuates thermal hyperalgesia and mechanical allodynia in different models of neuropathic pain in rats via a naloxone-sensitive mechanism, suggesting that lithium action is opioid receptor dependent.9,10 In addition, lithium prevents paclitaxel-induced peripheral neuropathy,11 increases survival by allowing the use of higher doses of paclitaxel, and prevents paclitaxel-induced cardiac abnormalities.
The present study reveals that a single injection of lithium alleviates neuropathic pain symptoms in a mouse model of sciatic nerve chronic constriction and demonstrates for the first time mu opioid receptor (MOR) involvement in lithium analgesia. In this context, the potential variations of endogenous opioid as well as monoamine levels have been studied after lithium injection.
Methods
Animals
Experiments were performed with 45-day-old adult male C57BL/6J mice (25 ± 4 g; Charles River, L’Arbresle, France). MOR null mice were a generous gift from Professor B. Kieffer (IGBMC, Illkirch-Graffenstaden, France). Animals were given free access to food and water, with a 12 h light-dark cycle at a temperature of 22°C ± 2°C. All procedures were performed in accordance with European directives (2010/63/EU) and were approved by the regional ethics committee and the French Ministry of Agriculture (license No. 00456.02 to Y.G.). The right sciatic nerve was cuffed with a section of polyethylene tubing (cuff group) as previously described.12 Briefly, surgeries were done under aseptic conditions and ketamine/xylazine was used for anesthesia (ketamine: 17 mg/mL, i.p., xylazine: 2.5 mg/mL, i.p., 4 mL/kg; Centravet, Taden, France). After performing a 1.5 cm skin incision of the right hind thigh, a 2 mm long polyethylene tubing was placed on the common branch of the right sciatic nerve (internal diameter = 0.38 mm, external diameter = 1.09 mm; PE-20, Harvard Apparatus, Les Ulis, France) and sutures were used to close the skin.12 Sham-operated mice underwent the same surgical procedure as cuffed animals without implantation of the cuff.
Response to mechanical stimuli
The mechanical threshold for hind paw withdrawal was determined using Von Frey hairs as previously described.12 Intraperitoneal injections of a solution of 100 mg/kg of lithium chloride (corresponding to 16.4 mg/ml of lithium; Sigma-Aldrich, St. Louis, USA) diluted in NaCl 0.9% (w/v; saline), naloxone (Sigma-Aldrich) diluted in saline or an equivalent volume of saline were performed at 10 am. Injections of naloxone13 (0.1 mg/kg, s.c.) were performed 4 h after the injections of lithium (corresponding to the peak of analgesia observed for neuropathic mice). Hind paw withdrawal was determined 15 min later.
Response to thermal stimuli
Mice were placed during 15 min in clear Plexiglas boxes (7 cm × 9 cm× 7 cm) on a glass surface.14 The infrared beam of the radiant heat source (7370 Plantar Test, Ugo Basile, Comerio, Italy) was applied to the plantar surface of each hind paw. The cut-off to prevent damage to the skin was set at 15 s. The paw withdrawal latency was tested 3 times 4 h after lithium injection and was averaged for each hind paw.
Sample preparation
Plasma was prepared from blood recovered in tubes containing 50 µl of 2% EDTA (w/v) and protease inhibitors (cOmplete Mini EDTA-free, Roche, Basel, Switzerland).
Each brain was homogenized in 2 ml of 0.5 µM ascorbic acid containing protease inhibitors and sonicated for 10 s at 90 W and centrifuged (20,000 g, 15 min, 4°C). Supernatant was recovered and protein content was determined using the Protein Assay kit (Bio-Rad, Marnes-la-Coquette, France).
Monoamines and catecholamines derivatization
The presence of L-DOPA, dopamine, adrenaline, noradrenaline, serotonin, and adenosine was studied. Twenty microliters of tissue extracts or plasma were derived with the AccQ-Tag Ultra Derivatization kit (Waters, Guyancourt, France). Twenty microliters of the sample were added to 30 µl of borate buffer (provided within the kit) and 10 µl of internal standards ([2H3]-L-DOPA, [2H4]-dopamine, [2H6]-adrenaline, [13C6]-noradrenaline, [2H4]-serotonin, [13C5]-adenosine; Sigma Aldrich and Alsachim, Illkirch, France). Derivatization was performed by addition of 10 µl of AccQtag Ultra reagent (10 min, 55°C under agitation). Ten microliters of this solution were analyzed using a LC-MS/MS approach.
