Abstract
Gastric antral vascular ectasia (GAVE) is an uncommon but important cause of chronic gastrointestinal bleeding. It is often associated with systemic diseases such as autoimmune diseases, liver cirrhosis, chronic renal insufficiency and cardiovascular disease. The etiology of GAVE has not been fully explored and remains controversial. Diagnosis is mainly based on endoscopic presentation with flat or raised erythematous stripes radiating from the pylorus to the antrum and resembles a watermelon. Clinical presentation may range from iron-deficiency anemia secondary to occult blood loss, melena to hematemesis. In past decades, many therapeutic modalities including medical, endoscopic and surgical intervention have been introduced for GAVE treatment with variable efficacy. Herein, we review the efficacy and safety of these treatment options for GAVE.
Keywords: antrectomy, endoscopic hemostasis, gastric antral vascular ectasia, gastrointestinal bleeding
Introduction
Gastric antral vascular ectasia (GAVE), known as watermelon stomach, is characterized endoscopically by parallel red stripes, angiomatous lesions at antral mucosal folds resembling watermelon stripes.1,2 The diagnosis is usually established by specific endoscopic appearance. For uncertain cases, biopsy will offer histological clues with dilated tortuous mucosal capillaries, focal thrombosis, spindle cell proliferation and fibrohyalinosis.3,4
It is a rare acquired vascular disease, responsible for around 4% of nonvariceal upper gastrointestinal bleeding and may cause chronic blood loss with iron-deficiency anemia.5–7 Clinical presentations differ widely, from asymptomatic occult blood loss to overt gastrointestinal bleeding. Most of these patients are elderly and females number roughly twice that of males. In addition, 60–70% of patients need blood transfusion due to recurrent anemia despite iron supplements. The etiology of GAVE is still unknown. However, certain comorbidities may exist including autoimmune disease (e.g. systemic sclerosis), liver cirrhosis, chronic renal failure, and cardiovascular disease.5,6,8,9 Several mechanisms have been suspected for the development of GAVE. Altered antrum motility and dysfunction inducing chronic mucosa trauma and subsequent submucosa fibromuscular hyperplasia and dilation of mucosal capillary are the main contributing factors.3,10
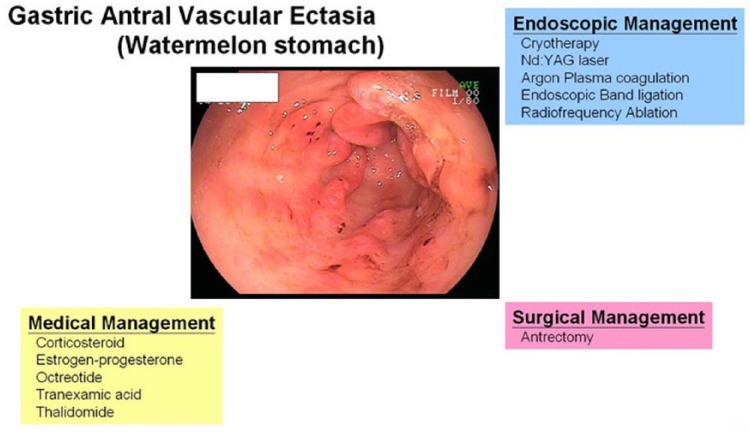
Management of GAVE-related gastrointestinal bleeding is a clinically challenging issue. In the last two decades, many therapeutic options and modalities have been applied for GAVE. The purpose of this paper is to review these therapeutic options including medical, endoscopic and surgical management for GAVE (Figure 1).
Figure 1.
Management of gastric antral vascular ectasia.
Nd: YAG, neodymium-yttrium-aluminum garnet laser.
Medical management
Variant medical treatments have been used to control GAVE bleeding; however, most of them were limited to case series and long-term effectiveness and safety are the major considerations for medical treatment.
