Short abstract
Objective
Pain catastrophizing is linked to many aspects of pain perception and defines a unique dimension in predicting pain intensity and physical disability. Pain Catastrophizing Scale (PCS) is an effective, validated,self-report measure, commonly used in clinical trials. Here, we present a Simplified Chinese PCS (SC-PCS) version developed in Chinese patients suffering from chronic pain.
Methods
The SC-PCS was generated in five steps and tested on an initial patient cohort (N = 30). A convenience sample (N = 200) of in-hospital patients with non-malignant pain lasting for more than 12 weeks were recruited for the study, of which 81 completed 5 additional pain questionnaires. A subset (N = 24) of the patients completed an additional SC-PCS, 10 days after the initial query to assess test–retest validation.
Results
Intra-class correlations coefficient indicated high reproducibility and temporal consistency, (0.97), for the total score. Cronbach’s alpha determined high internal consistency across the SC-PCS total score and its three subscales (0.87, 0.85, 0.62, and 0.65). The SC-PCS total score moderately or weakly (R = −0.2 to 0.49), but significantly, correlated with other measurements, such as pain Visual Analog Scale, Beck Depression Inventory, Pain Anxiety Symptoms Scales, Positive and Negative Affect Schedule, and education. We used exploratory factor analysis to examine the dimensionality of the SC-PCS, which indicated instability of the current three-factor model. However, a confirmatory factor analysis indicated that the three-factor model had the best goodness-fitting.
Conclusions
We demonstrate the successful translational adaptation from English to Simplified Chinese as well as the reliability and validity of SC-PCS. An important discovery was education level significantly correlated with SC-PCS, identifying a future consideration for other cross-cultural development of self-reported measures.
Keywords: Pain Catastrophizing Scale, Simplified Chinese version, chronic pain, education level
Introduction
Pain Catastrophizing Scale (PCS)1 is widely adopted self-report tool used to assess exaggerated negative conceptualization in response to ongoing, anticipated, or imaged pain. The original PCS is a 13-item questionnaire consisting of three subscales (helplessness, magnification, and rumination), each of which reflects a unique dimension of pain catastrophizing. Pain catastrophizing has been linked to many aspects of the pain experience including intensity, disability, anxiety, depression, and behavior.2 The PCS defines a unique dimension in predicting pain intensity and disability beyond gender and age in children3 and adults.4 A High score in pain catastrophizing is usually accompanied by an increased pain sensitivity, in turn, representing cognitive and emotional processes of the subjective pain experience. As a result, pain catastrophizing is thought to be reduced in conjunction with many successful treatment interventions. PCS provides an invaluable tool for exploring pain-related outcome measures in the clinical research.
The simplicity and usefulness of PCS has led to various translations across languages and cultures, for example, German,5 Catalan,6,7 Turkish,8,9 Malay,10 Italian,11 Portuguese,12,13 Sinhala,14 Korean,15 English-, Afrikaans- and Xhosa-speaking,16 French,17 Spanish,18 Dutch,19 and Traditional and Simplified Chinese (SC).20,21 In addition, researchers have developed a Child-version, (PCS-C)6,22,23 and a shortened version of the PCS.24,25 In this report, we provide a revised version of the Chinese SC-PSC, taking into consideration education and correcting misinterpretations that we have detected in a previous version.21
A rapid development of pain research in China asserts the need for self-reported pain outcome measures such as PCS. However, one shortcoming of conducting pain research in China is the lack of validated questionnaires. To our knowledge, eight pain-related questionnaires have been translated from English into SC which includes, Beck Depression Inventory (BDI),26,27 Chronic Pain Acceptance Questionnaire,28,29 the Leeds Assessment of Neuropathic Symptoms and Signs,30,31 McGill Quality-of-Life Questionnaire (McGill),32,33 the Oswestry Disability Index (ODI),34,35 the Pain Anxiety Symptoms Scale (PASS),36,37 the Pain Sensitivity Questionnaire (PSQ),38 and the Positive and Negative Affect Schedule (PANAS).39 Although a Traditional Chinese version of PCS20 was created in Hong Kong, it was not adopted in Mainland China due to cross-cultural differences between Mandarin and Cantonese.
The existing Chinese SC-PCS contains two flaws that nullify its clinical utility.21 First, it lacks instructions that are common in a majority of self-reported questionnaires. Second, the wording of some items is inaccurate, for example, item 4, “It’s awful and I feel that it overwhelms me.” was translated into “情况很糟糕, 我觉得被疼痛打到 (The situation is very bad and I feel that the pain overwhelms me).” Given such flaws, we sought to develop a more accurate and generalizable SC-PCS while exploring socioeconomic confounds relevant in China.
