Abstract
Background:
In relapsing–remitting multiple sclerosis (RRMS), ‘no evidence of disease activity’ (NEDA) is regarded as a key treatment goal. The increasing number of treatments allows for individualized treatment optimization in patients with suboptimal response to first-line disease-modifying therapies (DMTs). Therefore, monitoring of clinical and subclinical disease activity on DMTs has been recognized as an important component of long-term patient management.
Methods:
EPIDEM was a multicenter non-interventional retrospective study in a large cohort of RRMS patients receiving injectable DMTs for at least 2 years in outpatient centers throughout Germany. It documented measures and ratings of disease activity on DMTs to characterize the factors that made the treating neurologists consider to switch therapy towards potentially more effective or better-tolerated drugs.
Results:
The cohort included predominantly female patients with a mean age of 45 years and a mean disease duration of 9.6 years, who had been continuously treated with an injectable DMT for a median duration of 54 months. Overall, 34.0% of the patients had experienced ⩾1 relapse on any DMT in the previous 2 years; 21.0% exhibited magnetic resonance imaging (MRI) activity, and the Kurtzke Expanded Disability Status Scale (EDSS) score increased by at least 0.5 points in 20.1%. Overall, 50.3% of the patients with EDSS progression and 70.6% of the patients with relapses were assessed as clinically stable by the neurologists. A change of treatment was considered in a fraction of patients with disease activity: in 22.8% of those with relapse activity, in 37.8% of those with MRI activity and in 20.1% of those with EDSS progression.
Conclusion:
The results of EPIDEM underline the importance of standardized evaluation and documentation of ongoing disease activity and disability deterioration. Judged from the present data, the current paradigm of low tolerance for disease activity and recommendations for early treatment optimization have not been turned fully into action as yet. More widespread implementation of current guideline recommendations may allow patients to more benefit from the growing panel of effective treatment options.
Keywords: Multiple Sclerosis, Disease-modifying therapies, NEDA, treatment optimization
Introduction
In relapsing–remitting multiple sclerosis (RRMS), ‘no evidence of disease activity’ (NEDA) has been proposed as an increasingly important treatment goal.1–3 This paradigm is based on data indicating that uncontrolled disease activity in the early stages of the disease may be critical for the evolution of long-term disability.4 In line with this assumption, immunomodulatory treatments become less effective in more advanced stages of disease, where pathogenic mechanisms other than inflammation come into play.5
The increasing number of immunomodulatory and immunosuppressive treatments encompassing a wide array of different mechanisms of action allow for individualized treatment optimization in patients with suboptimal response to first-line disease-modifying therapies (DMTs). Therefore, monitoring of clinical and subclinical disease activity on DMTs has been recognized as an important component of long-term patient management.6
We report on a multicenter non-interventional retrospective study in a large cohort of RRMS patients receiving injectable DMTs in outpatient centers throughout Germany. The EPIDEM study investigated measures and ratings which were applied to assess disease activity while on DMT treatment, aiming to characterize the factors that made the treating neurologists consider to switch therapy towards potentially more effective or better-tolerated drugs.
Methods and patients
Study design
EPIDEM was a retrospective, non-interventional, open-label, multicenter study analyzing the course of RRMS patients treated with injectable DMTs for ⩾2 years with regard to relapses, magnetic resonance imaging (MRI) activity, quality of life and a number of other parameters as described below. Patients were enrolled between October 2011 and June 2014, and the database was locked in July 2014. The study protocol was approved by the Freiburg Ethics Commission International (FEKI code 011/1728).
Inclusion criteria
Adult patients with a diagnosis of RRMS according to the revised McDonald criteria7 who had been continuously treated with interferon beta or glatiramer acetate for at least 24 months before data acquisition were included in the study. All patients signed an informed consent form prior to inclusion. Due to the observational nature of the study, no further inclusion criteria were defined.
Data acquisition and analysis
Data were collected using electronic case report forms and hardcopy quality of life (MSQOL-54) questionnaires. The following items were documented: demographic data, duration of MS since diagnosis, Kurtzke Expanded Disability Status Scale (EDSS) score (current and 24 months before data acquisition), relapses in the past 24 months, MRI findings as available, neurologists’ global assessment of the disease course during the 24 months before data acquisition (based on clinical and radiological findings), type and duration of current MS medication, other previous MS therapies, and the neurologists’ consideration of changing the DMT to another drug.