Corticosteroid is a familiar medication for autoimmune disease. It may involve the augmentation of vascular sensitivity and improvement of integrity of vascular endothelium. A case report has described the complete resolution of GAVE in systemic sclerosis treated with pulse intravenous steroid and cyclophosphamide.11 There were also several case reports demonstrating that oral prednisolone improved blood loss and decreased blood transfusion requests in GAVE cases.12–14 However, long-term toxicity such as hyperglycemia, fluid retention and an immunocompromising effect has limited its clinical usage.
Hormone supply (estrogen–progesterone) has been used for postmenopausal women and has also been reported as successfully controlling recurrent gastrointestinal angiodysplasia bleeding and GAVE bleeding, maintaining hemoglobin concentrations in a case-series study.15–17 However, it did not modify the endoscopic appearance of GAVE and bleeding tended to recur at hormone treatment cessation.17,18 Long-term complications with gynecomastia and menorrhagia also present important clinical issues.
Octreotide, a long-acting somatostatin analogue, has many biological effects that indicate efficacy in bleeding of gastrointestinal vascular abnormalities. Nardone and colleagues reported 17 case series with gastrointestinal tract vascular abnormality bleeding and of those 3 cases were cirrhosis with watermelon stomach. Octreotide treatment achieved clinical response with halted bleeding or reduction in blood transfusion.19 However, another study showed disappointing results in a noncirrhotic patient with GAVE.20
Tranexamic acid, an antifibrinolytic agent, is widely used in clinical situations for traumatic or nontraumatic bleeding. Cases reports have shown its efficacy in reducing blood loss and transfusion requests in GAVE cases.21–23 However, the physician should be alerted to occasional severe complications such as central venous stasis retinopathy or pulmonary embolism in long-term treatment.
Thalidomide, an agent of angiogenesis inhibition, has been proven effective for treatment of gastrointestinal angiodysplasia bleeding.24 Small cases reports showed its efficiency in endoscopic refractory angiodysplasia-related gastrointestinal bleeding. It has also been reported in the successful salvage treatment for argon plasma coagulation (APC) refractory GAVE for cirrhotic patients.25,26 However, until now, most of these have been small case reports and more studies are required to evaluate the efficacy and safety in GAVE treatment.
Endoscopic management
Cryotherapy
Cryotherapy is an endoscopic technique using nitrous oxide to apply an extremely cold temperature on affected tissue and achieve hemostasis by thermal destruction or necrosis of the mucosa. Limited data of cryotherapy on GAVE are available and, as far as we know, there are only two studies evaluating cryotherapy on the management of GAVE. The pilot study was presented by Kantsevoy and colleagues27, and endoscopic cryotherapy was applied on 26 patients with mucosal vascular lesions of the gastrointestinal tract, including 7 patients with GAVE, who had a poor response to previous endoscopic therapy, including laser, thermal and electrosurgical coagulation, and had active bleeding. A total of five of the seven (71%) patients achieved cessation of bleeding after an average 3.6 sessions of cryotherapy and no recurrent bleeding or major complications were found during the 6-month follow-up period.27 The other study (in 2008) evaluated the efficacy of cryotherapy on 12 patients with GAVE, including 8 patients who had failed prior APC therapy. This study demonstrated that cryotherapy achieved a 50% complete response, defined as a significant improvement in endoscopic appearance associated with raising the hemoglobin level and no requirement for blood transfusion, and a 50% partial response, defined as incomplete ablation of GAVE on endoscopic assessment, with a stable hemoglobin level and reduced transfusions. In addition, there were no immediate or major complications after cryotherapy. It also found the mean hemoglobin level increased from 9.9 g/dl to 11.3 g/dl after cryotherapy, and large area lesions of GAVE, >70%, could be treated in an average 5-min treatment session.28
Neodymium-yttrium-aluminum garnet laser coagulation
