Abstract
Background/aims:
Non-alcoholic fatty liver disease is one of the most common chronic liver diseases. Some risk factors are known to influence the development of non-alcoholic fatty liver disease, but the effect of tobacco smoking on the progression of non-alcoholic fatty liver disease is controversial. The main goal of this systematic review and meta-analysis is to investigate the association between smoking and non-alcoholic fatty liver disease.
Method:
Electronic databases (PubMed, Scopus, and ISI Web of Science) were searched to find published articles on non-alcoholic fatty liver disease and smoking until December 2016. All relevant studies were screened by inclusion and exclusion criteria and compatible studies were chosen. The Newcastle–Ottawa Scale was used to assess the methodological quality of eligible articles. Subsequently, information was gathered based on the following: author, publication year, keywords, country, inclusion and exclusion criteria, main results, study design, conclusion, and confounder variables (age, body mass index, gender, ethnicity, and diabetes). Finally, analyses were performed using Comprehensive Meta-Analysis Software.
Results:
Data were extracted from 20 observational studies (9 cross-sectional, 6 case-control, 4 cohort studies, and 1 retrospective cohort study). A significant association was observed between smoking and non-alcoholic fatty liver disease with a pooled odds ratio of 1.110 (95% confidence interval, 1.028–1.199), p-value = 0.008. The statistical heterogeneity was medium with an I2 of 40.012%, p-heterogeneity = 0.074. Also there was a significant relation between non-alcoholic fatty liver disease and passive smoking with a pooled odds ratio of 1.380 (95% confidence interval, 1.199–1.588; p-value = 0.001; I2 = 59.41; p-heterogeneity = 0.117).
Conclusion:
Our meta-analysis demonstrated that smoking is significantly associated with non-alcoholic fatty liver disease. Further prospective studies exploring the underlying mechanisms of this association should be pursued. Also passive smoking increases the risk of non-alcoholic fatty liver disease about 1.38-fold. The effects of smoking cigarettes on active smokers (current smoker, former smoker, and total smoker) are less than passive smokers. Further studies are needed to compare the of effects of passive and active smoking on non-alcoholic fatty liver disease.
Keywords: Smoking, non-alcoholic liver disease, liver, fatty liver
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by accumulation of fat (steatosis) within liver cells due to causes other than alcohol.1 NAFLD is the most common chronic liver disease which includes a wide range of medical conditions from simple steatosis to hepatic fibrosis and hepatocellular carcinoma (HCC).2,3 Some risk factors have been proved to have a relationship with NAFLD. Obesity, based on the measure of body mass index (BMI), is an important risk factor for the pathogenesis and progression of NAFLD. However, some factors like lifestyle correction and regular caffeine consumption can decrease fibrosis of liver in patient with NAFLD.4 The prevalence of NAFLD shows a 4.6-fold increase among obese individuals.5 Other risk factors associated with NAFLD are waist circumference (more than 102 and 88 cm for males and females, respectively), hyperinsulinemia, hypertriglyceridemia, impaired glucose tolerance or type 2 diabetes, and smoking.6–9
According to a study in 2017, the prevalence of daily smoking in men and women are 25.0% and 5.4%, respectively. In 2015, smoking was the cause of death in 6.4 million (11.5%) of people.10 In Europe, where approximately half of the adult males are regular smokers, the prevalence varies from 63% in Russia to 17% in Sweden.11 In women, the pattern of the prevalence of tobacco smoking is different and the prevalence of smoking in developed countries and developing countries for women are 24% and 7%, respectively. In the last decade, smoking has become more common in many countries.10
Tobacco smoking is one of the major risk factors for chronic diseases such as cardiovascular disease, cancer, and type 2 diabetes. Several studies show that smoking is also associated with liver diseases such as neoplasm of liver and chronic liver disease.12–14 Basic and clinical research indicates that smoking affects some of the physiological pathways in the liver.15–18 Some studies on both humans and rats indicate that smoking has an association with the progression of NAFLD. However, the clinical correlation of these findings has been controversial. A cross-sectional study reported that active smoking was related to fibrosis in patients with NAFLD, while another study expresses a lack of significant relationships between active smoking and NAFLD.19,20
Regarding this controversy, no systematic review and meta-analysis of the literature were found demonstrating an “association between smoking and NAFLD.” Furthermore, because of the high prevalence of smoking in different populations and the importance of NAFLD in the progression of chronic liver disease, this study was designed in order to determine the association between smoking and NAFLD. Also, in this study, an effort was made to investigate the association between current, former, and passive smoking and NAFLD in observational studies.
Methods
Literature search
Electronic databases (Scopus, PubMed, and ISI Web of Science) were searched (until June 2016) by two independent investigators (B.H. and M.G.N.) for studies that provided information on the relationship between NAFLD and smoking in English literature. We improved our search strategy by hand-searching the reference lists of included papers to identify additional relevant studies.
Our search terms included variations of the concept of smoking (smoking, tobacco smoking, hookah smoking, water pipe smoking, pipe smoking, tobacco smoke pollution, environmental tobacco smoke pollution, passive smoking, secondhand smoking, involuntary smoking, cigarette smoking, and cigar smoking) and “fatty liver” concepts (fatty liver, steatohepatitis, steatohepatitides, steatosis of liver, visceral steatosis, visceral steatoses, liver steatosis, liver steatoses, NAFLD, non-alcoholic steatohepatitis (NASH), toxicant-associated steatohepatitis (TASH), and toxicant-associated fatty liver disease (TAFLD)). The strategy of search in ISI Web of Science is “(TS = (fatty liver) OR TS = (Steatohepatitis) OR TS = (Steatohepatitides) OR TS = (Steatosis of Liver) OR TS = (Visceral Steatosis) OR TS = (Visceral Steatoses) OR TS = (Liver Steatosis) OR TS = (Liver Steatoses) OR TS = (NAFLD) OR TS = (NAFLD) OR TS = (NASH) OR TS = (NASH) OR TS = (TASH) OR TS = (TAFLD)) AND (TS = (smoking) OR TS = (Tobacco Smoking) OR TS = (Hookah Smoking) OR TS = (Waterpipe Smoking) OR TS = (Pipe Smoking) OR TS = (Tobacco Smoke Pollutions) OR TS = (Environmental Tobacco Smoke Pollution) OR TS = (Passive Smokings) OR TS = (Secondhand Smoking) OR TS = (Involuntary Smoking) OR TS = (Cigarette Smoking)).”