Beta-endorphin enzyme-linked immunosorbent assay
Beta-endorphin concentrations in the brain and plasma were quantified using a direct enzyme-linked immunosorbent assay (ELISA) (M0184 ELISA, Clinisciences-Elabscience, Nanterre, France) according to the manufacturer’s instruction. Samples (50 µl) were analyzed in duplicate. All samples with a duplicate CV > 5% were retested to obtain a CV below or equal to 5%. Detection range was 15.63-1000 pg/ml and sensitivity was 9.38 pg/ml of beta-endorphin.
LC-MS/MS instrumentation and analytical conditions
Analyses were performed with a Dionex Ultimate, 3000 HPLC system (Thermo Scientific, San Jose, USA) coupled with a triple quadrupole Endura. The system was controlled by Xcalibur v2.0 software. Samples were loaded onto an Accucore RP-MS column (100 × 2.1 mm, 2 μm, Thermo Electron) heated at 50°C. Buffer A was H2O 99.9%/formic acid 0.1% (v/v), whereas buffer B was ACN 99.9%/formic acid 0.1% (v/v). Gradient used is detailed in Table 1.
Table 1.
LC and MS conditions for the purification and the detection of catecholamines and monoamines and their respective heavy tagged counterparts.
|
HPLC gradient | ||||||||||||
| Time (min) | 0 | 2.5 | 4.5 | 6.5 | 7.5 | 8 | 10 | |||||
| % B buffer | 1 | 1 | 30 | 99 | 99 | 1 | 1 | |||||
|
MS ionization, selection, fragmentation, and identification parameters | ||||||||||||
|
Compound |
Polarity |
Precursor (m/z) |
Product (m/z) |
Ion product type |
Collision energy (V) |
|||||||
| Adenosine | Positive | 268.25 | 136.07 | Qualification and Quantification | 26 | |||||||
| C5-Adenosine | Positive | 273.25 | 136.18 | Qualification and Quantification | 23 | |||||||
| AccQ-Tag-D4-dopamine | Positive | 328.25 | 171.16 | Qualification and Quantification | 35 | |||||||
| AccQ-Tag-Dopamine | Positive | 324.25 | 171.16 | Qualification and Quantification | 38 | |||||||
| AccQ-Tag-C6-noradrenaline | Positive | 346.25 | 171.16 | Qualification and Quantification | 35 | |||||||
| AccQ-Tag-noradrenaline | Positive | 340.25 | 171.16 | Qualification and Quantification | 35 | |||||||
| AccQ-Tag-D6-adrenaline | Positive | 360.25 | 171.16 | Qualification and Quantification | 36 | |||||||
| AccQ-Tag-adrenaline | Positive | 354.25 | 171.16 | Qualification and Quantification | 34 | |||||||
| AccQ-Tag-D4-serotonin | Positive | 351.25 | 171.16 | Qualification and Quantification | 40 | |||||||
| AccQ-Tag-serotonin | Positive | 347.25 | 171.16 | Qualification and Quantification | 38 | |||||||
Note: Buffer A corresponded to ACN 1%/H2O 98.9%/formic acid 0.1% (v/v/v), whereas buffer B was ACN 99.9%/formic acid 0.1% (v/v).
Electrospray ionization was achieved in the positive mode with the spray voltage set at 3750 V. Nitrogen was used as the nebulizer gas and the ionization source was heated to 250°C. Desolvation (nitrogen) sheath gas was set to 45 Arb and Aux gas was set to 15 Arb. Ion transfer tube was heated at 350°C. Q1 and Q2 resolutions were set at 0.7 full width at half maximum (FWHM), whereas collision gas (CID, argon) was set to 2 mTorr. Identification of the compounds was based on precursor ion, selective fragment ions, and retention times. Selection of the monitored transitions and optimization of collision energy and radio frequency (RF) lens parameters were manually determined (see Table 1 for details). Qualification and quantification were performed in multiple reaction monitoring (MRM) mode using Quan Browser software (Thermo Scientific). Limits of detection and of quantification for each compound are indicated in Table 2. All amounts of opiates present in samples fit within the standard curve limits. Precision values were <1% for same-day measurements and <5% for inter-day measurements.
Table 2.
Limits of detection (LOD) and limits of quantification (LOQ).
| Adenosine | Adrenaline | Dopamine | L-DOPA | Noradrenaline | Serotonin | |
|---|---|---|---|---|---|---|
| LOD (fmol ± SEM) | 9.77 ± 1.20 | 0.71 ± 0.29 | 4.64 ± 1.54 | 43.31 ± 1.04 | 3.39 ± 0.74 | 24.04 ± 0.22 |
| LOQ (fmol ± SEM) | 32.53 ± 3.99 | 2.36 ± 0.97 | 15.46 ± 5.14 | 144.24 ± 3.45 | 11.29 ± 2.45 | 80.05 ± 0.73 |
Note: LOD was defined as the lowest detectable amount of analyte with a signal-to-noise (S/N) ratio >3. LOQ was defined as the lowest detectable amount of analyte with a signal-to-noise (S/N) ratio >10. Data are presented as the mean ± SEM of five measurements.