Neodymium-yttrium-aluminum garnet (Nd: YAG) laser, a thermal device, causes tissue destruction by absorption of laser light without direct contact. It makes extensive mucosal injury, up to 4–6 mm and achieves coagulation of superficial and submucosal vessels.29 In the past, the Nd: YAG laser was widely used to treat gastrointestinal vascular ectasias and many case series reported >150 cases receiving Nd: YAG lasers for GAVE. In most case series, the Nd: YAG laser was effective both in stopping bleeding and decreasing the requirement for blood transfusions after 1–4 sessions of laser therapy.5,6,8,9,30–32 The most recent study presented in 2004 retrospectively analyzed 24 transfusion-dependent patients who were treated with Nd: YAG for GAVE over an 18-year period. After a median of two treatment sessions and a follow-up median of 55 months, 20 of 24 patients (83%) had resolution of bleeding and remained transfusion-free for a median of 16 months.33 However, most studies were retrospective case series with a small number of patients and there were no randomized control trials available for evaluating Nd: YAG lasers with other endoscopic modalities, all of which suggested insufficient evidence of the efficacy of Nd: YAG lasers for GAVE. Nd: YAG lasers have been largely replaced by other treatment modalities because of higher cost and serious complications. The reported severe complications included perforation, antral narrowing, and mortality.8,33 The development of hyperplastic polyps and multiple focal gastric neoplasia has also been reported after repeated laser therapies.31,34
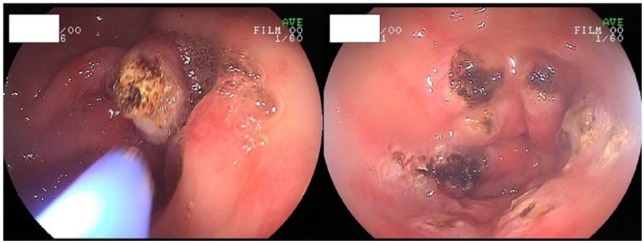
Argon plasma coagulation
APC is the other thermoablative method used as endoscopic therapy for GAVE. By using ionized argon gas (plasma) as a medium, APC produces high frequency electrical current flows to achieve tissue coagulation. APC is a noncontact thermal method and provides limited depth of mucosal injury, which reduces the risk of perforation.29 Comparing APC with Nd: YAG lasers, both methods allow for the treatment of large areas, but there are several advantages of APC in the treatment of GAVE (Figure 2). Overall, APC is easier for endoscopists to use, safer due to its favorable side-effect profile, and less expensive.35,36 Even though there is no available randomized control trial to compare Nd: YAG lasers and APC, currently, APC has been the treatment of choice and the most widely used in the treatment of GAVE. Many studies have demonstrated the efficacy of APC, including a mean increase in hemoglobin levels and a decreased need for blood transfusions.37–41 If bleeding recurs, APC was also useful to stop rebleeding.
Figure 2.
Argon plasma coagulation treatment of gastric antral vascular ectasia.
Notably, however, most studies were case series with small numbers of patients and several sessions of treatment were almost always required to achieve hemostasis. Studies investigating the long-term efficacy of APC for GAVE demonstrated unsatisfactory results and high recurrence rates of GAVE bleeding after APC treatment were reported.42–44 Chiu and colleagues had compared APC in the management of upper gastrointestinal angiodysplasia and GAVE. Patients with GAVE cases had a higher recurrence rate of 78.9% versus 7.4% in simple angiodysplasia.45 A study investigated 20 GAVE patients in whom GAVE eradication was achieved by a median of three sessions of APC and prospectively followed for a mean period of 28 months. Fuccio and colleagues showed that relapse of GAVE was found in 15% patients after a mean of 7.7 months.43 The other large case series enrolled 62 gastric vascular ectasia patients, including 31 GAVE patients, and followed these patients for a mean of 47 months. Boltin and colleagues demonstrated that only APC achieved successful treatment but in only 25.8% of patients, which was defined as absence of further gastrointestinal bleeding, coupled with a 30% increase in the hemoglobin level on follow up, and most patients did not experience long-term resolution of upper gastrointestinal bleeding and anemia.44 The recurrent bleeding following APC may be related to its limited depth of mucosal coagulation and GAVE usually involves deeper structures, including the submucosa, which could not be treated adequately by APC.46