Disease activity (i.e. the non-NEDA status) was defined as having one of the following: a clinical relapse, or an EDSS progression of ⩾0.5 points, or new/enlarging T2 lesions versus the previous MRI, or Gd-enhancing lesions during the observation period. In clinical trials, EDSS progression is usually defined as in increase by ⩾1.0 point. However, since there is no generally accepted uniform definition of NEDA, we intentionally chose an EDSS progression of ⩾0.5 point in order to be able to also detect incomplete recovery from relapses rather than progressive disability. Patient-reported items included quality of life measures using the MSQOL-54 questionnaire and changes in overall health status over 1 year.
SAS software was used for all data processing and analysis. Correlations between treatment decisions and the clinical course of disease were analyzed using the Chi-square test and Fisher’s exact test for homogeneity. Multivariate logistic regression analyses were performed using the SAS/STAT procedure ‘Logistic’.
Results
A total of 4215 RRMS patients were enrolled in 131 participating centers. The median patient number per center was 24 (range 1–102). Patient and disease characteristics at the time of data acquisition are shown in Table 1. The cohort was a typical RRMS population consisting of predominantly female patients with a mean age of 45 years and a mean disease duration of 9.6 years, who had been continuously treated with an injectable DMT for a median duration of 54 months. Treatment duration was below the 24 months predefined inclusion threshold in 3.9% of the patients (20–24 months).
Table 1.
Patient and disease characteristics at the time of data acquisition.
| Patient characteristics | N = 4215 |
|---|---|
| Sex, female, % | 73.1 |
| Age, mean, years (SD) | 44.8 (10.3) |
| Time since RRMS diagnosis, mean, years (SD) | 9.6 (6.6) |
| DMT ongoing for ⩾24 months, n (%) | |
| Interferon beta | 70.0 |
| Glatiramer acetate | 30.0 |
| Duration of current therapy, median, months | 54.0 (20.1–245.6) |
| EDSS score 24 months before data acquisition (n = 2316) | |
| Median, points (range) | 2.0 (0–8.5) |
| Interquartile range, points | 1.0–3.0 |
| Patients with EDSS > 6.0, n | 42 |
DMT, disease-modifying therapy; EDSS, Kurtzke Expanded Disability Status Scale; SD, standard deviation.
Course of disease in the 2 years before data acquisition
Of the total patient population, 34.0% had experienced one or more relapses while receiving DMT treatment in the previous 2 years. Most patients with relapse activity had one relapse (21.7%). 21.0% of those patients in whom MRI data were available (n = 2686), exhibited MRI activity, and the EDSS score increased by at least 0.5 points in 20.1% of the subgroup of patients in whom EDSS scores were available (n = 2316) (Table 2).
Table 2.
MS disease activity measures during the 2 years before data acquisition.
| MS activity measures | Results | ||||||
|---|---|---|---|---|---|---|---|
| Relapses | n = 4215 | ||||||
| Proportion with relapses, % of pts (n) | 34.0 (1434) | ||||||
| Number of relapses, % of pts | 0 | 1 | 2 | 3 | 4 | 5 | 6–9 |
| 66.0 | 21.7 | 7.8 | 3.0 | 1.1 | 0.3 | 0.2 | |
| EDSS | n = 2316 | ||||||
| Increase in EDSS (points), % of pts* | 0 | +⩾0.5 | +⩾1 | +⩾2 | |||
| 78.9 | 20.1 | 8.7 | 2.3 | ||||
| MRI activity | n = 2686 | ||||||
| MRI lesion activity, % of pts** | no | yes | |||||
| 74.3 | 21.0 | ||||||
data missing for 22 patients; **data missing for 124 patients.
EDSS, Kurtzke Expanded Disability Status Scale; MRI, magnetic resonance imaging; pts, patients.