Statistics
Statistical analysis was performed using GraphPad Prism 6 Software. Results were presented as mean values ± standard error of the mean. Groups were compared using analysis of variance tests with Bonferroni correction.
Results
Effect of lithium on the mechanical nociceptive threshold in the cuff neuropathic pain model
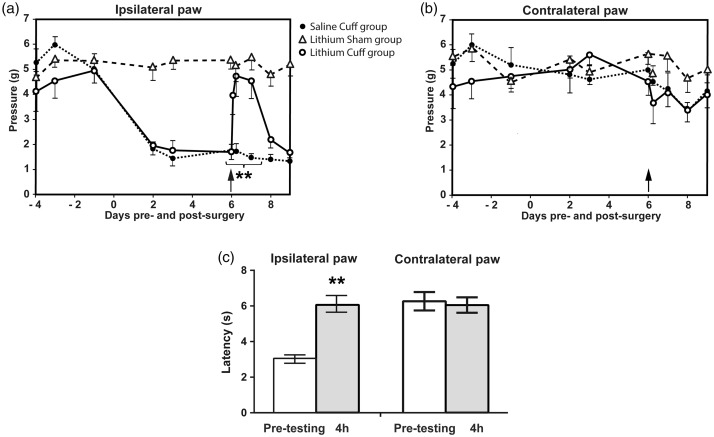
Cuff and Sham mice were tested for mechanical pain threshold using the Von Frey filaments test. The cuff group showed a significant mechanical allodynia compared to the sham group (Figure 1(a)). Lithium injection performed six days after surgery did not affect the mechanical pain threshold of the sham group. Conversely, in the neuropathic cuff group, lithium normalized the ipsilateral mechanical pain threshold to sham group values. This analgesic effect of a single injection lasted for about 24 h (Figure 1(a)). The contralateral paw mechanical pain threshold was unaffected (Figure 1(b)), and no statistical difference before and after lithium injection was observed. Additional experiments were designed to determine the effect of lithium 4 h after administration on thermal hyperalgesia (6 days after the surgery). The Hargreaves test (Figure 1(c)) showed a significant relief of thermal hyperalgesia for the ipsilateral paw compared to the pretest condition (before lithium administration; n = 6; Mann–Whitney U test; p < 0.01). Heat-nociceptive threshold of the contralateral paw was not affected by lithium.
Figure 1.
Antinociceptive effect of lithium chloride (100 mg/kg, i.p., day 6) on sham and cuffed mice. Effect of lithium administration on (a) ipsilateral paw mechanical allodynia and (b) contralateral paw (n = 6; two-way ANOVA test with a Bonferroni correction; **p < 0.01). (c) Effect of lithium 4 h after administration on thermal hyperalgesia (6 days after the surgery; Hargreaves test; n = 6; Mann–Whitney U test; **p < 0.01). Values are means ± SEM.
Effect of naloxone on lithium-induced analgesia
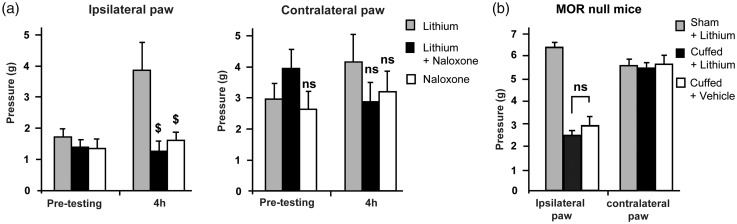
In order to determine if this analgesic effect involves the endogenous opioid system, naloxone (0.1 mg/kg, s.c.), a non-specific opioid receptor antagonist, was administered 4 h after lithium injections (corresponding to the peak of analgesia observed for neuropathic mice; Figure 1(a)). Naloxone alone did not modify the mechanical threshold but significantly decreased the analgesic effect of lithium (Figure 2(a)). The contralateral paw mechanical pain threshold was not affected.
Figure 2.
Mu opioid receptor-dependent analgesic effect of lithium chloride on neuropathic mice. (a) Effect of naloxone (0.1 mg/kg, s.c.) on lithium-induced analgesia (100 mg/kg, i.p.; n = 6 per group; two-way ANOVA test with a Bonferroni correction; $p < 0.01). (b) Effect of lithium chloride on MOR null neuropathic mice (n = 6 per group, ANOVA test; n = 6. ns: non-significant). Values are means ± SEM. Pretesting group corresponds to mechanical threshold before lithium and/or naloxone injections. The 4-h group corresponds to mechanical threshold observed 4 h after lithium and/or naloxone injections. MOR: mu opioid receptor.