Although APC presumably has a favorable side-effect profile due to its noncontact method and limited depth of mucosal injury, some major complications are still reported. Gastric outlet obstruction and hyperplastic polyps were reported as serious adverse events after APC treatment.43,47,48 Furthermore, Fuccio and colleagues also found hyperplastic polyps caused active bleeding in two of three patients who had hyperplastic polyps.43
Endoscopic band ligation
Endoscopic band ligation (EBL) is initially designed for esophageal varices eradication. It has also been applied in the hemostasis for angiodysplasia, Dieulafoy’s lesion and Mallory–Weiss syndrome.49,50 The first case using EBL as salvage treatment for GAVE was reported in 2006. After two sessions of EBL with a Multi-Band Ligator, hemoglobin became stable and serum ferritin normalized in 16 months of follow up.51 A subsequent retrospective study compared the efficacy of EBL with endoscopic thermal therapy for GAVE. It showed EBL had higher rates of bleeding cessation (67% versus 23%) and fewer treatment sessions (1.9 versus 4.7).52 Another two retrospective studies showed similar results with EBL superior to APC.53,54 In 2005, a prospective study enrolled 21 patients with GAVE who were treated with EBL. Clinical response was achieved in 19 patients (91%). Significant improvements in hemoglobin level and decreased blood transfusion were noted without serious complications.55
Radiofrequency ablation
The principles and rationales of radiofrequency ablation (RFA) is to apply high-energy coaptive coagulation (power density of 40 W/cm2) to destroy the superficial mucosal capillary ectasia with the subsequent regeneration of epithelium composed of a normal capillary structure. The advantages of RFA include broader coverage of the mucosa surface and the uniform distribution of energy.56 One pilot prospective study of RFA using the HALO90 ablation system as a GAVE treatment was published in 2008. A total of six patients were included and five patients were no longer dependent on blood transfusions, increasing hemoglobin levels after a median of 1.6 RFA treatment sessions.57 The two other larger studies included 21 and 24 patients, many of whom were refractory to APC and were transfusion-dependent. A clinical success rate of 80% (36/45), with increased hemoglobin levels and without further transfusions was achieved after a median of two treatment sessions. Only 1 out of 45 patients was reported to have complications of ulcer bleeding after RFA.58,59 The latest published study in 2017 included 15 patients, all of whom were refractory to APC. The clinical success with increasing hemoglobin levels and without further transfusions achieved an 80% rate after a median of two treatment sessions, and no procedure-related complications were reported.60
The definition of clinical success in the documented literature is “a transfusion-free condition without breakthrough gastrointestinal bleeding”. However, whether the RFA was able to achieve endoscopic success, meaning the disappearance of watermelon mucosa, and histological success, meaning the normal microvascular structure in the regenerated epithelium, was still not evaluated. In other words, the current evidence indicates that RFA management achieves hemostasis and results in a transfusion-free condition but the mucosal capillary ectasia may not be completely eradicated. Also, GAVE can be divided into striped-pattern and diffused-pattern based on endoscopic appearance. Whether both subtypes respond equally well to RFA is not known and remains unstudied.
At present, the data regarding RFA efficacy are promising but only limited to case series. There are no randomized controlled studies comparing the clinical success rates and complications of different treatment modalities such as band ligation, APC and RFA. Owing to the rarity of GAVE, such double-blinded and head-to-head comparison studies are difficult to conduct. Consequently, based on the current evidence, RFA yields a satisfying clinical success rate without subsequent blood transfusions. The minimal time interval of 6 weeks between each treatment session is recommended and proton pump inhibitors should be prescribed.61 RFA is a well-tolerated and feasible method for patients with GAVE with poor response to APC and band ligation.