Changes in EDSS correlated with the number of relapses: 34.1% of the patients with one to two relapses, 40.7% of those with three to four relapses, and 83.3% of those with five or more relapses had an increase in EDSS scores. MRI activity was reported in 34.7% of patients with relapses, and in 35.6% of the patients with an increase of EDSS.
Neurologist’s assessment of the course of disease
In patients with relapses in the previous 2 years (n = 1434), the course of disease was assessed as worse in 28.9%, stable in 67.8%, and improved in 2.6% by the treating neurologists. Assessments were given at a median of 199 days (range 0–730) after the last relapse. Stable/improved assessments decreased with an increasing number of relapses [Figure 1(a)].
Figure 1a.
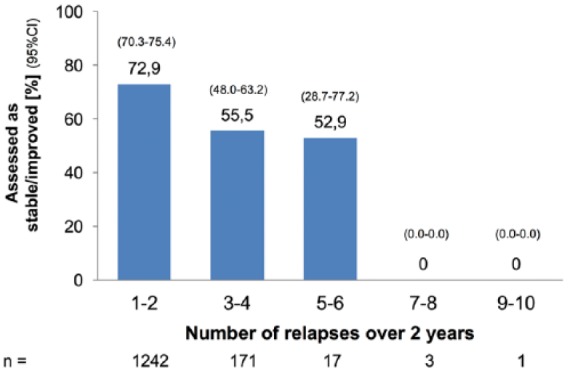
Rating of the disease course by the treating neurologist: proportion of ratings ‘stable’ or ‘improved’. (a) By number of relapses* in the past 24 months.
CI, confidence interval.
In patients with documented EDSS progression by ⩾0.5 points in the previous 2 years (n = 466; 43.3% increased by ⩾1 point, 11.6% by ⩾2 points), 48.7 % were assessed as worse, 49.1% as stable, and 0.2% as improved [Figure 1(b)].
Figure 1b.
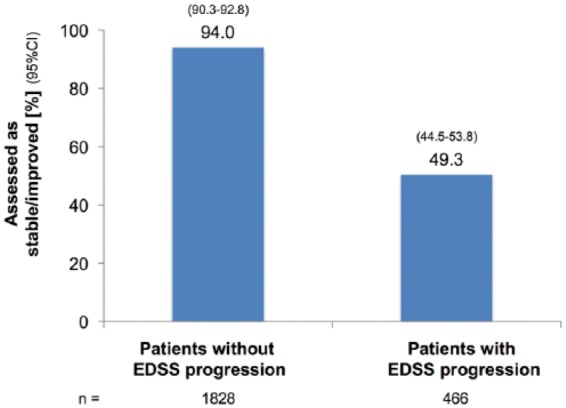
(b) By status of EDSS progression (of at least 0.5 points) in the past 24 months.
CI, confidence interval; EDSS, Kurtzke Expanded Disability Status Scale.
In patients with increased MRI activity as compared with previous MRIs (n = 565)* in the previous 2 years, 47.4% were assessed as worse, 50.1% as stable, and 1.9% as improved compared with 8.0, 87.9 and 3.7%, respectively, of those without MRI activity (n = 1997)** [Figure 1(c)]
Figure 1c.
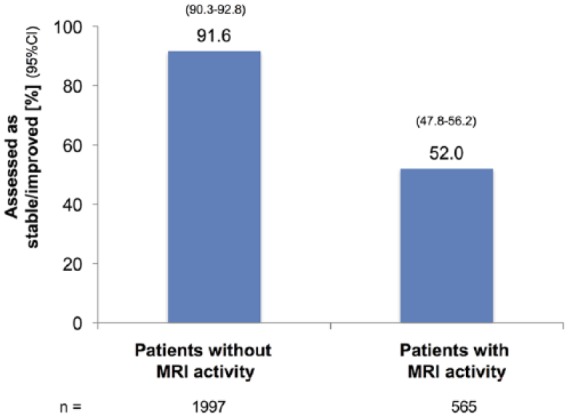
(c) By MRI activity detected in the past 24 months.
CI, confidence interval; MRI, magnetic resonance imaging.