Inclusion and exclusion criteria
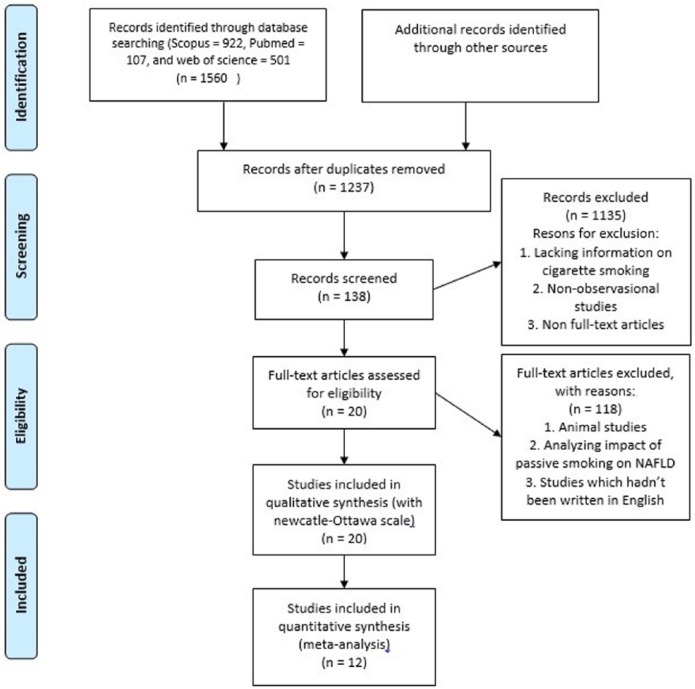
Studies were included in systematic review and meta-analysis if they met the following criteria: (1) observational studies with a comparison (case-control and retrospective/prospective cohort studies). (2) Prevalence of smoking individuals in NAFLD patients and control group or Odds ratios are reported. (3) NAFLD patients were diagnosed by abdominal ultrasound or pathological report of liver biopsies. (4) The reported amount of cigarette smoking can be transformed to pack-year (Brinkman Index). The studies in which their NAFLD patients were under 18 years old and animal studies and non-available full-text articles were excluded. Other exclusion criteria were lacking information on cigarette smoking. Letter to editors, comments, position papers, unstructured papers, proceeding papers, thesis, and dissertation were also excluded. After checking for eligibility, the full text of qualified studies was obtained. The finally selected papers were read, tagged, and hand-noted by two reviewers (A.A.R.) and (M.T.N.) then verified by the second reviewer (K.A.R.). A brief flow diagram of the strategy is depicted in Figure 1.
Figure 1.
Flow diagram of included and excluded studies.
Search strategy development and study screening
After developing methods of study identification, source selection, and search combinations, two reviewers (A.A.R. and M.S.) performed the search for the literature. All search results were exported into the reference manager software, EndNote X7 (Thomson Reuters, New York, NY, USA). These studies were screened and compared to meet inclusion and exclusion criteria by two independent reviewers (A.A.R. and M.T.N.). Any disagreement was reconciled with the third reviewer (B.H.) who was also responsible for the supervision of the research.
Data extraction, quality assessment, and risk of bias assessment
Total of 1273 studies were identified and reviewed. Reference lists of all the final articles (20 articles) were hand-searched for any additional studies.
Quantitative papers selected for retrieval will be assessed by two independent reviewers for methodological validity prior to inclusion in the review using Newcastle–Ottawa Scale.21 Methodological quality assessment using the newcastle ottawa scale (NOS) is based on the selection of study groups, comparability of study groups, and the ascertainment of the exposure/outcome of interest. Any discrepancy between two reviewers was evaluated by B.H.
NOS scores of 1–3, 4–6, and 7–10 show low-, intermediate-, and high-quality studies, respectively.4 Also publication bias was assessed with funnel plot.
Primary data extraction form was designed and were used to extract data. Information was gathered for the following terms: author, publication year, keywords, country main results, confounder variables, study design, number of passive and active smokers, current and formers smokers, and light and heavy smokers. The main outcome measurement was the prevalence of smoking in patients with and without NAFLD.
Quality scores were assigned by two reviewers (A.A.R.) and (M.T.N.) and verified by K.A.R. The summary of our quality assessment approach has been outlined in Table 1.
Table 1.