Effect of lithium on neuropathic MOR-null mice
To assess whether MORs were necessary for lithium analgesia, sham and cuff MOR null mice were treated with a single injection of lithium. Lithium-induced analgesia was never observed in MOR-null mice (Figure 2(b)) as illustrated by the stability of the mean mechanical threshold observed in lithium and vehicle-treated cuffed mice. No effect was observed on the contralateral paw mechanical pain threshold.
Effect of lithium on the level of endogenous mediators
While MORs have been long known to promote analgesia, opioid ligands such as beta-endorphin as well as non-opioid neurotransmitters may be produced locally or after recruitment of classical pain controls acting in central nervous system (CNS) circuits or at the periphery. Plasma and brains of saline and lithium-treated neuropathic animals were analyzed using biochemical approaches aimed at measuring levels of noradrenaline, adenosine, serotonin, and beta-endorphin.
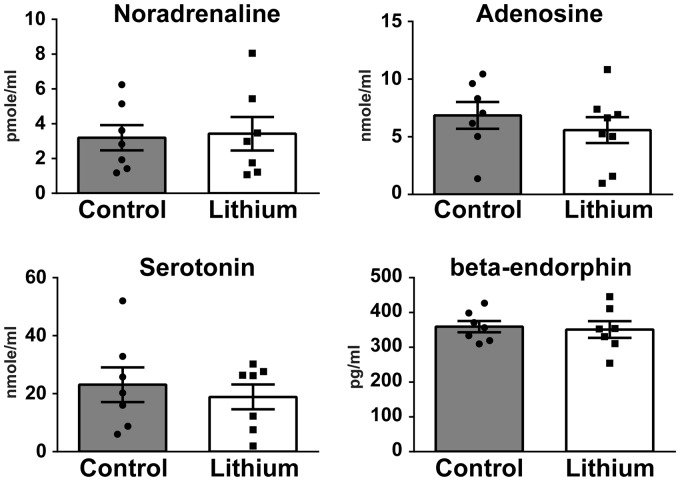
In the plasma, no differences between saline- and lithium-treated cuff groups were found (Figure 3). Adrenaline, dopamine, and L-DOPA were below detection levels. Values obtained for beta-endorphin, serotonin, noradrenaline, and adenosine were in agreement with values published in the literature.15–18
Figure 3.
Effect of lithium chloride on noradrenaline, adenosine, serotonin, and beta-endorphin plasma levels of neuropathic mice. Li: lithium chloride (n = 7 or 8), Mann–Whitney U test.
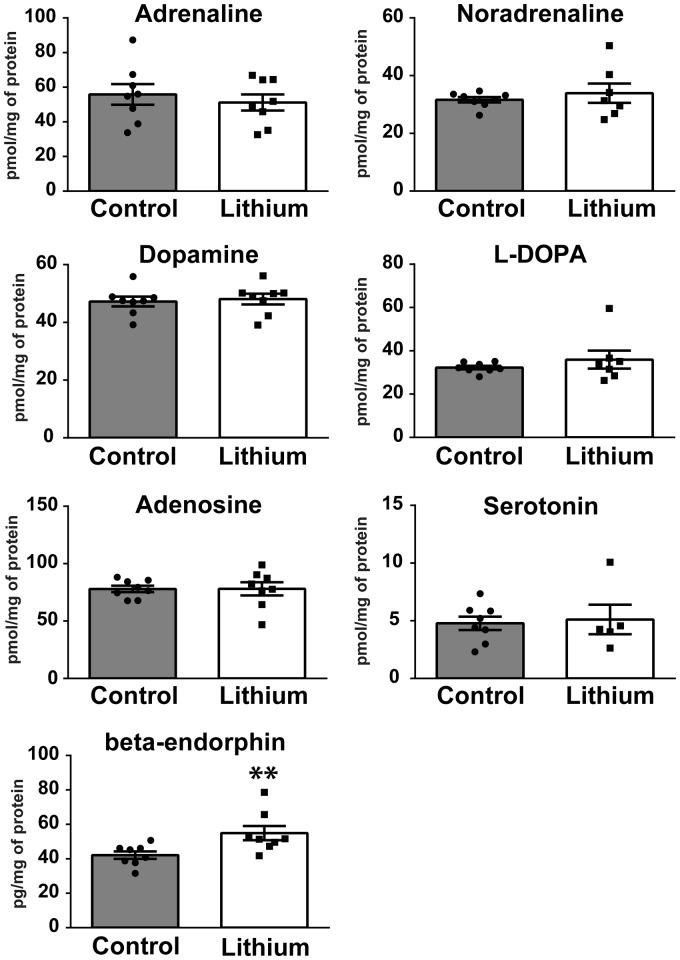
In the brain, adrenaline, noradrenaline, dopamine, L-DOPA, adenosine, and serotonin levels were not affected by a lithium treatment (Figure 4) and were in agreement with levels described for healthy animals.19–21 In sharp contrast, beta-endorphin levels were significantly increased by 30% (p = 0.0047; 54.9 ± 4.1 pg/mg of brain protein) in lithium-treated cuff mice compared to the saline group (42.1 ± 2.1 pg/mg). Similarly, the values of beta-endorphin brain levels (low pg/mg physiological range) are consistent with previous studies.22
Figure 4.