Materials and methods
Translation and cross-cultural adaptation
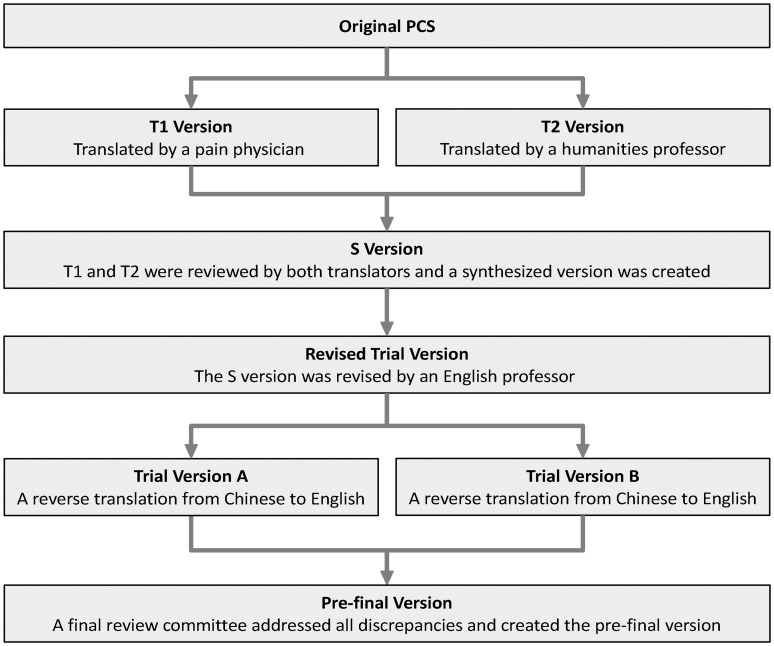
The translation and cross-cultural adaptation processes followed the recommendations by Beaton et al.,40 and Wild et al.41 In principle, the objective is to minimize any semantic language misinterpretations and adaptation if a direct (word-to-word) translation leads to misunderstanding. As shown in Figure 1, in the first stage, two independent versions (T1 and T2) were carried out by two bilingual translators, a pain clinician from China and a humanities professor at an American university from China, respectively. In the second stage, both the T1 and T2 versions were reviewed by both translators and a synthesized version (S version) was created after all discrepancies between T1 and T2 were addressed. In the third stage, the S version was reviewed by a bilingual English professor and a revised synthesized version was created. In the fourth stage, a reverse translation from SC to English was performed by two bilingual American-Born-Chinese undergraduates and any inconsistencies were addressed. In the final stage, a review committee comprising all translators reviewed the SC-PCS version and agreed on the pre-final test version prior to its implementation in the final patient cohort.
Figure 1.
Chart of translation and cross-cultural adaptation flow.
Test of the pre-final version
A total of 30 non-malignant chronic pain patients completed the pre-final version of SC-PCS. There was no significant difference between the pre-final test group and the final test group in terms of age, education, residence, gender, pain Visual Analog Scale (VAS), pain duration, or locations. Cognitive debriefing was completed by a physician with more than 10 years of experience in patient counseling and pain management to check understandability and cultural relevance of the translation .41 All findings from this phase were evaluated by the committee prior to finalizing the SC-PCS. To be noted, the 30 patients were not included in the validation.
Participants
A total of 271 in-hospital patients with non-malignant pain were recruited over an eight-month period, among which 30 patients were exclusively used for testing the pre-final version of SC-PCS; 37 patients were removed due to exclusionary criteria and 4 due to floor and ceiling effects. The remaining 200 patients were used in all analysis presented. The inclusion criteria were as follows: (1) age over 18 years, (2) ability to read and write SC and accurately understand SC-PCS and other questionnaires, and (3) pain duration greater than 12 weeks. Exclusion included tumor, inability to comprehend instructions or lack of consent for study participation. All study procedures were approved by the Institutional Review Board of the Second Affiliated Hospital and Yuying’s Children Hospital, Wenzhou Medical University.
The minimum number of participants for evaluating factor analysis and internal consistency, for assessing concurrent validity, and for testing temporal reliability is 100, 52, and 30, respectively.42,43 We anticipated to recruit 150, 75, and 30 patients who would complete SC-PCS, SC-PCS with five other questionnaires, and additional SC-PCS 10 days after the initial query, respectively. Since participants were recruited from a hospital setting, the first two numbers were easily exceeded (N = 200 and 81). However, because most patients were hospitalized for less than one week, the number of patients for assessing temporal reliability was slightly below the anticipation (N = 24).
All patients completed a battery of questionnaires including a 10-item demographic survey regarding age, gender, residence (urban/rural), marriage, education, and employment status as well as pain VAS, pain duration, and the SC-PCS. Eighty-one patients were chosen to complete five additional five questionnaires (BDI, PANAS, PASS, PSQ, and ODI) in order to construct an internal validity of the SC-PCS. A total of 24 patients were chosen to complete the SC-PCS a second time 10 days after the initial completion to assess temporal stability. In order to minimize pain influence in the temporal stability analysis, only patients whose pain VAS remained invariant over a 10-day period completed a second SC-PCS. All questionnaires were collected on an electronic tablet device and were collected using Research Electronic Data Capture (REDCap).44 REDCap is a secure, convenient, and efficient online/offline web application for capturing electronic survey data and is recommended by the National Institutes of Health for data collection in clinical trials.
Missing data and removal of floor and ceiling effects
Due to the effort made by our staff and the convenience of using a tablet device, the rate of missing data was very low: 7 patients missed 10 questions of BDI, most of which covered the item 21 of BDI with regard to sexual activity and 2 patients missed 2 questions of PANAS. The above missing data were replaced by the mean value of variables corresponding to that questionnaire or subscale. A total of 12 patients did not provide residence information .
The effects of floor and ceiling on the data were also addressed. The floor and ceiling is the limit under and above which variance in SC-PCS would no longer be accurately estimated, a total of four patients were removed at this stage.
Data descriptive statistics
The descriptive statistics were performed on the full 200 patients, which included the mean, standard deviation, skewness, kurtosis, Kolmogorov–Smirnov test for the total score and each subscale.