Quality assessment of the studies.
| Study | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|
| Chavez-Tapia et al.20 | 5 | 1 | 3 | 9 |
| Caballería et al.22 | 5 | 2 | 2 | 9 |
| Liu et al.23 | 5 | 2 | 3 | 10 |
| Oniki et al.24 | 4 | 2 | 2 | 8 |
| Hamabe et al.25 | 5 | 2 | 2 | 9 |
| Zhang et al.26 | 5 | 2 | 2 | 9 |
| Otgonsuren et al.27 | 5 | 2 | 2 | 9 |
| Ozturk et al.28 | 3 | 2 | 2 | 7 |
| Lin et al.29 | 3 | 0 | 2 | 5 |
| Koch et al.30 | 3 | 2 | 3 | 8 |
| Zhang et al.31 | 4 | 2 | 3 | 9 |
| Singh et al.32 | 4 | 2 | 2 | 8 |
| Koehler et al.33 | 5 | 2 | 2 | 9 |
| Zhang et al.34 | 1 | 2 | 2 | 5 |
| Zhang et al.26 | 1 | 2 | 2 | 5 |
| Wu et al.35 | 2 | 2 | 3 | 7 |
| Chang et al.36 | 3 | 2 | 2 | 7 |
| Hung et al.37 | 3 | 1 | 1 | 5 |
| Yang et al.38 | 4 | 2 | 2 | 8 |
| Arslan et al.39 | 3 | 2 | 2 | 7 |
| Total (mean) | 73 | 36 | 44 | 153 |
Data synthesis
To assess the association, summary data from individual studies were pooled using a fixed effect model. All continuous data are summarized as odds ratio (OR) along with 95% confidence intervals (CIs). The inconsistency index (I2) was used to measure heterogeneity, with values of I2 > 50% indicating substantial heterogeneity.40 All analyses were performed using comprehensive meta-analysis with a p-value < 0.05 considered statistically significant.
Result
Characteristics of the studies
We initially identified a total of 1273 studies that met our search criteria. After performing a title and abstract review, 1135 studies were excluded, which resulted in 138 studies that underwent full-text review. Finally, 20 (9 cross-sectional, 6 case-control, 4 cohort, and 1 retrospective cohort) studies20,22–41 and 12 (7 cross-sectional, 3 case-control, and 2 cohort) studies20,22,23,24,27,28,30,32,33,35,39,41 were included in the systematic review and meta-analysis, respectively. Characteristics of the studies included in this meta-analysis are described in Table 2.
Table 2.
General information of the non-alcoholic fatty liver disease and control groups (n (%)).
| Author, publication year, country | Sample size | NAFLD, n (%) | Non-NAFLD, n (%) | Odds ratio | Confidence interval | Adjusting | Diagnostic methods | Study design |
|---|---|---|---|---|---|---|---|---|
| Hamabe et al., 2011, Japan25 | 1553 | A, R, CC | Abdominal ultrasonography | Retrospective cohort | ||||
| Total smoker | 93 (5.99) | 216 (13.90) | 2.683 | (2.00–3.59) | ||||
| Light | 24 (1.55) | 57 (3.67) | 0.94 | (0.59–1.48) | ||||
| Heavy | 69 (4.44) | 159 (10.24) | 2.7 | (1.95–3.74) | ||||
| Non-smoker | 172 (11.1) | 1072 (69) | ||||||
| Liu et al., 2013, China23 | 2426 | A, I, R, Z, AA, BB, CC, DD, EE, FF | Ultrasonography | Cross-sectional | ||||
| Total smoker | 420 (17.31) | 962 (39.65) | 1.047 | (0.88–F1.25) | ||||
| Light | 162 (6.67) | 421 (17.35) | 0.92 | (1.39–1.30) | ||||
| Heavy | 258 (10.63) | 541 (22.30) | 1.14 | (3.98–7.98) | ||||
| Current | 420 (17.31) | 962 (39.65) | 1.05 | (0.87–1.25) | ||||
| Former | 106 (4.36) | 204 (8.40) | 1.25 | (0.95–1.63) | ||||
| Passive | 5 | 70 | 1.364 | |||||
| Non passive smokers | 4 | 225 | ||||||
| Non-smoker | 294 (12.12) | 705 (29.06) | ||||||
| Zhang et al., 2015, China26 | 800 | A, B, D, E, G, I, L, M, N, O, P, Q, T, U, W, Y | Ultrasonography | Case-control | ||||
| Total smoker | 408 (51) | 55 (6.87) | 5.602 | (3.93–7.98) | ||||
| Light | 171 (21.38) | 31 (3.87) | 4.24 | (2.68–6.46) | ||||
| Heavy | 237 (29.63) | 24 (3.00) | 7.71 | (4.66–11.95) | ||||
| Non-smoker | 192 (24.00) | 145 (18.13) | ||||||
| Chavez-Tapia et al., 2006, Mexico20 | 885 | Not mention | Ultrasonography | Cross-sectional | ||||
| Total smoker | 87 (9.83) | 232 (26.21) | 0.888 | (0.66–1.21) | ||||
| Non-smoker | 168 (18.98) | 398 (44.97) | ||||||
| Caballería et al., 2010, Spain22 | 766 | A, E, G (male), S | Ultrasonography | Cross-sectional | ||||
| Total smoker | 92(12.01) | 250(32.63) | 0.104 | (0.80–1.53) | ||||
| Current | 39(5.09) | 150 (19.58) | 0.78 | (0.40–0.93) | ||||
| Former | 53(6.91) | 100 (13.05) | 1.59 | (0.83–1.87) | ||||
| Non-smoker | 106 (13.83) | 318 (41.51) | ||||||
| Oniki et al., 2013, Japan24 | 696 | A, G, B, EE, DD, R | Ultrasonography | Cross-sectional case-control | ||||
| Total smoker | 61 (8.76) | 221 (31.75) | 1.38 | (0.94–2.02) | ||||
| Current | 21 (3.01) | 57 (8.18) | 1.84 | (0.86–1.18) | ||||
| Former | 40 (5.74) | 164 (23.56) | 1.22 | (0.79–1.87 | ||||
| Non-smoker | 69 (9.91) | 345 (49.56) | ||||||
| Zatu et al., 2014, South Africa41 | 195 | A, G | Not mentioned | Cross-sectional | ||||