Effect of lithium chloride on adrenaline, noradrenaline, dopamine, L-DOPA, adenosine, serotonin, and beta-endorphin brain levels of cuffed mice. Li: Lithium chloride; n = 8, 7, or 6 (please see graphs). Mann–Whitney U test. *p < 0.05; **p < 0.01. Values are means ± SEM.
Discussion
In addition to the physiological processing of acute pain by the nociceptive system, inflammatory and neuropathic insults may sometimes result in chronic pain that persists long after recovery from the initial lesions.23 In such pathological states, pain no longer plays its physiological warning role. Most therapeutic approaches aimed at alleviating chronic pain symptoms have beneficial effects but suffer from adverse side effects.24,25 This is the case for morphine and related opiates that are widely prescribed to chronic pain patients and may lead to the development of tolerance, opioid-induced hyperalgesia, and addiction.26,27 Therefore, alternative therapeutic strategies are needed.
Studies examining the role of lithium on pain responses are unfortunately contradictory so far. On one side, lithium seems to induce hyperalgesia and decrease morphine-induced analgesia.5,28,29 In addition, lithium overdose can, in a few cases, cause peripheral neuropathy or myopathy in patients.30 In mice and rats, it has been hypothesized that lithium induces a biphasic effect on morphine-induced analgesia that is dependent on lithium concentration and on the pain test used.6,31,32 On the other hand, several groups did not observe any direct effect of lithium on pain perception using a mouse model.33
Interestingly, lithium was shown to relieve cluster headache, which causes pain episodes of extreme intensity. Lithium also reduced the associated autonomic symptoms in humans.34 Moreover, acute and chronic administration of lithium induce a direct analgesia in neuropathic pain states,9,10,35 and potentiate morphine analgesia in mouse and rat models.33,36 Lithium treatments also reversed thermal hyperalgesia as well as the mechanical and cold allodynia induced by a partial sciatic nerve ligation in rats in a naloxone-sensitive manner.9,10 Furthermore, lithium has been shown to prevent paclitaxel-induced peripheral neuropathy in mice.11 In humans, a clinical trial performed with lithium medication had a favorable effect on sciatic nerve injury neuropathic pain.37 Moreover, lithium was able to relieve tricyclic antidepressants-refractory fibromyalgia.38 Our present data confirm the long-lasting analgesic effect of acute lithium administration on a well-characterized mouse neuropathic model.12 In addition, we show that MORs are necessary for lithium-induced analgesia, as no analgesic effect was observed in MOR-null mice.
Lithium acts on numerous targets that have been recently reviewed.39,40 Different mechanisms of action have been proposed and include a possible substitution of Na+ by Li+ impacting homeostasis of electrolyte balance and therefore neuronal firing, or a modulation of the membrane transport of different ions and neurotransmitter precursors.41 In rats, lithium-aversive effects (place-preference conditioning procedure) were abolished by naloxone, suggesting a beta-endorphin-dependent mechanism.42 In addition to being a modulator of NO production in the brain, lithium inhibits Gi and Gs proteins leading to an inhibition of adenylate and guanylate cyclases and of different protein kinases.43 Moreover, lithium affects GSK-3β (glycogen synthase kinase 3)44 acting on both Akt (protein kinase B) and Wingless-related integration site (Wnt) signaling.45 This compound is also able to inhibit inositol monophosphatase and inositol polyphosphate-1-phosphatase,46,47 influencing inositol-dependent regulatory processes. It also reduces cyclic AMP-responsive element-binding protein (CREB) phosphorylation and decreases CREB-dependent gene expression.48 Lithium’s anti-inflammatory properties lead to a downregulation of both proinflammatory cytokines and TNF-alpha interleukin and intracellular mechanisms including GSK-3β.4 Finally, lithium has also been shown to regulate the biosynthesis of different neurotransmitters and/or associated receptors (e.g., modulation of serotonin and glutamate synthesis and secretion).42,49– 52
Our results indicate that acute lithium treatment has a strong anti-allodynic effect as well as a stimulatory effect (+30%) on the production of brain beta-endorphin, a MOR agonist displaying strong analgesic properties. In good agreement with our data, it has been described that stress-induced analgesia is absent in mice lacking beta-endorphin53 and that ultraviolet light induces both analgesia and addiction through a 35% increase of plasma beta-endorphin levels.15 More recently, photobiomodulation therapy performed on the chronic constriction injury mouse model correlated the level of pain relief to an increase of beta-endorphin levels.54 However, this effect is still unclear since previous studies reported that in vivo chronic treatment with lithium did not modify beta-endorphin levels in different rat brain structures,55 whereas in vitro and ex vivo experiments demonstrated that an acute stimulation with lithium increases the release of hypothalamic beta-endorphin.56 As a 35% blood beta-endorphin increase induces a strong elevation of mechanical and thermal thresholds,15 the 30% increase in beta-endorphin brain content we observed after lithium injection should likely be sufficient to induce robust analgesia.