Note: *data missing for three patients; **data missing for seven patients
Assessments of the course of disease including the treating neurologists’ considerations to switch treatment were available in 1594 patients: 810 of these showed evidence of disease activity as defined by ⩾1 relapse or an EDSS increase by ⩾0.5 points or MRI activity (Figure 2). Out of these 810 patients 31.9% were assessed as worsened, 64.8% as stable, and 2.3% as improved (Figure 3).
Figure 2.
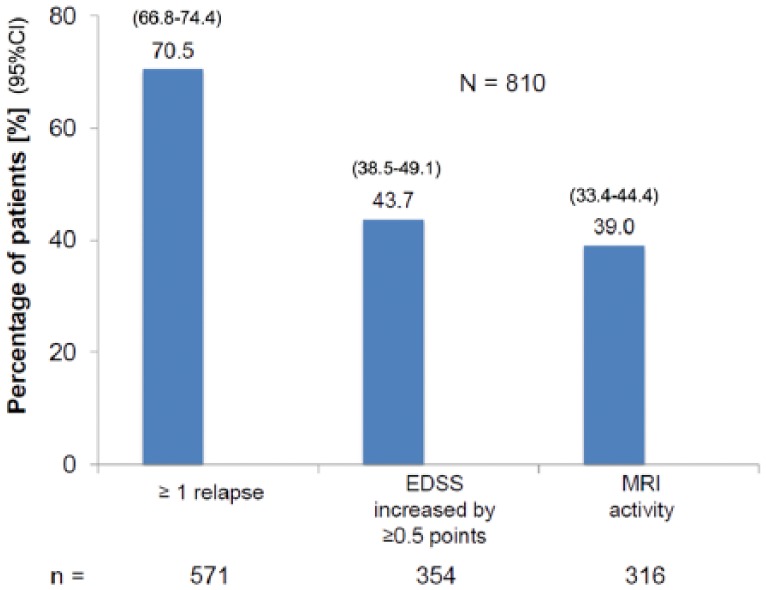
Distribution of types of disease activity in patients with any documented disease activity in the past 24 months (patients could have >1 type of disease activity).
CI, confidence interval; EDSS, Kurtzke Expanded Disability Status Scale; MRI, magnetic resonance imaging.
Figure 3.
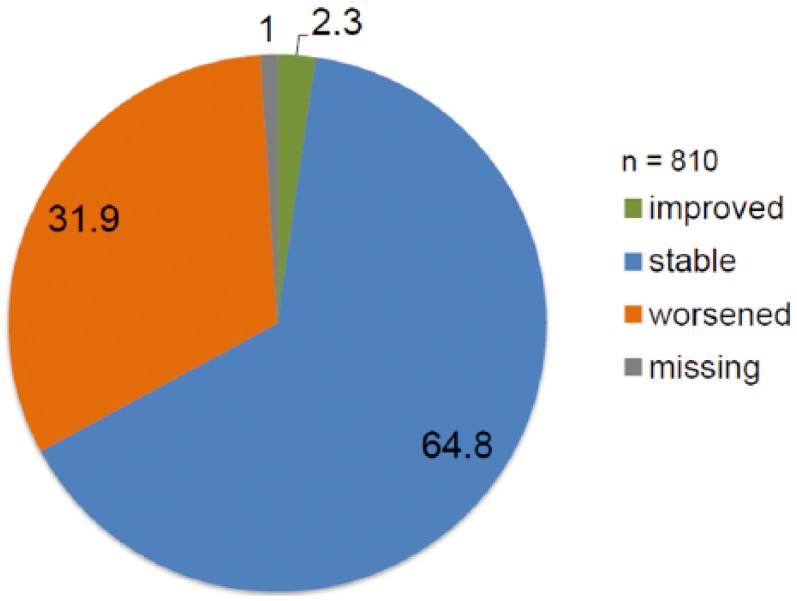
Physician’s assessment of the course of disease in patients with disease activity (any type) in the past 2 years.
Patients’ self-assessment of overall health status
The patients’ self-assessment of general health status correlated with MRI activity, EDSS deterioration and the number of relapses in the previous 2 years (data not shown). Among the patients with an increase of EDSS scores, 33% assessed their general health status as ‘less good’ or ‘poor’. Overall, 25.2% of the patients with one or two relapses and 35.4% of those with three or more relapses gave an assessment of ‘less good’ or ‘poor’. Patients’ self-assessment of the changes in overall health status corresponded to the treating neurologists’ assessment of the overall course of disease (Figure 4).