| Total smoker | 17 | 44 | 0.629 | (0.33–1.22) | ||||
| Non-smoker | 51 | 83 | ||||||
| Zhang et al., 2015, China34 | 1800 | A, G, HH | Based on guidelines for the diagnosis and treatment of NAFLD revised by the Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association in 201042 | Case-control | ||||
| Total smoker | 447 (49.67) | 207 (23) | 3.303 | (2.70–4.05) | ||||
| Light | 144 (16.00) | 116 (12.89) | 1.899 | (1.37–2.55) | ||||
| Heavy | 303 (33.67) | 91 (10.11) | 5.0937 | (3.02–8.35) | ||||
| Non-smoker | 453 (50.33) | 693 (77.00) | ||||||
| Wu et al., 2015, China35 | 587 | H, J, Q, X | Diagnosis of NAFLD was made according to criteria proposed by the fatty liver and alcoholic liver disease study group of the Chinese Liver Disease Association43,44 | Case-control | ||||
| Total smoker | 129 (52.43) | 124 (36.47) | 1.275 | (0.93–1.75) | ||||
| Non-smoker | 177 (49.57) | 217 (63.53) | ||||||
| Koehler et al., 2012, Netherland33 | 2811 | Not mention | Ultrasonography | cross-sectional | ||||
| Total smoker | 660 (23.48) | 1123 (40.00) | 1.266 | (1.08–1.49) | ||||
| Current | 75 (2.67) | 165 (2.31) | ||||||
| Former | 585 (20.81) | 958 (34.08) | ||||||
| Non-smoker | 326 (11.60) | 702 (24.97) | ||||||
| Chang et al., 2013, Korea36 | 43,166 | G, AA, BB, HH | Abdominal ultrasonography | Cohort | ||||
| Smoker | 5133 (44) | 8068 (25.6) | 2.288 | (2.19–2.39) | ||||
| Non-smoker | 6519 (56) | 23,446 (74.4) | ||||||
| Hung et al., 2013, Taiwan37 | 521 | B-mode ultrasonography | Cross-sectional | |||||
| Smoker | 32 (12.3) | 54 (20.8) | 0.533 | (0.33–0.86) | ||||
| Non-smoker | 229 (87.7) | 206 (79.2) | ||||||
| Yang et al., 2012, China38 | 903 | A, B, G | B-mode ultrasonography | Case-control | ||||
| Smoker | 82 (19.2) | 55 (11.8) | 1.735 | (1.20–2.51) | ||||
| Non-smoker | 354 (80.8) | 412 (88.2) | ||||||
| Arslan et al., 2014,Turkey39 | 145 | G, HH | Based on biochemical, radiological, and histological criteria | Cohort | ||||
| Smoker | 29 (29) | 20 (44.4) | 0.511 | (0.25–1.06) | ||||
| Non-smoker | 71 (71) | 25 (55.6) | ||||||
| Koch et al., 2015, Germany30 | 354 | A, G | MRI | Cohort | ||||
| Smoker | 95 (50.8) | 91 (54.5) | 0.862 | (0.57–1.31) | ||||
| Non-smoker | 92 (49.2) | 76 (45.5) | ||||||
| Singh et al., 2015, India32 | 645 | Not mentioned | Ultrasonography and histological confirmation whenever possible | Case–Control | ||||
| Smoker | 49 (10.6) | 12 (6.6) | 1.663 | (0.86–3.20) | ||||
| Non-smoker | 415 (89.4) | 169 (93.4) | ||||||
| Zhang et al., 2014, China31 | 17,920 | Confounding factors | Abdominal ultrasonography | Prospective cohort | ||||
| Smoker | 2178 (66.4) | 11,716 (80) | 0.494 | (0.46–54) | ||||
| Non-smoker | 1101 (33.6) | 2925 (20) | ||||||
| Otgonsuren et al., 2013, United States27 | 10,565 | A, G,L, EE, DD, II | Ultrasonography | Cross-sectional | ||||
| Total smoker | 2241 (89.3) | 7137 (88.6) | 1.072 | (0.93–1.24) | ||||
| Heavy/moderate smoker | 733 (29.2) | 2658 (33) | ||||||
| Light smoker | 1508 (60.1) | 4479 (55.6) | ||||||
| Never smoker | 269 (10.7) | 918 (11.4) | ||||||
| Lin et al., 2014, United States29 | 304 | A | Abdominal ultrasonography | Cross-sectional | ||||
| Smoking exposure | 5 (55.6) | 70 (23.7) | 4.018 | (1.05–15.07) | ||||
| No smoking exposure | 4 (44.4) | 225 (76.3) | ||||||
| Ozturk et al., 2016, Turkey28 | 74 | A, B, C, D, E, F, I, K, L, M, O, P, X, Y, HH, MM,NN, OO | Liver biopsy | Cross-sectional | ||||
| Smoker | 20 (27.03) | 4 (5.40) | 2.353 | (0.69–8.03) | ||||
| Non-smokers | 34 (45.45) | 16 (21.62) |
NAFLD: non-alcoholic fatty liver disease; MRI: magnetic resonance imaging.
A: age; B: body mass index; C: uric acid; D: aspartate transaminase; E: alanine transaminase; F: alkaline phosphatase; G: gender; H: high-density lipoprotein cholesterol; I: fasting serum insulin; J: white blood cell; K: glucose; L: waist circumference; M: hip circumference; N: waist-to-hip ratio; O: systolic blood pressure; P: diastolic blood pressure; Q: fasting blood glucose; R: dyslipidemia; S: metabolic syndrome; T: triglyceride; U: total cholesterol; W: low-density lipoprotein cholesterol; X: homeostatic model assessment (HOMA-IR); Y: high-sensitivity C-reactive protein; Z: education status; AA: alcohol consumption; BB: physical activity; CC: obesity; DD: hypertension; EE: diabetes; FF: use of anti-diabetic medication; HH: smoking status; II: race/ethnicity; MM: ferritin; NN: 2-h oral glucose tolerance test; OO: lipids.