Surgical treatments
With the advances of endoscopic techniques, surgery is now reserved as the last resort for those patients with extensive severity in whom medical and endoscopic treatments fail. Antrectomies including Billroth I, II, and Roux-en-Y reconstructions were reported as being performed in the majority of patients (89%, 40/45).62 Other surgical approaches such as partial gastrectomy, total gastrectomy and esophagogastrectomy were also reported in some case reports.14 A laparoscopic approach with the advantage of minimal invasiveness instead of conventional open gastrectomy was reported.63 This yields shorter hospitalization times and also better functional recovery. The results of surgical hemostasis were satisfying. Of the 45 patients treated with a surgical procedure, all of them were transfusion-dependent before treatment and no transfusion requirement was reported in each patient during the follow-up period of 1–48 months.62 The 30-day mortality rate after operation was 6.6% (3/45) and the causes of mortality were multiple organ failures instead of gastrointestinal bleeding.64 The postoperative complications are similar to other benign etiologies for gastric resection. Late dumping syndromes developed at a 2.4% rate and no continual diarrhea case was ever reported in a 20-year follow up.65 Nutrition deficiencies such as vitamin D, vitamin B12 and iron are concerning and consequently, dietary counseling should be carefully given to improve nutritional outcomes.
Summary
Although GAVE is uncommon in clinical practice, it is frequently characterized with chronic gastrointestinal bleeding and transfusion requests tormenting both patients and physicians. In past decades, multiple treatment modalities have been used for bleeding control (Figure 1). Overall, medical treatment does not alter the endoscopic/histologic figures of GAVE and has no definite role in the cure of GAVE-related bleeding. Small case-series reports have shown variable improvement of gastrointestinal blood loss but often patients drop out because of medication side-effects. In recent years, endoscopic therapy has become the mainstay of GAVE management with acceptable success rates and few complications (Table 1). Nd: YAG lasers, APC, and RFA are based on thermal therapy to eradicate antral vascular ectasia. EBL is a mechanical method to obliterate the submucosal vascular plexus. These modalities have shown different numbers of treatment sessions and success rates in the management of GAVE bleeding. However, most of these studies had small numbers of patients, and as retrospective studies, might provide insufficient evidence to compare each method. Despite surgical antrectomy being more invasive and having higher complications than endoscopic therapy, it is the only method to achieve a complete cure for GAVE. Hence, we should weigh the pros and cons in different treatment modalities when it comes to refractory cases.
Table 1.
Endoscopic treatment for gastric antral vascular ectasia.
| Method | Bleeding cessation ratea | Mean number of sessions | Complicationsb |
|---|---|---|---|
| Cryotherapy | 50–71% | 2–6 | Major: ~0%; minor: 0–8% |
| Nd: YAG laser | 60–100% | 1–5 | Major: 0–13%; minor: ~0% |
| Argon plasma coagulation | 30–100% | 2–6 | Major: ~0%; minor: ~0% |
| Endoscopic band ligation | 65–95% | 2–3 | Major: ~0%; minor: 8–12% |
| Radiofrequency ablation | 67–86% | 2–3 | Major: ~0%; minor: 1–2% |
Definition of bleeding cessation: rising hemoglobin level and no requirement for blood transfusion.
Major complication: death, perforation, stenosis; minor complication: gastrointestinal upset.
Nd: YAG, neodymium-yttrium-aluminum garnet.
Footnotes
Funding: This work was supported by grants from the Kaohsiung Medical University Hospital, Taiwan (KMUH102-2M02).
ORCID iD: Deng-Chyang Wu  https://orcid.org/0000-0003-3742-0634
https://orcid.org/0000-0003-3742-0634
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Wen-Hung Hsu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yao-Kuang Wang, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Internal Medicine, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung, Taiwan.
Meng-Shu Hsieh, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Fu-Chen Kuo, Shool of Medicine, College of Medicine, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan.
Meng-Chieh Wu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan.
Hsiang-Yao Shih, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
I-Chen Wu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Fang-Jung Yu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Huang-Ming Hu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yu-Chung Su, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, TaiwanDepartment of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Deng-Chyang Wu, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, 100 Tz-You 1st road, Kaohsiung 807, Taiwan.