Plasma levels of beta-endorphin are tightly linked to secretions from the pituitary and adrenal gland, whereas brain and cerebrospinal fluid levels are mainly dependent on the arcuate nucleus of the hypothalamus and of the brainstem nucleus tractus solitaris. In addition, beta-endorphin displays a short half-life in rodents’ blood (2 to 10 min)57,58 while in the CNS, degradation of beta-endorphin is described to be extremely long.59,58
Therefore, the beta-endorphin elevation observed in the brain 4 h after a lithium injection is likely due to an upregulation of beta-endorphin production from the arcuate nucleus and the nucleus tractus solitaris associated with a longer brain half-life. While the cuff is a model of peripheral neuropathy, CNS beta-endorphin secretion can normalize the ipsilateral paw threshold by acting on supraspinal (e.g., periaqueductal gray, rostral ventromedial medulla) or spinal nociceptors.
Together with other studies showing the analgesic effect of acute lithium treatment on chronic pain, our results suggest that lithium analgesia involves the upregulation of beta-endorphin synthesis in the CNS. This would explain, at least in part, the MOR-dependent nature of the analgesic properties of lithium.
Acknowledgment
Mu opioid receptor null mice were a generous gift from Professor B. Kieffer (IGBMC, Illkirch-Graffenstaden, France).
Author contributions
YG, ALB, IW, MOP, PP, and MA were involved in conceptualization. Methodology was designed by IW, MA, ALB, VC, JM, NPD, and YG. Investigation was done by IW, ALB, MA, NPD, VC, TM, and YG. Writing the original draft was done by YG, IW, MOP, and AG; review and editing were performed by PP, PD, TM, AG, AC, MOP, and YG. YG and MOP were involved in funding acquisition. Resources were from YG, AC, and PP. YG was responsible for supervision.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Inserm, CNRS (Postdoctoral fellowship to ALB), University of Strasbourg, French Ministère délégué à la Recherche et à l'Enseignement Supérieur (PhD fellowship to ALB, MA, and IW ITMO Cancer (YG), National Council for Scientific Research of Lebanon (CNRS-Lebanon, PhD fellowship to JM), National Research Foundation of South Africa (NRF, PhD fellowship to TM), and USIAS (AC).
References
- 1.Breslow R andGuo T.. Surface-tension measurements show that chaotropic salting-in denaturants are not just water-structure breakers. Proc Natl Acad Sci U S A 1990; 87: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu CT andChuang DM.. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 2010; 128: 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leone M, Franzini A, Proietti Cecchini A, et al. Management of chronic cluster headache. Curr Treat Options Neurol 2011; 13: 56–70. [DOI] [PubMed] [Google Scholar]
- 4.Nassar A andAzab AN.. Effects of lithium on inflammation. ACS Chem Neurosci 2014; 5: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston IN andWestbrook RF.. Inhibition of morphine analgesia by lithium: role of peripheral and central opioid receptors. Behav Brain Res 2004; 151: 151–158. [DOI] [PubMed] [Google Scholar]
- 6.Dehpour AR Farsam H andAzizabadi-Farahani M.. The effect of lithium on morphine-induced analgesia in mice. Gen Pharmacol 1994; 25: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 7.Dehpour AR, Farsam H, Azizabadi-Farahani M. Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology 1995; 34: 115–121. [DOI] [PubMed] [Google Scholar]
- 8.Alborzi A, Mehr SE, Rezania F, et al. The effect of lithium chloride on morphine-induced tolerance and dependence in isolated guinea pig ileum. Eur J Pharmacol 2006; 545: 123–128. [DOI] [PubMed] [Google Scholar]
- 9.Banafshe HR, Mesdaghinia A, Arani MN, et al. Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacol Biochem Behav 2012; 100: 425–430. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Shibata M, Wakisaka S, et al. Intrathecal lithium reduces neuropathic pain responses in a rat model of peripheral neuropathy. Pain 2000; 85: 59–64. [DOI] [PubMed] [Google Scholar]
- 11.Mo M, Erdelyi I, Szigeti-Buck K, et al. Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment. Faseb J 2012; 26: 4696–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benbouzid M, Pallage V, Rajalu M, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 2008; 12: 591–599. [DOI] [PubMed] [Google Scholar]