Systematic review and meta-analysis studies that were included generated a total study population of 92,125 and 20,149 subjects, respectively. Among these, four studies22–24,33 with 6699 subjects were included in the analysis for prevalence of former smoking, current smoking, and non-smoking among patients with and without NAFLD. “Former smoker” is defined as those who had not smoked in the 6 months leading to the study, while “current smoker” refers to those who had smoked in the 6 months prior to the study (Table 3).The other two studies23,29 with 2730 subjects were used for the analysis of the prevalence of passive smoking between patients with and without NAFLD (Table 3).
Table 3.
Main results of the subgroups and total analysis included in this meta-analysis.
| Subgroup | Studies, n | Heterogeneity |
Model of meta-analysis | Pooled OR (95% CI) | Z analysis | p-value | ||
|---|---|---|---|---|---|---|---|---|
| % | p | |||||||
| Current smokers | 4 | 49.618 | 0.114 | Fixed | 1.034 (0.899–1.188) | 0.465 | 0.642 | |
| Former smokers | 4 | 0.00 | 0.768 | Fixed | 1.316 (1.158–1.496) | 4.211 | 0.001 | |
| Passive smokers | 2 | 59.41 | 0.117 | Fixed | 1.380 (1.199–1.588) | 4.503 | 0.001 | |
| Light smoker | 3 | 20.924 | 0.282 | Fixed | 1.074 (0.991–1.332) | 1.262 | 0.207 | |
| Heavy smoker | 2 | 54.98 | 0.136 | Fixed | 1.014 (0.895–1.149) | 0.219 | 0.826 | |
| Study design | Cohort | 2 | 10.81 | 0.29 | Fixed | 2.97 (2.2–2.4) | 37.066 | 0.001 |
| Case-control | 4 | 0.00 | 0.622 | Fixed | 1.451 (1.94–1.762) | 3.748 | 0.001 | |
| Cross-sectional | 9 | 43.83 | 0.001 | Fixed | 1.113 (1.025–1.208) | 2.545 | 0.011 | |
| Total | 12 | 40.012 | 0.074 | Fixed | 1.110 (1.029–1.199) | 2.672 | 0.008 | |
OR: odds ratio; CI: confidence interval.
Meta-analysis results
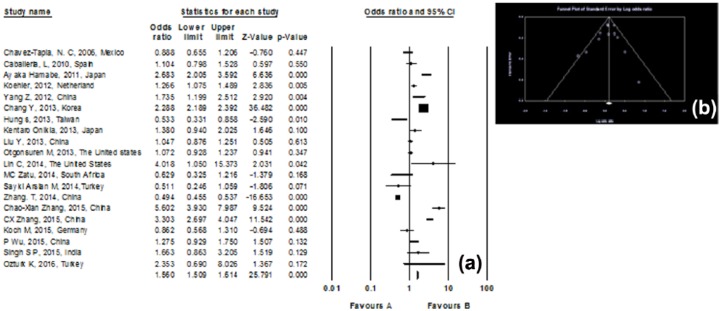
Among 20 studies, 12 observational studies involving 20,149 people were included in the data analysis with cumulative meta-analysis. There was a significant association between Smoking and NAFLD with pooled OR of 1.110 (95% CI, 1.028–1.199), p value = 0.008. The statistical heterogeneity was medium with I2 of 40.012%, p heterogeneity = 0.074 (Figure 2) and funnel plot show publication bias in study (Figure 3).
Figure 2.
(a) Forest plot of the included studies assessing the association between smoking and non-alcoholic fatty liver disease; a diamond data marker represents the overall OR, 95% CI, and relative weight for the outcome of interest. (b) Funnel plot of the included studies represents the tau score = −0.075, z-value for tau = 0.34, and p-value (two-tailed) = 0.73 in Begg and Mazumdar rank correlation test that show publication bias does not exist in this study.
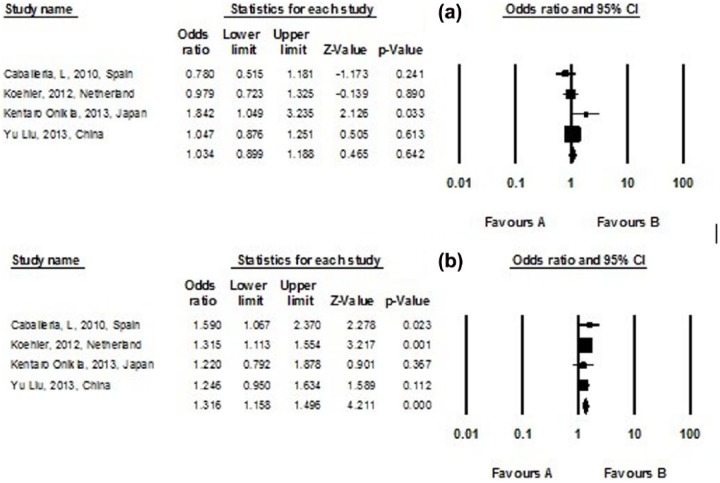
Figure 3.
Subgroup analysis assessing (a) current and (b) former smokers for the risk of non-alcoholic fatty liver disease in included studies; a diamond data marker represents the overall OR and 95% CI for the outcome of interest.