Figure 4.
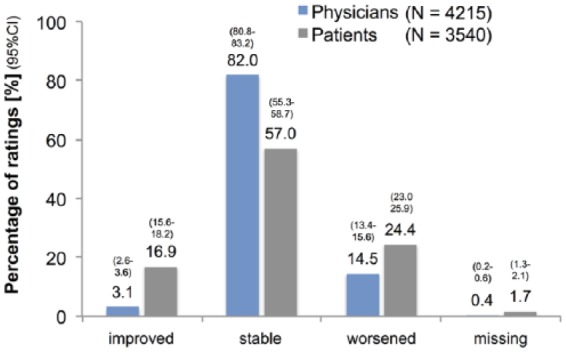
Physicians rating of disease course and patients’ rating of change in overall health status.
CI, confidence interval.
Consideration to change treatment
A change of treatment was considered in 22.8% (327/1434) of the patients with relapses in the previous 2 years including 40.1% (77/192) of patients with three or more relapses. Among the patients with an EDSS progression of at least 0.5 points in the previous 2 years (n = 466), a change of treatment was considered in 31.1% (145/466). In patients with MRI activity (565/2686), a change of treatment was considered in 37.9% (214/565). In patients in whom the treating neurologist assessed the course of disease as ‘worse’, a change of treatment was considered in 59.1% (361/610). In patients reporting quality of life (MSQOL) self-assessments as ‘less good’ or ‘poor’, a change of treatment was considered in 19.6% (155/791).
For patients in whom a change of treatment was considered, multivariate logistic regression analyses showed significant correlations with the following variables (p < 0.0001): current EDSS score (< versus > median), number of relapses (< versus > median), MRI activity in the previous 2 years (yes versus no); MSQOL PCS and MCS (< versus > median). No significant correlations were observed for duration of disease and treatment, and number of therapies.
Discussion
NEDA has been proposed as a primary objective in RRMS treatment owing to the association of long-term disability to uncontrolled disease activity in the early stages of MS. However, in the large population of RRMS patients included in EPIDEM, the majority of patients (64.8%) showing evidence of clinical or subclinical MS activity were nonetheless considered as having a stable course of disease by treating neurologists. This may in part be related to the fact that a uniform definition of the NEDA status has not been established as yet. However, as for example the majority of patients with relapses were categorized as stable, at least a subgroup of neurologists did not use highly sensitive criteria of disease activity. In this regard, some patients with ‘mild relapses’, that is relapses without functional impairment such as pure sensory symptoms, may have been considered stable despite clinical evidence of disease activity.
Consistently, only a minority of patients with evidence of clinical or subclinical disease activity were considered suitable for a change of treatment (24.7%). This result is in line with a previous retrospective analysis (TYPIC) in a large cohort of RRMS patients from German outpatient centers receiving first-line DMTs. In this survey, approximately 25% of patients showing clinical and MRI activity while receiving DMTs would have qualified for a change to a second-line treatment according to licensed indications.8
Derived from the TYPIC data which date back to data collection between 2007 and 2008 and the more recent EPIDEM data, it appears that in everyday clinical practice the current paradigm of low tolerance for disease activity and recommendations for early treatment optimization have not turned into action as yet. This finding is surprising taken into account that current guidelines and expert panels advocate a more stringent approach of early treatment optimization in patients with disease activity on treatment, that is switching treatment to drugs indicated for active disease, to minimize irreversible damage already in early stages of MS.9–11 Many patients assessed as stable in the EPIDEM study by the treating neurologist showed evidence of disease activity according to NEDA criteria.
The interpretation of the results presented here is limited due to the retrospective non-interventional design of the study that entails various potential biases (e.g. selection and recall bias, nonstandardized algorithms used for decisions on treatment change) as well as incomplete datasets, particularly with regard to MRI. The latter becomes evident when considering the fact that the proportion of patients with relapses was higher than the percentage of those with MRI activity.
Data on brain atrophy, which is increasingly discussed as a marker of disease progression, were not acquired.