- 13.Desmeules JA Kayser V andGuilbaud G.. Selective opioid receptor agonists modulate mechanical allodynia in an animal-model of neuropathic pain. Pain 1993; 53: 277–285. [DOI] [PubMed] [Google Scholar]
- 14.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 15.Fell GL, Robinson KC, Mao J, et al. Skin β-endorphin mediates addiction to UV light. Cell 2014; 157: 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grouzmann E, Cavadas C, Grand D, et al. Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch 2003; 447: 254–258. [DOI] [PubMed] [Google Scholar]
- 17.Ziu E, Hadden C, Li Y, et al. Effect of serotonin on platelet function in cocaine exposed blood. Sci Rep 2014; 4: 5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Adebiyi MG, Luo JL, et al. Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro-immune interaction. Cell Reports 2016; 16: 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A 1998; 95: 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaney SM Shepel PN andGeiger JD.. Levels of endogenous adenosine in rat striatum. I. Regulation by ionotropic glutamate receptors, nitric oxide and free radicals. J Pharmacol Exp Ther 1998; 285: 561–567. [PubMed] [Google Scholar]
- 21.Bengel D, Murphy DL, Andrews AM, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 1998; 53: 649–655. [DOI] [PubMed] [Google Scholar]
- 22.Gong N, Xiao Q, Zhu B, et al. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci 2014; 34: 5322–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. IASP Subcommittee on Taxonomy. Classification of chronic pain. Descriptors of chronic pain syndromes and definition of pain terms. Pain 1986; 3: S1–S225. [PubMed] [Google Scholar]
- 24.Dworkin RH, O'connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010; 85: S3–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho KY andSiau C.. Chronic pain management: therapy, drugs and needles. Ann Acad Med Singap 2009; 38: 929–930. [PubMed] [Google Scholar]
- 26.Roeckel LA, Le Coz GM, Gavériaux-Ruff C, et al. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338: 160–182. [DOI] [PubMed] [Google Scholar]
- 27.Williams JT, Ingram SL, Henderson G, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 2013; 65: 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiertelak EP, Smith KP, Furness L, et al. Acute and conditioned hyperalgesic responses to illness. Pain 1994; 56: 227–234. [DOI] [PubMed] [Google Scholar]
- 29.McNally GP andWestbrook RF.. Test type influences the expression of lithium chloride-induced hyperalgesia. Pharmacol Biochem Behav 1998; 61: 385–394. [DOI] [PubMed] [Google Scholar]
- 30.Timmer RT andSands JM.. Lithium intoxication. J Am Soc Nephrol 1999; 10: 666–674. [DOI] [PubMed] [Google Scholar]
- 31.de Gandarias JM, Acebes I, Echevarria E, et al. Lithium alters mu-opioid receptor expression in the rat brain. Neurosci Lett 2000; 279: 9–12. [DOI] [PubMed] [Google Scholar]
- 32.Raffa RB andMartinez RP.. Morphine Antinociception Is Mediated through a Licl-Sensitive, Ip3-Restorable Pathway. Eur J Pharmacol 1992; 215: 357–358. [DOI] [PubMed] [Google Scholar]
- 33.Karakucuk EH, Yamanoglu T, Demirel O, et al. Temporal variation in drug interaction between lithium and morphine-induced analgesia. Chronobiol Int 2006; 23: 675–682. [DOI] [PubMed] [Google Scholar]
- 34.Robbins MS, Starling AJ, Pringsheim TM, et al. Treatment of cluster headache: The American Headache Society evidence-based guidelines. Headache 2016; 56: 1093–1106. [DOI] [PubMed] [Google Scholar]
- 35.Männistö PT andSaarnivaara L.. Effect of lithium on the analgesia caused by morphine and two antidepressants in mice. Pharmacology 1972; 8: 329–335. [DOI] [PubMed] [Google Scholar]
- 36.Jensen J. The effect of prolonged lithium ingestion on morphine actions in the rat. Acta Pharmacol Toxicol (Copenh) 1974; 35: 395–402. [DOI] [PubMed] [Google Scholar]
- 37.Yang ML, Li JJ, So KF, et al. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: a double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012; 50: 141–146. [DOI] [PubMed] [Google Scholar]
- 38.Tyber MA. Lithium carbonate augmentation therapy in fibromyalgia. CMAJ 1990; 143: 902–904. [PMC free article] [PubMed] [Google Scholar]