Internal consistency, reproducibility, and concurrent validity
Internal consistency was assessed with Cronbach’s alpha, which measures the strength of inter-item homogeneity. Cronbach’s alpha of the total score and of the three subscales was calculated. A commonly accepted rule for describing internal consistency using Cronbach’s alpha is as follows: alpha ≥0.9, excellent; 0.8≤ alpha < 0.9, good; 0.7≤ alpha < 0.8, acceptable; and alpha < 0.5, unacceptable.45
Reproducibility validity, i.e. the extent of the agreement of scores between the two time points, was tested by the intra-class correlation coefficient (ICC). Usually, ICC ≥0.7 is regarded as acceptable for test-retest reliability and ≥0.9 as excellent.46
Concurrent validity was assessed by Pearson’s correlation coefficients (R) with other pain-related measurements. PCS measures unique aspects of a patient’s pain experience and perception such that pain catastrophizing theoretically should be moderately or weakly correlated to other pain-related questionnaires. In order to validate the effectiveness and correlative assumptions of SC-PCS, a battery of questionnaires was administered, which included pain VAS, SC-PSQ, SC-BDI, SC-PANAS, SC-PASS, and SC-ODI questionnaires. Usually, the correlation is regarded as positive weak, moderate, or strong when R < 0.30, 0.30 < R < 0.60, and R > 0.60, respectively.47
Factor structure analysis
Principal component analysis (PCA) with the varimax rotation method by Kaiser normalization was used to find latent dimensions. Overall, the PCA converts a set of possibly correlated components into linearly uncorrelated, orthogonal, principal components, which correspond to the latent dimensions of the data. In our study, only components with an eigenvalue greater than one were determined as principal components or factors. Following this, structural equation modeling was performed with AMOS software,48 a confirmatory factorial analysis with maximum-likelihood estimation was carried out to test the adequacy of the model and was tested against five models, a null model in which all the 13 observed items are uncorrelated, one-factor model in which all 13 items are indicated by one latent factor, two-factor model from our exploratory factor analysis above, two-factor model,49 and original three-factor model.1 The chi-square/degree of freedom (df), normalized fit index (NFI), comparative fit index (CFI), and the root-mean-square error of approximation (RMSEA) were used to quantify the goodness-of-fit for the five-factor models. A best-fit model was expected to have the following indices: chi-square/df < 2.0, NFI > 0.90, CFI > 0.90, and RMSEA < 0.08.50
Other analysis
We evaluated the effects of demographic variables (age, gender, pain duration, residence, and education) on the total and three subscales of SC-PCS, determining potential confounds and to increase the generalizability and accuracy of this version. A multiple linear regression model predicted pain intensity (VAS) from the total of SC-PCS and all demographic variables (age, gender, pain duration, residence, and education), which provides sound statistical evidence that our SC-PCS version can be used to identify clinical pain intensity.
Results
Socioeconomic background and pain characteristics
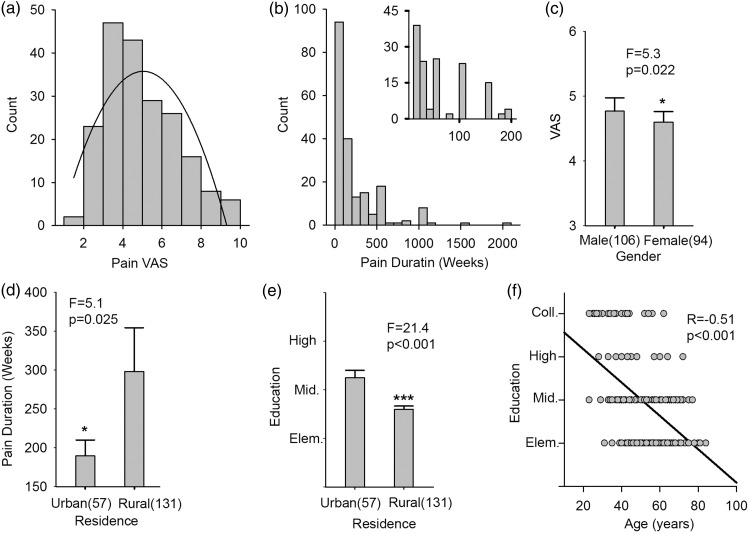
Table 1 summarizes demographics of the patient cohort. Figure 2 shows pain properties of the cohort. Mean VAS (0–10; 0 = no pain, 10 = worst pain imaginable) was 4.5/10 with a standard deviation (SD) of 1.5; mean pain duration was 215 weeks with SD of 300 weeks. Pain intensity was gender dependent (VAS scores, F = 5.3 and p = 0.022 from one-way analysis of variance (ANOVA), Figure 2(c)). Additionally, there was a significant difference in pain duration (F = 5.1 and p < 0.025 obtained from a one-way ANOVA, Figure 2(d)) and in education (F = 21.4 and p < 0.001, Figure 2(e)) between participants who lived in urban and in rural areas. An inverse relation was seen between education and age (Pearson's R = −0.51 and p < 0.001, Figure 2(f)). All other interrelationships between demographic variables and pain-related outcomes were not significant. These results imply a strong educational and residential differences in our patient population, and that majority were of rural residence with elementary education.
Table 1.
Socioeconomic background and pain characteristics.
| Age | Mean (52.1 years), Standard Deviation (13.9 years) |
| Gender | Male (53%), Female (47%) |
| Education | Elementary (43%), Middle (35.5%), High (7.5%), College (14%) |
| Marriage | Married (96.5%), Unmarried (3.5%) |
| Residence | Urban (28.5%), Rural (65.5%), N/A (6%) |
| Job | Employee (30%), Employer (6.5%), Self-employed (35%), Retired (28%), Students (0.5%) |
| Number of pain position | One (59.5%), Two (34.5%), Three (4%), Above Three (2%) |
| Pain location | Back (40.7%), Leg (33.4%), Neck (7.6%), Arm (5.5%),Head (3.5%), Shoulder (2.9%), Pelvic (2.9%),Foot (1.7%), Sacrococcygeal (1.5%), Abdomen (0.3%) |
Figure 2.
Pain-related and socioeconomic information about participants. (a) A histogram of pain Visual Analogue Scale (0–10; no pain to worst imaginable pain) of all participants, the mean and standard deviation = 4.5 ± 1.5. (b) A histogram of pain duration in the right corner is an expanded version of the histogram in which the duration was limited to 200 weeks and less, which covered 70% of the participants. (c) There was a significant pain VAS difference between males and females (F = 5.3 and p = 0.022 from a one-way ANOVA). (d) There was a significant pain duration difference between participants who lived in urban and in rural areas (F = 5.1 and p < 0.025 from a one-way ANOVA). (e) There was a significant education degree difference between participants who lived in urban and rural areas (F = 21.4 and p < 0.001 resulted from a one-way ANOVA). (f) The education degree of participates was significantly inversely correlated with age. The Pearson’s correlation coefficient = −0.51 and p < 0.001 resulted from a one-way ANOVA.Note: 12 of the total 200 participants did not provide residence information.