Prospective studies analyzing real-life treatment patterns are required to further elucidate the reasons why treatment decisions deviate from current therapeutic recommendations in substantial proportion of MS cases.
Conclusion
The results of EPIDEM underline the importance of standardized detection, evaluation and documentation of ongoing disease activity and disability deterioration,12 that is the monitoring of treatment efficacy in patients with RRMS by algorithms which can be implemented in daily clinical practice. These tools should include patient related outcome measures and are expected to allow patients to more benefit from the growing panel of effective treatment options.
Acknowledgments
The authors would like to dedicate this work to the memory of Petra Schicklmaier who passed away in 2015. She is greatly missed.
Footnotes
Funding: This study was sponsored by Biogen Inc. who also provided funding for editorial support by Markus Fischer, Fischer BioMedical, Homburg/Saar, Germany.
Conflict of interest statement: B. Kallmann has received honoraria for serving on advisory boards and as speaker from Merck Serono, Biogen, Sanofi/Genzyme, TEVA and Novartis. J. Koehler has received honoraria for lecturing or travel expenses for attending meetings from Almirall, Bayer, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, and TEVA. He is a consultant for Allmiral, Bayer, Genzyme, Novartis and Roche. S. Schmidt has received speaking honoraria, travel compensations and fees for serving on advisory boards from BayerVital, Biogen, Merck Serono, Novartis and TEVA. P. Schicklmaier and C. Winterstein were employees of Biogen GmbH, Germany.
Contributor Information
Stephan Schmidt, Neurologische Gemeinschaftspraxis, Kölnstr. 54, 53111 Bonn, Germany.
Jürgen Koehler, Behandlungszentrum Kempfenhausen für Multiple Sklerose Kranke Gemeinnützige GmbH, Kempfenhausen, Germany.
Christine Winterstein, Biogen, Medical Department, Munich, Germany.
Petra Schicklmaier, Biogen, Medical Department, Munich, Germany.
Boris Kallmann, Multiple Sklerose Zentrum Bamberg (MSZB), Bamberg, Germany.
References
- 1. Bevan CJ, Cree BA. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol 2014; 71: 269–270. [DOI] [PubMed] [Google Scholar]
- 2. Havrdova E, Galetta S, Stefoski D, et al. Freedom from disease activity in multiple sclerosis. Neurology 2010; 74(Suppl. 3): S3–S7. [DOI] [PubMed] [Google Scholar]
- 3. Lublin F. Disease activity free status in MS. Mult Scler Relat Dis 2012; 1: 6–7. [DOI] [PubMed] [Google Scholar]
- 4. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 5. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010; 133: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gold R, Hartung HP, Stangel M, et al. ; For the participants of an expert meeting. Therapeutic goals of baseline and escalation therapy for relapsing–remitting multiple sclerosis. Akt Neurol 2012; 39: 342–350. [Google Scholar]
- 7. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mäurer M, Dachsel R, Domke S, et al. ; TYPIC Study Investigators. Health care situation of patients with relapsing–remitting multiple sclerosis receiving immunomodulatory therapy: a retrospective survey of more than 9000 German patients with MS. Eur J Neurol 2011; 18: 1036–1045. [DOI] [PubMed] [Google Scholar]
- 9. Therapeutic goals of baseline and escalation therapy for relapsing–remitting multiple sclerosis German Neurological Society (DGN). Guidelines diagnosis and therapy of multiple sclerosis. https://www.dgn.org/images/red_leitlinien/LL_2012/pdf/030-050l_S2e_Multiple_Sklerose_Diagnostik_Therapie_2014-08_verlaengert.pdf (accessed 4 January 2018).
- 10. Gold R, Hartung HP, Stangel M, et al. Therapeutic goals of baseline and escalation therapy for relapsing–remitting multiple sclerosis. Akt Neurol 2012; 39: 342–350. [Google Scholar]
- 11. Gold R, Gass A, Haupts M, et al. Therapeutic goals of baseline and escalation therapy for relapsing-remitting multiple sclerosis. Nervenheilkunde 2015; 34: 915–923. [Google Scholar]
- 12. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord 2015; 8: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