- 39.Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry 2015; 20: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oruch R, Elderbi MA, Khattab HA, et al. Lithium: a review of pharmacology, clinical uses, and toxicity. Eur J Pharmacol 2014; 740: 464–473. [DOI] [PubMed] [Google Scholar]
- 41.Jope RS, Jenden DJ, Ehrlich BE, et al. Choline accumulates in erythrocytes during lithium therapy. N Engl J Med 1978; 299: 833–834. [PubMed] [Google Scholar]
- 42.Shippenberg TS, Millan MJ, Mucha RF, et al. Involvement of beta-endorphin and mu-opioid receptors in mediating the aversive effect of lithium in the rat. Eur J Pharmacol 1988; 154: 135–144. [DOI] [PubMed] [Google Scholar]
- 43.Schubert T Stoll L andMüller WE.. Therapeutic concentrations of lithium and carbamazepine inhibit cGMP accumulation in human lymphocytes. A clinical model for a possible common mechanism of action? Psychopharmacology (Berl) 1991; 104: 45–50. [DOI] [PubMed] [Google Scholar]
- 44.Klein PS andMelton DA.. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 1996; 93: 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valvezan AJ andKlein PS.. GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Front Mol Neurosci 2012; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Coronado JM, Bellés JM, Lesage F, et al. A novel mammalian lithium-sensitive enzyme with a dual enzymatic activity, 3′-phosphoadenosine 5′-phosphate phosphatase and inositol-polyphosphate 1-phosphatase. J Biol Chem 1999; 274: 16034–16039. [DOI] [PubMed] [Google Scholar]
- 47.Shaltiel G, Deutsch J, Rapoport SI, et al. Is phosphoadenosine phosphate phosphatase a target of lithium's therapeutic effect? J Neural Transm (Vienna) 2009; 116: 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Böer U, Cierny I, Krause D, et al. Chronic lithium salt treatment reduces CRE/CREB-directed gene transcription and reverses its upregulation by chronic psychosocial stress in transgenic reporter gene mice. Neuropsychopharmacology 2008; 33: 2407–2415. [DOI] [PubMed] [Google Scholar]
- 49.Lenox RH andWang L.. Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol Psychiatry 2003; 8: 135–144. [DOI] [PubMed] [Google Scholar]
- 50.Massot O, Rousselle JC, Fillion MP, et al. 5-HT1B receptors: a novel target for lithium. Possible involvement in mood disorders. Neuropsychopharmacology 1999; 21: 530–541. [DOI] [PubMed] [Google Scholar]
- 51.Scheuch K, Höltje M, Budde H, et al. Lithium modulates tryptophan hydroxylase 2 gene expression and serotonin release in primary cultures of serotonergic raphe neurons. Brain Res 2010; 1307: 14–21. [DOI] [PubMed] [Google Scholar]
- 52.Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry 1999; 4: 117–128. [DOI] [PubMed] [Google Scholar]
- 53.Rubinstein M, Mogil JS, Japón M, et al. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A 1996; 93: 3995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Andrade ALM, Bossini PS, do Canto De Souza ALM, et al. Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci 2017; 32: 865–872. [DOI] [PubMed] [Google Scholar]
- 55.Shippenberg TS andHerz A.. Influence of Chronic Lithium Treatment Upon the Motivational Effects of Opioids – Alteration in the Effects of Mu-Opioid but Not Kappa-Opioid Receptor Ligands. J Pharmacol Exp Therapeut 1991; 256: 1101–1106. [PubMed] [Google Scholar]
- 56.Burns G Herz A andNikolarakis KE.. Stimulation of hypothalamic opioid peptide release by lithium is mediated by opioid autoreceptors – evidence from a combined in vitro, ex vivo study. Neuroscience 1990; 36: 691–697. [DOI] [PubMed] [Google Scholar]
- 57.Houghten RA Swann RW andLi CH.. beta-Endorphin: stability, clearance behavior, and entry into the central nervous-system after intravenous-injection of the tritiated peptide in rats and rabbits. Proc Natl Acad Sci U S A 1980; 77: 4588–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silman RE, Chard T, Landon J, et al. ACTH and MSH peptides in the human adult and fetal pituitary gland. Front Horm Res 1977; 4: 179–187. [DOI] [PubMed] [Google Scholar]
- 59.Burbach JPH, Loeber JG, Verhoef J, et al. Schizophrenia and degradation of endorphins in cerebrospinal-fluid. Lancet 1979; 2: 480–481. [DOI] [PubMed] [Google Scholar]