Distribution of the SC-PCS scores
The distribution of SC-PCS scores is shown in Table 2. The mean score and S.D. of the total score and three subscales (helplessness, magnification, and rumination) were 26.89 ± 10.63, 11.58 ± 5.90, 5.39 ± 2.87, and 9.92 ± 3.53, respectively. Except for magnification, the Kolmogoro–Smirnov normality test indicated that SC-PCS scores were distributed normally given a predefined threshold of significance (p < 0.05), which satisfies the assumption for linear regression models to be implemented.
Table 2.
Score mean and standard deviation and normality test results.
| Measure | PCS total score |
PCS subscale |
||
|---|---|---|---|---|
| Helplessness | Magnification | Rumination | ||
| Mean (SD) | 26.89(10.63) | 11.58(5.90) | 5.39(2.87) | 9.92(3.53) |
| Skewness | −0.05 | −0.11 | 0.12 | −0.34 |
| K-S NormalityTest (p value) | 0.26 | 0.08 | 0.03* | 0.18 |
K-S: Kolmogorov–Smirnov; PCS: Pain Catastrophizing Scale.
*p < 0.05.
Internal consistency, reproducibility, and concurrent validity
Cronbach’s alpha results showed good internal consistency with 0.87, 0.85, 0.62, and 0.65 for the total score and the three subscales, helplessness, magnification, and rumination, respectively.
For reproducibility validity, ICC, mean difference (MD) between test and retest scores, and standard error of measurement (standard deviation of test score × sqrt(1−correlation between test and retest scores)) were calculated for the total score, three subscales, and each item independently. All ICCs, with the exception of items of 3, 4, and 12, were greater than 0.90 with MD being close to 0 and small SEM, indicating excellent reproducibility and temporal consistency (Table 3).
Table 3.
Measurements of reproducibility validity.
| ICC (95% CI) | MD | SEM | |
|---|---|---|---|
| PCS total score | 0.97 (0.92–0.99) | −0.24 | 0.20 |
| Helplessness | 0.95 (0.89–0.98) | 0.03 | 0.24 |
| Magnification | 0.95 (0.88–0.98) | −0.40 | 0.26 |
| Rumination | 0.98 (0.95–0.99) | 0.16 | 0.16 |
| Item 1 | 0.97 (0.92–0.98) | 0.05 | 0.21 |
| Item 2 | 0.96 (0.92–0.98) | −0.08 | 0.21 |
| Item 3 | 0.73 (0.48–0.87) | 0.02 | 0.57 |
| Item 4 | 0.83 (0.65–0.92) | 0.08 | 0.46 |
| Item 5 | 0.92 (0.82–0.92) | −0.12 | 0.30 |
| Item 6 | 0.94 (0.88–0.98) | −0.04 | 0.26 |
| Item 7 | 0.91 (0.81–0.96) | −0.24 | 0.33 |
| Item 8 | 0.98 (0.95–0.99) | 0.04 | 0.17 |
| Item 9 | 0.99 (0.97–0.99) | 0.04 | 0.14 |
| Item 10 | 0.95 (0.90–0.98) | 0.04 | 0.24 |
| Item 11 | 0.93 (0.85–0.97) | 0.04 | 0.29 |
| Item 12 | 0.71 (0.44–0.86) | 0.12 | 0.60 |
| Item 13 | 0.92 (0.83–0.96) | −0.12 | 0.31 |
PCS: Pain Catastrophizing Scale; ICC: Intra-class correlation coefficient; MD: mean difference between test and retest scores; SEM: standard error of measurement (standard deviation of test score × sqrt(1−correlation between test and retest scores)).
Pain catastrophizing is associated with pain disability, anxiety, depression, and pain intensity, and similar studies have shown moderate or weak correlations between their version of PCS and pain-related outcomes.5,15,20 For concurrent validity, as shown in Table 4, the PCS total and the three subscales were weakly or moderately, but significantly, correlated with the other questionnaires (pain VAS, BDI, PASS, and PANAS_N). Therefore, we expect a moderate or weak correlation between PCS and relevant parameters. We observed a correlation range from 0.19 to 0.52, providing evidence of the concurrent validity of the SC-PCS.
Table 4.
Correlation coefficients (R) with other related measures.
| Other Measures | PCS total score |
PCS subscale |
||
|---|---|---|---|---|
| Helplessness | Magnification | Rumination | ||
| Pain VAS | 0.19** | 0.21** | 0.01 | 0.20** |
| BDI | 0.32** | 0.24** | 0.34** | 0.25* |
| PASS | 0.49*** | 0.28** | 0.52*** | 0.49*** |
| PANAS_N | 0.26* | 0.18 | 0.36*** | 0.13 |
Pain VAS: Pain Visual Analog Scale; BDI: Beck Depression Index; PASS: Pain Anxiety Symptoms Scales; PANAS_N: Negative part of Positive and Negative Affect Schedule; PAS: Pain Catastrophizing Scale; PCS: Pain Catastrophizing Scale; PCS: Pain Catastrophizing Scale.
*p < 0.05; **p < 0.01; ***p < 0.001.
Factor structure analysis
Exploratory factor analysis was done using PCA, which suggested a two-factor structure in the SC-PCS with an eigenvalue threshold greater than one. As shown in Table 5, factor I consisted of items of 1, 2, 3, 4, 5, 9, and 10 most related to the subclass of helplessness and factor II consisted of items of 6, 7, 8, 11, 12, and 13 most related to magnification. The two factors accounted for 37.83% and 17.84% of the variance, respectively.