Subgroup analysis
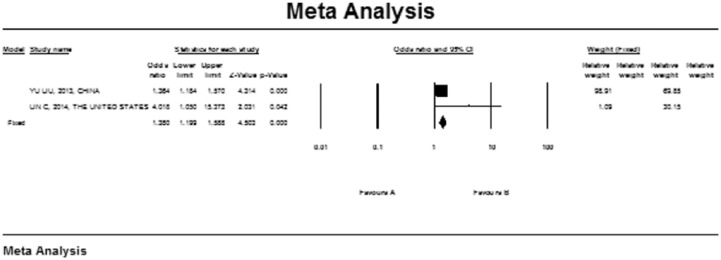
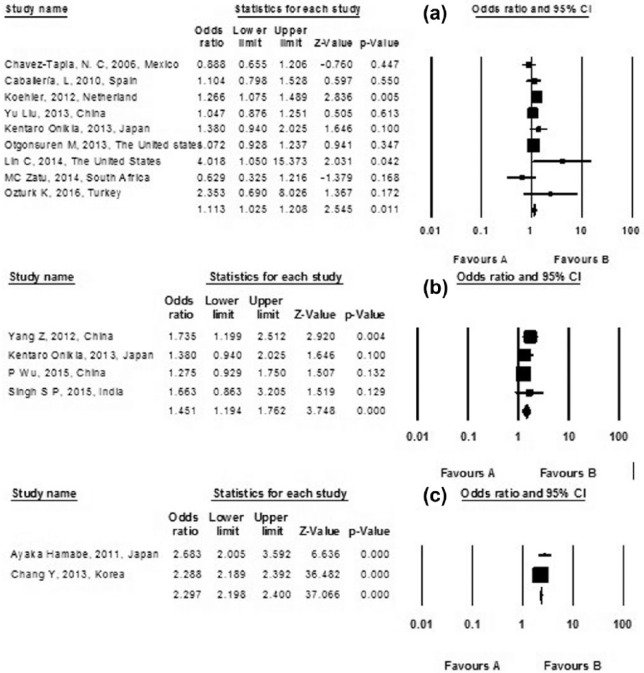
Classification of smoking varies between studies, which may impact the overall estimates. We, therefore, stratified the risk of NAFLD based on the study’s classification of smoking. Four studies classify smoking into a current subgroup and a former subgroup.22–24,33 There was a significant association between former smoking and NAFLD with pooled OR of 1.316 (95% CI, 1.158–1.496; I2 = 0.001; p-heterogeneity = 0.007), p-value = 0.768. However, in the current subgroup, no significant association between smoking and NAFLD was observed (pooled OR 1.034; 95% CI, 0.899–1.188; I2 = 49.618; p-heterogeneity = 0.114), p-value = 0.642 (Figure 3). Also, a subgroup analysis for passive smokers was performed including two studies23,29 yielding a significant relation between NAFLD and passive smoking with a pooled OR of 1.380 (95% CI, 1.199–1.588; p-value = 0.001; I2 = 59.41; p-heterogeneity = 0.117) (Figures 4). Also, an analysis was performed according to study design (cross-sectional studies, case-control studies, and cohort studies) for the risk of non-alcoholic fatty liver disease in included studies (Figure 5).
Figure 4.
Subgroup analysis assessing passive smokers for the risk of non-alcoholic fatty liver disease in included studies; a diamond data marker represents the overall OR and 95% CI for the outcome of interest.
Figure 5.
Analysis assessing according to study design ((a) cross-sectional studies, (b) case-control studies, and (c) cohort studies) for the risk of non-alcoholic fatty liver disease in included studies; a diamond data marker represents the overall OR and 95% CI for the outcome of interest.
Discussion
There are controversial data on the association between smoking and NAFLD. In this study, we systematically reviewed 20 studies and meta-analyzed 12 studies, to further evaluate the association. Although our results supported a putative relationship between NAFLD and smoking, exhibiting a stronger relation in cohort studies (OR = 2.97) and in case-control studies (OR = 1.451), there was no significant association between smoking and NAFLD in current smokers. However, this association was considerable in former smokers. In addition, in passive smokers, NAFLD and smoking showed a significant relation.
Several studies demonstrated different conclusions about the association between smoking and NAFLD. Liu et al.23 observed a positive association between heavy active smoking and NAFLD in the Chinese population. These results may be due to the fast deposition of fat in the liver after using tobacco. Besides, although higher Brinkman Index is associated with NAFLD, cessation of smoking may lead to NAFLD by increasing BMI.22 As a result, a clear history of previous smoking habits is crucial to prevent NAFLD development.
Another possible mechanism that may play a role in sex-related NAFLD is the possible influence of sex hormones on smoking-induced NAFLD.45,46 The amount of body fat can change as a result of smoking cigarettes due to the anti-estrogenic properties of cigarette smoke, which could potentially explain the independent role of BMI in the association between active smoking and NAFLD.47–49
Glucose oxidative metabolism can be induced by long-term smoking, which causes the inhibition of the non-oxidative reactions and ultimately leads to higher levels of plasma free fatty acid (FFA). Hepatocytes and adipose tissue absorb these FFAs and turn them into triglycerides causing insulin resistance (IR).26 IR frequently occurs in patients with NAFLD and mostly results from deposition of fat, FFAs production in skeletal muscle, decreased glucose absorption, and suppressed gluconeogenesis in liver cells.50
A study20 did not find any association between smoking, smoking intensity (number of packs/year), and the prevalence of NAFLD. However, univariate regression analysis showed that NAFLD and smoking were not associated with each other.20 In these studies, the samples were very similar, and the primary variable of these studies (smoking) did not influence the prevalence of NAFLD. Limitations of these studies should be considered, which could explain the absence of an association between smoking and NAFLD. First, the studies did not evaluate IR, which can partially affect the results. The other factor that helps explain this discrepancy is the fact that physical activity was not evaluated in these studies, which is another factor related to IR and can possibly be affected by smoking.20
In our study, current smoking did not have any association with NAFLD. A possible explanation for this finding could be unknown confounding factors (period of smoking, amount of smoking, etc.) which were not considered. Another explanation is that because NAFLD is a chronic liver disease; having a history of smoking for a period of time could be a risk factor for NAFLD. An increase in body weight and BMI as a consequence of cessation of smoking may be another explanation for the development of NAFLD in former smokers.