References
- 1. Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology 1984; 87: 1165–1170. [PubMed] [Google Scholar]
- 2. Chawla SK, Ramani K, Lo Presti P. The honeycomb stomach: coalesced gastric angiodysplasia. Gastrointest Endosc 1990; 36: 516–518. [DOI] [PubMed] [Google Scholar]
- 3. Suit PF, Petras RE, Bauer TW, et al. Gastric antral vascular ectasia. A histologic and morphometric study of “the watermelon stomach”. Am J Surg Pathol 1987; 11: 750–757. [PubMed] [Google Scholar]
- 4. Gilliam JH, III, Geisinger KR, Wu WC, et al. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia. The “watermelon stomach”. Dig Dis Sci 1989; 34: 885–888. [DOI] [PubMed] [Google Scholar]
- 5. Gostout CJ, Viggiano TR, Ahlquist DA, et al. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol 1992; 15: 256–263. [DOI] [PubMed] [Google Scholar]
- 6. Liberski SM, Mcgarrity TJ, Hartle RJ, et al. The watermelon stomach: long-term outcome in patients treated with Nd:YAG laser therapy. Gastrointest Endosc 1994; 40: 584–587. [DOI] [PubMed] [Google Scholar]
- 7. Dulai GS, Jensen DM, Kovacs TO, et al. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy 2004; 36: 68–72. [DOI] [PubMed] [Google Scholar]
- 8. Sargeant IR, Loizou LA, Rampton D, et al. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut 1993; 34: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourke MJ, Hope RL, Boyd P, et al. Endoscopic laser therapy for watermelon stomach. J Gastroenterol Hepatol 1996; 11: 832–834. [DOI] [PubMed] [Google Scholar]
- 10. Quintero E, Pique JM, Bombi JA, et al. Gastric mucosal vascular ectasias causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology 1987; 93: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 11. Lorenzi AR, Johnson AH, Davies G, et al. Gastric antral vascular ectasia in systemic sclerosis: complete resolution with methylprednisolone and cyclophosphamide. Ann Rheum Dis 2001; 60: 796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rawlinson WD, Barr GD, Lin BP. Antral vascular ectasia: the “Watermelon” stomach. Med J Aust 1986; 144: 709–711. [DOI] [PubMed] [Google Scholar]
- 13. Kruger R, Ryan ME, Dickson KB, et al. Diffuse vascular ectasia of the gastric antrum. Am J Gastroenterol 1987; 82: 421–426. [PubMed] [Google Scholar]
- 14. Cales P, Voigt JJ, Payen JL, et al. Diffuse vascular ectasia of the antrum, duodenum, and jejunum in a patient with nodular regenerative hyperplasia. Lack of response to portosystemic shunt or gastrectomy. Gut 1993; 34: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet 1990; 335: 953–955. [DOI] [PubMed] [Google Scholar]
- 16. Manning RJ. Estrogen/progesterone treatment of diffuse antral vascular ectasia. Am J Gastroenterol 1995; 90: 154–156. [PubMed] [Google Scholar]
- 17. Tran A, Villeneuve JP, Bilodeau M, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol 1999; 94: 2909–2911. [DOI] [PubMed] [Google Scholar]
- 18. Moss SF, Ghosh P, Thomas DM, et al. Gastric antral vascular ectasia: maintenance treatment with oestrogen-progesterone. Gut 1992; 33: 715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nardone G, Rocco A, Balzano T, et al. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther 1999; 13: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 20. Barbara G, De Giorgio R, Salvioli B, et al. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol 1998; 26: 345–346. [DOI] [PubMed] [Google Scholar]
- 21. Park RH, Danesh BJ, Upadhyay R, et al. Gastric antral vascular ectasia (watermelon stomach): therapeutic options. Postgrad Med J 1990; 66: 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mccormick PA, Ooi H, Crosbie O. Tranexamic acid for severe bleeding gastric antral vascular ectasia in cirrhosis. Gut 1998; 42: 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan S, Vaishnavi A. Pharmacotherapy for gastric antral vascular ectasia: dramatic response to tranexamic acid. Gastrointest Endosc 2009; 70: 191; author reply 191–192. [DOI] [PubMed] [Google Scholar]