Table 5.
Exploratory factor analysis of the SC-PCS.
| Item No. | Factor I loading | Factor II loading | Communality |
|---|---|---|---|
| Item 1 | 0.62 | 0.25 | 0.44 |
| Item 2 | 0.83 | 0.15 | 0.71 |
| Item 3 | 0.80 | 0.06 | 0.64 |
| Item 4 | 0.85 | 0.08 | 0.73 |
| Item 5 | 0.85 | 0.14 | 0.74 |
| Item 6 | 0.50 | 0.52 | 0.53 |
| Item 7 | 0.29 | 0.34 | 0.20 |
| Item 8 | −0.09 | 0.77 | 0.60 |
| Item 9 | 0.79 | 0.23 | 0.68 |
| Item 10 | 0.81 | 0.22 | 0.71 |
| Item 11 | 0.02 | 0.79 | 0.62 |
| Item 12 | 0.24 | 0.44 | 0.25 |
| Item 13 | 0.28 | 0.55 | 0.38 |
| Eigenvalue | 4.92 | 2.32 | |
| Variance (%) | 37.83 | 17.84 |
PCS: Pain Catastrophizing Scale.
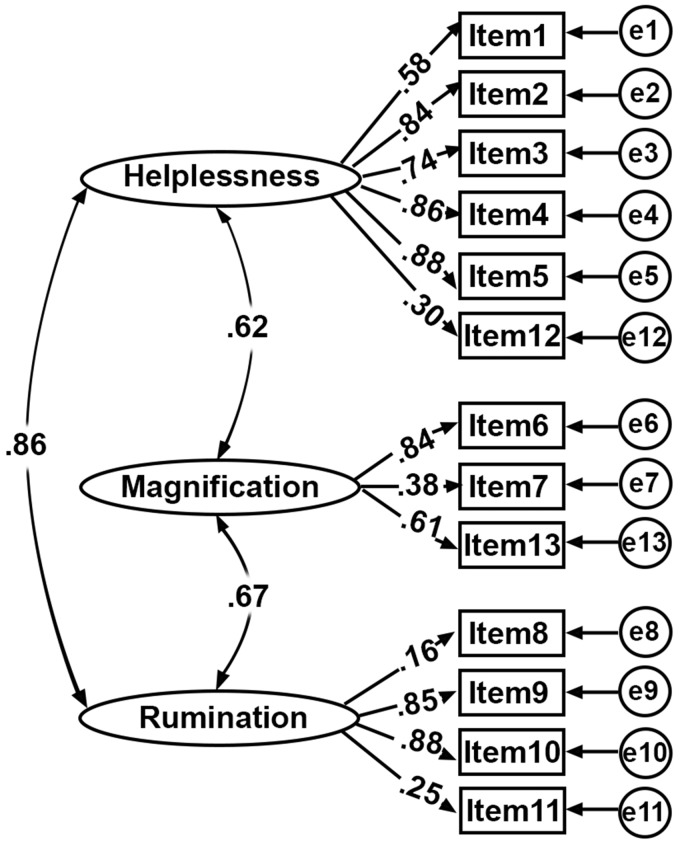
Confirmatory factor analysis was done using AMOS software, which tested five models that provided the best fit to the data. The four criterions (chi-square/df, NFI, CFI, and RMSEA) were computed and shown in Table 6, which suggested the original three-factor model explained the most variance and its model structure with standardized parameter estimates are illustrated in Figure 3.
Table 6.
Goodness-of-fit values for different models.
| Model Type | Chi-square | df | Chi-square/df | NFI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| Null | 1276.86 | 78 | 16.37 | – | – | 0.28 |
| One-factor | 261.36 | 66 | 3.96 | 0.80 | 0.84 | 0.12 |
| Two-factor (Osman) | 259.20 | 64 | 4.05 | 0.80 | 0.84 | 0.12 |
| Two-factor (Current) | 192.64 | 64 | 3.01 | 0.85 | 0.89 | 0.10 |
| Three-factor | 182.28 | 62 | 2.94 | 0.86 | 0.90 | 0.10 |
Null: 13 uncorrelated items; One-factor: 13 items are indicated by one latent factor; Two-factor: suggested by Osman et al.,49 Two-factor (Currently): suggested by current study; Three-factor: suggested by Sullivan et al1; NFI: normalized fit index; CFI: comparative fit index; RMSEA: root-mean square error of approximation.
Figure 3.
Three-factor model with standardized parameter estimates. The observed 13 items were determined by three latent factors (Helplessness, Magnification, and Rumination) and their measurement error. The Pearson’s correlation coefficients between three factors were 0.62, 0.67, and 0.86, respectively. The factor loadings from each factor to 13 items are shown in the middle of the figure, the range of which was between 0.25 and 0.88.
Correlations with demographic variables of SC-PCS
As shown in Table 7, the total score and two of its three subscales (helplessness and rumination) were significantly correlated with age and education level.
Table 7.
Correlation coefficients (R) with demographic variables.
| PCS total score |
PCS subscale |
|||
|---|---|---|---|---|
| Helplessness | Magnification | Rumination | ||
| Age | 0.23*** | 0.26*** | 0.02 | 0.23*** |
| Gender | 0.08 | 0.06 | 0.13 | 0.03 |
| Pain Duration | −0.07 | −0.03 | −0.14 | −0.06 |
| Residence | −0.03 | 0.01 | −0.07 | −0.04 |
| Education | −0.20** | −0.22** | −0.04 | −0.20** |
PCS: Pain Catastrophizing Scale.