In this study, we discovered that passive smoking has a significant relation with NAFLD. We also found that passive smoking increases the risk of NAFLD about 1.38-fold. However, the effects of smoking cigarettes on active smokers (current smoker, former smoker, and total smoker) are less than passive smokers. This result may be due to a factor which is discussed in a study which showed side stream smoke has higher concentration of harmful chemicals than mainstream smoke.51 A study performed by Liu et al.23 demonstrated that passive smokers have more liver steatosis than light and moderate active smokers. Further study is needed to compare the effects of passive and active smoking on NAFLD.29
This systematic review has several limitations. First, the included studies were all case-control, cross-sectional, or cohort studies using questionnaires to evaluate smoking habits in participants, so our results are based on self-reported data until laboratory and clinical data are collected. Second, some studies only investigated the association between smoking and NAFLD in male participants. Several of our studies diagnosed NAFLD based on ultrasonography without the requirement for a pathologic confirmation after liver biopsy. Some factors such as physical activity, diet, caffeine consumption, or socioeconomic factors may play the role of confounders; however, more studies were needed to determine the exact place of smoking in NAFLD; nevertheless in this study, these confounder variables were not included. The inclusion of over 92,125 patients was a significant strength of this meta-analysis. While cross-sectional, case-controlled, and cohort studies have their inherent limitations, with the utilization of 12 studies in meta-analysis, we were able to generate a much greater statistical power compared with a single study.
In conclusion, our results show that smoking is significantly associated with NAFLD. While we concluded that there was an association between smoking and NAFLD in former smokers, there was not any correlation in current smokers.
For clinicians, it is obvious that smoking is correlated with NAFLD, which is one of the most common chronic liver diseases. Physicians should warn their patients based on the potential effects of smoking on the pathogenesis and progression of NAFLD and the high prevalence of smoking in different populations. This study enables researchers to investigate the mechanisms of smoking-related NAFLD and run cohort studies considering confounders such as physical activity, diet, and socioeconomic factors.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Mashhad University of Medical Science.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Research Council of Mashhad University of Medical Science, Dr Azita Ganji and Gastrology and Enterology Research Center, Emam Reza Hospital of Mashhad.
References
- 1. Ludwig J, Viggiano TR, Mcgill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980; 55: 434–438. [PubMed] [Google Scholar]
- 2. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003; 98: 960–967. [DOI] [PubMed] [Google Scholar]
- 3. Chen S, Teoh NC, Chitturi S, et al. Coffee and non-alcoholic fatty liver disease: brewing evidence for hepatoprotection? J Gastroen Hepatol 2014; 29: 435–441. [DOI] [PubMed] [Google Scholar]
- 4. Shen H, Rodriguez AC, Shiani A, et al. Association between caffeine consumption and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Ther Adv Gastroenter 2016; 9: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994; 74: 761–811. [DOI] [PubMed] [Google Scholar]
- 6. Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology 1998; 27: 1463–1466. [DOI] [PubMed] [Google Scholar]
- 7. Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocr Metab 2004; 89: 2563–2568. [DOI] [PubMed] [Google Scholar]
- 8. Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uysal KT, Wiesbrock S, Marino M, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610–614. [DOI] [PubMed] [Google Scholar]
- 10. Ali R, Hay S. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study. Lancet 2015; 389: 1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. The European report on tobacco control policy. In: WHO European ministerial conference for a tobacco-free Europe, Warsaw, 18–19 February 2002, pp. 18–19. Geneva: World Health Organization. [Google Scholar]
- 12. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008; 300: 2765–2778. [DOI] [PubMed] [Google Scholar]
- 13. Jatoi NA, Jerrard-Dunne P, Feely J, et al. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007; 49: 981–985. [DOI] [PubMed] [Google Scholar]
- 14. Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007; 298: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 15. Avti PK, Kumar S, Pathak CM, et al. Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci 2006; 89: 547–553. [DOI] [PubMed] [Google Scholar]
- 16. Chen ZM, Liu BQ, Boreham J, et al. Smoking and liver cancer in China: case-control comparison of 36,000 liver cancer deaths vs. 17,000 cirrhosis deaths. Int J Cancer 2003; 107: 106–112. [DOI] [PubMed] [Google Scholar]
- 17. Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005; 42: 218–224. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Liu Y, Shi J, et al. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol M 2002; 227: 823–829. [DOI] [PubMed] [Google Scholar]
- 19. Azzalini L, Ferrer E, Ramalho LN, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology 2010; 51: 1567–1576. [DOI] [PubMed] [Google Scholar]
- 20. Chavez-Tapia NC, Lizardi-Cervera J, Perez-Bautista O, et al. Smoking is not associated with nonalcoholic fatty liver disease. World J Gastroenterol 2006; 12: 5196–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000, https://www.medicine.mcgill.ca/rtamblyn/Readings/The%20Newcastle%20-%20Scale%20for%20assessing%20the%20quality%20of%20nonrandomised%20studies%20in%20meta-analyses.pdf