- 24. Ge ZZ, Chen HM, Gao YJ, et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology 2011; 141: 1629–1637.e1621–1624. [DOI] [PubMed] [Google Scholar]
- 25. Moser S, Tischer A, Karpi A, et al. Evidence that thalidomide is effective in recurrent bleeding from watermelon stomach associated with liver cirrhosis. Endoscopy 2014; 46(Suppl. 1 UCTN): E384. [DOI] [PubMed] [Google Scholar]
- 26. Dunne KA, Hill J, Dillon JF. Treatment of chronic transfusion-dependent gastric antral vascular ectasia (watermelon stomach) with thalidomide. Eur J Gastroenterol Hepatol 2006; 18: 455–456. [DOI] [PubMed] [Google Scholar]
- 27. Kantsevoy SV, Cruz-Correa MR, Vaughn CA, et al. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc 2003; 57: 403–406. [DOI] [PubMed] [Google Scholar]
- 28. Cho S, Zanati S, Yong E, et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc 2008; 68: 895–902. [DOI] [PubMed] [Google Scholar]
- 29. American Society for Gastrointestinal Endoscopy Technology. Mucosal ablation devices. Gastrointest Endosc 2008; 68: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 30. Potamiano S, Carter CR, Anderson JR. Endoscopic laser treatment of diffuse gastric antral vascular ectasia. Gut 1994; 35: 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geller A, Gostout CJ, Balm RK. Development of hyperplastic polyps following laser therapy for watermelon stomach. Gastrointest Endosc 1996; 43: 54–56. [DOI] [PubMed] [Google Scholar]
- 32. Calamia KT, Scolapio JS, Viggiano TR. Endoscopic YAG laser treatment of watermelon stomach (gastric antral vascular ectasia) in patients with systemic sclerosis. Clin Exp Rheumatol 2000; 18: 605–608. [PubMed] [Google Scholar]
- 33. Mathou NG, Lovat LB, Thorpe SM, et al. Nd:YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Lasers Med Sci 2004; 18: 213–218. [DOI] [PubMed] [Google Scholar]
- 34. Bernstein CN, Pettigrew N, Wang KK, et al. Multifocal gastric neoplasia after recurrent laser therapy for the watermelon stomach. Can J Gastroenterol 1997; 11: 403–406. [DOI] [PubMed] [Google Scholar]
- 35. Pavey DA, Craig PI. Endoscopic therapy for upper-Gi vascular ectasias. Gastrointest Endosc 2004; 59: 233–238. [DOI] [PubMed] [Google Scholar]
- 36. Rosenfeld G, Enns R. Argon photocoagulation in the treatment of gastric antral vascular ectasia and radiation proctitis. Can J Gastroenterol 2009; 23: 801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roman S, Saurin JC, Dumortier J, et al. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy 2003; 35: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 38. Sebastian S, Mcloughlin R, Qasim A, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis 2004; 36: 212–217. [DOI] [PubMed] [Google Scholar]
- 39. Kwan V, Bourke MJ, Williams SJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol 2006; 101: 58–63. [DOI] [PubMed] [Google Scholar]
- 40. Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc 2008; 68: 440–446. [DOI] [PubMed] [Google Scholar]
- 41. Naga M, Esmat S, Naguib M, et al. Long-term effect of argon plasma coagulation (APC) in the treatment of gastric antral vascular ectasia (GAVE). Arab J Gastroenterol 2011; 12: 40–43. [DOI] [PubMed] [Google Scholar]
- 42. Chaves DM, Sakai P, Oliveira CV, et al. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol 2006; 43: 191–195. [DOI] [PubMed] [Google Scholar]
- 43. Fuccio L, Zagari RM, Serrani M, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion 2009; 79: 143–150. [DOI] [PubMed] [Google Scholar]