**p < 0.01; ***p < 0.001.
Prediction of pain VAS
As shown in Tables 8 and 9, only two independent variables, total of PCS and gender, statistically significantly predicted pain VAS, F(2, 197) = 6.94, p < 0.001, R2 = 0.07.
Table 8.
Estimated coefficients of pain VAS prediction model.
| Unstandardized coefficients/Standard error | t Value | Significance | |
|---|---|---|---|
| Constant | 3.17/0.39 | 8.07 | 0.000 |
| Total of PCS | 0.36/0.01 | 2.89 | 0.004 |
| Gender | 0.67/0.26 | 2.56 | 0.011 |
PCS: Pain Catastrophizing Scale; VAS: Visual Analog Scale. Dependent variable: Pain VAS; independent variable: total of PCS and gender (Male = 1 and Female = 0).
Table 9.
Summary of pain VAS prediction model.
| R | R2 | F value | Significance |
|---|---|---|---|
| 0.26 | 0.07 | 6.94 | 0.000 |
VAS: Visual Analog Scale.
Discussion
Pain research continues its rapid development in China and the need to generate validated and generalizable self-reported measure is of great importance. The aim of the current study was to cross-culturally adapt and validate the PCS into an SC version and to examine its psychometric properties in a chronic pain population. The final version of SC-PCS is available for public use and can be found in online Appendix.
Participants and translation
The translation and implementation of the PCS began in a Chinese population that varied in terms of education and residence compared to Western countries. The original PCS was generated in an urban and college-educated population. Therefore, we considered education level during the creation and validation process. Socioeconomic status of chronic pain patients in China is disproportionately economically and socially disadvantaged. We explored how educational background may negatively impact comprehension and mitigate usage of complicated language in the SC-PCS. For instance, item 1 “I worry all the time about whether the pain will end”, the first version (v1) translated into:: “我一直对疼痛是否会结束而忧心忡忡”. The v1 translation used a common Chinese idiom (忧心忡忡), which may not be fully understood by patients who have only completed elementary education. The second version (v2): “我一直担心疼痛是否会结束” replaced the idiom with a more common word (担心), but the logic sounded slightly complicated. In the final version (v3), incorporated a direct word-to-word translation that was more straightforward: “我一直担心疼痛不会结束”. During the validation, we observed an extremely low miss rate, and 95% of participants did not report any misunderstandings. In addition, with the assistance of a research coordinator, the remaining 5% finished the questionnaire without any comprehension issues. Generally, this cross-cultural adaptation, translation, and validation of PCS were determined a success.
Internal consistency and reproducibility of the SC-PCS
The internal consistency of the SC-PCS was evaluated using Cronbach’s alpha, which was also reported in the original study validating PCS.1 However, our study had a lower alpha coefficient compared to other reports.20,21 The lower alpha coefficient of magnification could be explained by the small number of items and its minimum item redundancy. Consistent low alpha coefficients of magnification in other studies may challenge the reliability of the magnification subscale as an independent subscale, and warrants investigation of a two-factor alternative.5
The reproducibility of the SC-PCS was assessed using ICCs, indicating excellent reproducibility. These results were stronger than other studies replicating PCS validation,1,5,20,21 which represents an improved temporal consistency. However, the value of ICC is a function of the interval between test and retest, longer interval periods inherently increase the variability and in turn decrease ICC. For our study, the interval was 10 days, shorter than 21 days as shown in Meyer et al.,5 and longer than 7 days as shown in Yap et al.20 It is safe to assume that the agreement of scores between test and retest is reliable.
Factor structure analysis of SC-PCS
Other investigators have raised concerns regarding the stability of a three-factor PCS.49 Numerous studies have demonstrated a similar decomposition of the PCS into a two-factorial model.2,8,22 Furthermore, in the child version for PCS adaptation, only one factor was found in a German version,23 but in an English version,3 the typical three factors were revealed. Undoubtedly, the agreed number of PCS subcomponents is debatable. In our dataset, we observed a similar instability of the three-factor model. Additionally, educational differences may bias the dimensionality analysis, and we divided the 200 completed participants into two groups (participants with only middle-school education or less vs. who those with high-school education or more). However, the effects were marginal, implying that education is not related to the PCS structure.
Although PCA suggested two factors in the SC-PCS, a confirmatory factor analysis indicated a three-factor model had the best fit compared with a null, one-factor, and two-factor models (current and Osman’s study). Regarding goodness-of-fit values of model fitting values (Table 5), the three-factor structure was the only one with an acceptable CFI (0.90). Similarly, the Spanish,18 German,5 Catalan,7 French,17 Norwegian,51 Brazilian Portuguese,13 Sinhala,14 and Hindi45 PCS versions had approximately the same comparative fit indices using a three-factor model. Overall, this convergence implies that the original three-factor model has the best goodness-of-fit values when testing in model fitting.
Education interaction with SC-PCS
Although the majority of our patients were from a lower socioeconomic background, the strong inverse correlation between education and SC-PCS is a novel finding. To our knowledge, this is the first such evidence. This observation may help explain the failure of assessment of the reliability and validity of the Chinese version of Beck Depression Inventory (SC-BDI),27 although the authors attributed the failure of application of the SC-BDI to China’s cultural sensitivity and cultural bias. This confound may occur in other cross-culturally translated questionnaires, which needs to be addressed in future studies.
Pain intensity prediction
PCS is linked to pain perception and its contribution to predicting pain intensity can be explored through linear regression analysis, as indicated by normality test. A multiple regression analysis with SC-PCS scores and demographic variables significantly modeled pain intensity. However, the small coefficient determination (R2= 0.07) of the pain intensity model indicates a poor level of prediction, suggesting PCS may be more related to affective dimensions of pain rather than the intensity.