- 22. Caballería L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroen Hepat 2010; 22: 24–32. [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Dai M, Bi Y, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol 2013; 23: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oniki K, Hori M, Saruwatari J, et al. Interactive effects of smoking and glutathione S-transferase polymorphisms on the development of non-alcoholic fatty liver disease. Toxicol Lett 2013; 220: 143–149. [DOI] [PubMed] [Google Scholar]
- 25. Hamabe A, Uto H, Imamura Y, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol 2011; 46: 769–778. [DOI] [PubMed] [Google Scholar]
- 26. Zhang C-X, Guo L-K, Qin Y-M, et al. Association of polymorphisms of adiponectin gene promoter-11377C/G, glutathione peroxidase-1 gene C594T, and cigarette smoking in nonalcoholic fatty liver disease. J Chin Med Assoc 2016; 79: 195–204. [DOI] [PubMed] [Google Scholar]
- 27. Otgonsuren M, Stepanova M, Gerber L, et al. Anthropometric and clinical factors associated with mortality in subjects with nonalcoholic fatty liver disease. Digest Dis Sci 2013; 58: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 28. Ozturk K, Kurt O, Dogan T, et al. Pentraxin 3 is a predictor for fibrosis and arterial stiffness in patients with nonalcoholic fatty liver disease. Gastroenterol Res Pract 2016; 2016: 1417962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin C, Rountree CB, Methratta S, et al. Secondhand tobacco exposure is associated with nonalcoholic fatty liver disease in children. Environ Res 2014; 132: 264–268. [DOI] [PubMed] [Google Scholar]
- 30. Koch M, Borggrefe J, Schlesinger S, et al. Association of a lifestyle index with MRI-determined liver fat content in a general population study. J Epidemiol Commun H 2015; 69: 732–737. [DOI] [PubMed] [Google Scholar]
- 31. Zhang T, Zhang Y, Zhang C, et al. Prediction of metabolic syndrome by non-alcoholic fatty liver disease in northern urban Han Chinese population: a prospective cohort study. PLoS ONE 2014; 9: e96651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh SP, Singh A, Misra D, et al. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case-control study. J Clin Exp Hepatol 2015; 5: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koehler EM, Schouten JN, Hansen BE, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol 2012; 57: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 34. Zhang CX, Guo LK, Qin YM, et al. Interaction of polymorphisms of resistin gene promoter -420C/G, glutathione peroxidase -1 Gene Pro198Leu and cigarette smoking in nonalcoholic fatty liver disease. Chin Med J 2015; 128: 2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu P, Hua Y, Tan S, et al. Interactions of smoking with rs833061 polymorphism on the risk of non-alcoholic fat liver disease in Hubei Han population: a preliminary case-control study. Iran J Basic Med Sci 2015; 18: 1112–1117. [PMC free article] [PubMed] [Google Scholar]
- 36. Chang Y, Jung H-S, Yun KE, et al. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol 2013; 108: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 37. Hung S-C, Lai S-W, Chen M-C, et al. Prevalence and related factors of non-alcoholic fatty liver disease among the elderly in Taiwan. Eur Geriatr Med 2013; 4: 78–81. [Google Scholar]
- 38. Yang Z, Wen J, Li Q, et al. PPARG gene Pro12Ala variant contributes to the development of non-alcoholic fatty liver in middle-aged and older Chinese population. Mol Cell Endocrinol 2012; 348: 255–259. [DOI] [PubMed] [Google Scholar]
- 39. Arslan MS, Turhan S, Dincer I, et al. A potential link between endothelial function, cardiovascular risk, and metabolic syndrome in patients with Non-alcoholic fatty liver disease. Diabetol Metab Syndr 2014; 6: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003. September 6;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zatu MC, van Rooyen JM, Greeff M, et al. A comparison of the cardiometabolic profile of black South Africans with suspected non-alcoholic fatty liver disease (NAFLD) and excessive alcohol use. Alcohol 2015; 49: 165–172. [DOI] [PubMed] [Google Scholar]
- 42. Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases. Chin J Hepatol 2006; 3: 917–923. [PubMed] [Google Scholar]
- 43. Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. Diagnostic criteria of nonalcoholic fatty liver disease. Chin J Hepatol 2003; 11: 71. [PubMed] [Google Scholar]
- 44. Jiang L-L, Li L, Hong X-F, et al. Patients with nonalcoholic fatty liver disease display increased serum resistin levels and decreased adiponectin levels. Eur J Gastroen Hepat 2009; 21: 662–666. [DOI] [PubMed] [Google Scholar]
- 45. Manjer J, Johansson R, Lenner P. Smoking as a determinant for plasma levels of testosterone, androstenedione, and DHEAs in postmenopausal women. Eur J Epidemiol 2005; 20: 331–337. [DOI] [PubMed] [Google Scholar]
- 46. Economou F, Xyrafis X, Livadas S, et al. In overweight/obese but not in normal-weight women, polycystic ovary syndrome is associated with elevated liver enzymes compared to controls. Hormones 2009; 8: 199–206. [DOI] [PubMed] [Google Scholar]
- 47. Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat: III. Effects of cigarette smoking. JAMA 1989; 261: 1169–1173. [PubMed] [Google Scholar]
- 48. Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause 2004; 11: 104–109. [DOI] [PubMed] [Google Scholar]
- 49. Windham GC, Mitchell P, Anderson M, et al. Cigarette smoking and effects on hormone function in premenopausal women. Environ Health Perspect 2005; 113: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Facchini FS, Hollenbeck CB, Jeppesen J, et al. Insulin resistance and cigarette smoking. Lancet 1992; 339: 1128–1130. [DOI] [PubMed] [Google Scholar]
- 51. Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control 2005; 14: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]