- 44. Boltin D, Gingold-Belfer R, Lichtenstein L, et al. Long-term treatment outcome of patients with gastric vascular ectasia treated with argon plasma coagulation. Eur J Gastroenterol Hepatol 2014; 26: 588–593. [DOI] [PubMed] [Google Scholar]
- 45. Chiu YC, Lu LS, Wu KL, et al. Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol 2012; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patwardhan VR, Cardenas A. Review article: the management of portal hypertensive gastropathy and gastric antral vascular ectasia in cirrhosis. Aliment Pharmacol Ther 2014; 40: 354–362. [DOI] [PubMed] [Google Scholar]
- 47. Baudet JS, Salata H, Soler M, et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE). Endoscopy 2007; 39(Suppl. 1): E320. [DOI] [PubMed] [Google Scholar]
- 48. Farooq FT, Wong RC, Yang P, et al. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc 2007; 65: 1090–1092. [DOI] [PubMed] [Google Scholar]
- 49. Matsui S, Kamisako T, Kudo M, et al. Endoscopic band ligation for control of nonvariceal upper GI hemorrhage: comparison with bipolar electrocoagulation. Gastrointest Endosc 2002; 55: 214–218. [DOI] [PubMed] [Google Scholar]
- 50. Junquera F, Brullet E, Campo R, et al. Usefulness of endoscopic band ligation for bleeding small bowel vascular lesions. Gastrointest Endosc 2003; 58: 274–279. [DOI] [PubMed] [Google Scholar]
- 51. Sinha SK, Udawat HP, Varma S, et al. Watermelon stomach treated with endoscopic band ligation. Gastrointest Endosc 2006; 64: 1028–1031. [DOI] [PubMed] [Google Scholar]
- 52. Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc 2008; 68: 231–236. [DOI] [PubMed] [Google Scholar]
- 53. Keohane J, Berro W, Harewood GC, et al. Band ligation of gastric antral vascular ectasia is a safe and effective endoscopic treatment. Dig Endosc 2013; 25: 392–396. [DOI] [PubMed] [Google Scholar]
- 54. Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc 2012; 24: 237–242. [DOI] [PubMed] [Google Scholar]
- 55. Zepeda-Gomez S, Sultanian R, Teshima C, et al. Gastric antral vascular ectasia: a prospective study of treatment with endoscopic band ligation. Endoscopy 2015; 47: 538–540. [DOI] [PubMed] [Google Scholar]
- 56. Peter S, Wilcox CM. Radiofrequency ablation therapy: the grave for GAVE (gastric antral vascular ectasia)? Endosc Int Open 2015; 3: E128–E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gross SA, Al-Haddad M, Gill KR, et al. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc 2008; 67: 324–327. [DOI] [PubMed] [Google Scholar]
- 58. Mcgorisk T, Krishnan K, Keefer L, et al. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video). Gastrointest Endosc 2013; 78: 584–588. [DOI] [PubMed] [Google Scholar]
- 59. Dray X, Repici A, Gonzalez P, et al. Radiofrequency ablation for the treatment of gastric antral vascular ectasia. Endoscopy 2014; 46: 963–969. [DOI] [PubMed] [Google Scholar]
- 60. Markos P, Bilic B, Ivekovic H, et al. Radiofrequency ablation for gastric antral vascular ectasia and radiation proctitis. Indian J Gastroenterol 2017; 36: 145–148. [DOI] [PubMed] [Google Scholar]
- 61. Becq A, Camus M, Rahmi G, et al. Emerging indications of endoscopic radiofrequency ablation. United Eur Gastroenterol J 2015; 3: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Novitsky YW, Kercher KW, Czerniach DR, et al. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg 2003; 7: 652–661. [DOI] [PubMed] [Google Scholar]
- 63. Sherman V, Klassen DR, Feldman LS, et al. Laparoscopic antrectomy: a novel approach to treating watermelon stomach. J Am Coll Surg 2003; 197: 864–867. [DOI] [PubMed] [Google Scholar]
- 64. Spahr L, Villeneuve J, Dufresne M, et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut 1999; 44: 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Makharia G, Behra A, Kaman L, et al. Watermelon stomach: a rare cause of upper gastrointestinal bleeding. Indian J Gastroenterol 1999; 18: 86–87. [PubMed] [Google Scholar]