Study limitations
Certain limitations may have affected the study, such as a lack of formal cognitive debriefing training during testing the pre-final version. Another limitation was the number of patients for test-retest analysis (N = 24), below the minimum requirement of 30. Therefore, the chance of the size effect on the test-retest reliability cannot be ruled out although the ICC results are still in the right range. In addition, some indices of the best-fit model (three-factor model) are short of reaching to the lower zone, which needs to be explored in future studies.
Conclusions
In this study, the original English version of the PCS was semantically translated into an SC version and tested its reliability and validity in a representative patient population. We observed socioeconomic status confounds and addressed educational background of the patient population during the creation of the SC-PCS. We explored the SC-PCS structure through PCA and demonstrated an instability in the three-factor model. We improved on previous attempts of translating the PCS and statistically validated the SC-PCS in patients suffering from chronic pain.
Supplementary Material
Acknowledgements
We would like to convey our gratefulness to staff members in China-USA Neuroimaging Research Institute of Wenzhou Medical University, Ms Shishi Tang, Dr Lili Yang, Dr Binbin Wu, Dr Xiaozheng Liu, and Biao Xia in the Department of Orthopedics of Wenrong Hospital, Hengdian, Jianghua, Zhejiang, for helping in collecting the data.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material
Supplementary material is available for this article.
References
- 1.Sullivan MJL Bishop SR andPivik J.. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995; 7: 524–532. DOI: 10.1037//1040-3590.7.4.524. [Google Scholar]
- 2.Chibnall JT andTait RC.. Confirmatory factor analysis of the Pain Catastrophizing Scale in African American and Caucasian Workers’ Compensation claimants with low back injuries. Pain 2005; 113: 369–375. DOI: 10.1016/j.pain.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Crombez G, Bijttebier P, Eccleston C, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003; 104: 639–646. [DOI] [PubMed] [Google Scholar]
- 4.Severeijns R, van den Hout MA, Vlaeyen JWS, et al. Pain catastrophizing and general health status in a large Dutch community sample. Pain 2002; 99: 367–376. DOI: Pii S0304-3959(02)00219-1 Doi 10.1016/S0304-3959(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 5.Meyer K Sprott H andMannion AF.. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res 2008; 64: 469–478. DOI: 10.1016/j.jpsychores.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Sole E, Castarlenas E, Miro J. A Catalan adaptation and validation of the Pain Catastrophizing Scale for Children. Psychol Assess 2016; 28: e119–e126. DOI: 10.1037/pas0000243. [DOI] [PubMed] [Google Scholar]
- 7.Miro J, Nieto R, Huguet A. The Catalan version of the Pain Catastrophizing Scale: a useful instrument to assess catastrophic thinking in whiplash patients. J Pain 2008; 9: 397–406. DOI: 10.1016/j.jpain.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Ilcin N, Gurpinar B, Bayraktar D, et al. Cross-cultural adaptation and validation of the Turkish version of the pain catastrophizing scale among patients with ankylosing spondylitis. J Phys Ther Sci 2016; 28: 298–303. DOI: 10.1589/jpts.28.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suren M, Okan I, Gokbakan AM, et al. Factors associated with the pain catastrophizing scale and validation in a sample of the Turkish population. Turk J Med Sci 2014; 44: 104–108. [DOI] [PubMed] [Google Scholar]
- 10.Mohd Din FH, Hoe VC, Chan CK, et al. Cultural adaptation and psychometric assessment of Pain Catastrophizing Scale among young healthy Malay-speaking adults in military settings. Qual Life Res 2015; 24: 1275–1280. DOI: 10.1007/s11136-014-0850-1. [DOI] [PubMed] [Google Scholar]
- 11.Meroni R, Piscitelli D, Bonetti F, et al. Rasch Analysis of the Italian version of Pain Catastrophizing Scale (PCS-I). J Back Musculoskelet Rehabil 2015; 28: 661–673. DOI: 10.3233/BMR-140564. [DOI] [PubMed] [Google Scholar]
- 12.Lopes RA, Dias RC, Queiroz BZ, et al. Psychometric properties of the Brazilian version of the Pain Catastrophizing Scale for acute low back pain. Arq Neuro-Psiquiatr 2015; 73: 436–444. DOI: 10.1590/0004-282X20150026. [DOI] [PubMed] [Google Scholar]
- 13.Sehn F, Chachamovich E, Vidor LP, et al. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med 2012; 13: 1425–1435. DOI: 10.1111/j.1526-4637.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 14.Pallegama RW, Ariyawardana A, Ranasinghe AW, et al. The Sinhala version of the pain catastrophizing scale: validation and establishment of the factor structure in pain patients and healthy adults. Pain Med 2014; 15: 1734–1742. DOI: 10.1111/pme.12529. [DOI] [PubMed] [Google Scholar]
- 15.Cho S, Kim HY, Lee JH. Validation of the Korean version of the Pain Catastrophizing Scale in patients with chronic non-cancer pain. Qual Life Res 2013; 22: 1767–1772. DOI: 10.1007/s11136-012-0308-2. [DOI] [PubMed] [Google Scholar]
- 16.Morris LD, Grimmer-Somers KA, Louw QA, et al. Cross-cultural adaptation and validation of the South African Pain Catastrophizing Scale (SA-PCS) among patients with fibromyalgia. Health Qual Life Outcomes 2012; 10: 137. DOI: 10.1186/1477-7525-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremblay I, Beaulieu Y, Bernier A, et al. Pain Catastrophizing Scale for francophone adolescents: a preliminary validation. Pain Res Manag 2008; 13: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Campayo J, Rodero B, Alda M, et al. [Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia]. Med Clin (Barc) 2008; 131: 487–492. [DOI] [PubMed] [Google Scholar]
- 19.Van Damme S, Crombez G, Bijttebier P, et al. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain 2002; 96: 319–324. [DOI] [PubMed] [Google Scholar]
- 20.Yap JC, Lau J, Chen PP, et al. Validation of the Chinese Pain Catastrophizing Scale (HK-PCS) in patients with chronic pain. Pain Med 2008; 9: 186–195. DOI: 10.1111/j.1526-4637.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Wei X, Wang F, et al. Validation of a simplified Chinese version of the pain catastrophizing scale and an exploration of the factors predicting catastrophizing in pain clinic patients. Pain Physician 2015; 18: E1059–E1072. [PubMed] [Google Scholar]
- 22.Pielech M, Ryan M, Logan D, et al. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain 2014; 155: 2360–2367. DOI: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroner-Herwig B, Maas J. The German Pain Catastrophizing Scale for Children (PCS-C) – psychometric analysis and evaluation of the construct. Psychosoc Med 2013; 10: Doc07. DOI: 10.3205/psm000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliams LA, Kowal J, Wilson KG. Development and evaluation of short forms of the Pain Catastrophizing Scale and the Pain Self-efficacy Questionnaire. Eur J Pain 2015; 19: 1342–1349. DOI: 10.1002/ejp.665. [DOI] [PubMed] [Google Scholar]
- 25.George SZ, Lentz TA, Zeppieri G, et al. Analysis of shortened versions of the tampa scale for kinesiophobia and pain catastrophizing scale for patients after anterior cruciate ligament reconstruction. Clin J Pain 2012; 28: 73–80. DOI: 10.1097/AJP.0b013e31822363f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory – 25 years of evaluation. Clin Psychol Rev 1988; 8: 77–100. DOI: 10.1016/0272-7358(88)90050-5. [Google Scholar]
- 27.Zheng YP, Wei LA, Goa LG, et al. Applicability of the Chinese Beck Depression Inventory. Compr Psychiatry 1988; 29: 484–489. [DOI] [PubMed] [Google Scholar]
- 28.McCracken LM Vowles KE andEccleston C.. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004; 107: 159–166. [DOI] [PubMed] [Google Scholar]
- 29.Liu YQ, Wang L, Wei YB, et al. Validation of a Chinese version of the Chronic Pain Acceptance Questionnaire (CAPQ) and CPAQ-8 in chronic pain patients. Medicine 2016; 95: DOI: ARTN e4339 10.1097/MD.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001; 92: 147–157. DOI: 10.1016/S0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Feng Y, Han J, et al. Linguistic adaptation, validation and comparison of 3 routinely used neuropathic pain questionnaires. Pain Physician 2012; 15: 179–186. [PubMed] [Google Scholar]
- 32.Cohen SR, Mount BM, Strobel MG, et al. The Mcgill Quality-of-Life Questionnaire – a measure of quality-of-life appropriate for people with advanced disease. A preliminary-study of validity and acceptability. Palliat Med 1995; 9: 207–219. DOI: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- 33.Hu L, Li J, Wang X, et al. Prior study of cross-cultural validation of McGill Quality-of-Life Questionnaire in Mainland Mandarin Chinese patients with cancer. Am J Hosp Palliat Care 2015; 32: 709–714. DOI: 10.1177/1049909114537400. [DOI] [PubMed] [Google Scholar]
- 34.Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980; 66: 271–273. [PubMed] [Google Scholar]
- 35.Lue YJ, Hsieh CL, Huang MH, et al. Development of a Chinese version of the Oswestry Disability Index version 2.1. Spine 2008; 33: 2354–2360. [DOI] [PubMed] [Google Scholar]
- 36.Mccracken LM Zayfert C andGross RT.. The Pain Anxiety Symptoms Scale – development and validation of a scale to measure fear of pain. Pain 1992; 50: 67–73. DOI: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- 37.Zhou XY, Xu XM, Wang F, et al. Validations and psychological properties of a simplified Chinese version of pain anxiety symptoms scale (SC-PASS). Medicine (Baltimore) 2017; 96: e5626. DOI: 10.1097/MD.0000000000005626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruscheweyh R, Marziniak M, Stumpenhorst F, et al. Pain sensitivity can be assessed by self-rating: development and validation of the Pain Sensitivity Questionnaire. Pain 2009; 146: 65–74. DOI: 10.1016/j.pain.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect – the Panas Scales. J Pers Soc Psychol 1988; 54: 1063–1070. DOI: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 40.Beaton DE, Bombardier C, Guillemin F, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000; 25: 3186–3191. DOI: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 41.Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for translation and cultural adaptation. Value Health 2005; 8: 94–104 2005/04/05. DOI: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 42.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34–42. 2006/12/13. DOI: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Fleiss JL. The design and analysis of clinical experiments. New York, NY: Wiley, 1999, p. xiv, 432 pp. [Google Scholar]
- 44.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. DOI: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bansal D, Gudala K, Lavudiya S, et al. Translation, adaptation, and validation of Hindi version of the pain Catastrophizing Scale in patients with chronic low back pain for use in India. Pain Med 2016; 17: 1848–1858. DOI: 10.1093/pm/pnv103. [DOI] [PubMed] [Google Scholar]
- 46.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994; 6: 7 [Google Scholar]
- 47.Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil 2000; 81: S15–S20. 2000/12/29. [DOI] [PubMed] [Google Scholar]
- 48.Arbuckle JL. Amos - Analysis of Moment Structures. Am Stat 1989; 43: 66–67. [Google Scholar]
- 49.Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997; 20: 589–605. [DOI] [PubMed] [Google Scholar]
- 50.Stevens J. Applied multivariate statistics for the social sciences. 4th ed Mahwah, NJ: Lawrence Erlbaum Associates, 2002, p. xiv, 699 pp. [Google Scholar]
- 51.Fernandes L, Storheim K, Lochting I, et al. Cross-cultural adaptation and validation of the Norwegian pain catastrophizing scale in patients with low back pain. BMC Musculoskelet Disord 2012; 13: 111. DOI: 10.1186/1471-2474-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.