Abstract
Objective:
To explore the effect of exercise on cognition in depression as well as the impact of potential moderators and intervention type.
Method:
Controlled and uncontrolled interventional studies that described an exercise intervention and cognitive outcomes in participants with major depressive disorder (MDD) were included following a search of Pubmed, Ovid Medline, PsycInfo and Embase from inception to January 2017. Meta-analyses were conducted to calculate Hedges’ g using a random-effects model. Meta-regression explored the relationships among age, baseline cognition, frequency and duration of exercise, and cognitive outcomes. Subgroup analyses were also conducted according to type and intensity of exercise interventions.
Results:
Of 12 controlled studies and 3 uncontrolled studies that met inclusion criteria, 9 (642 patients) were included in the meta-analysis. No significant effect of exercise was found on global cognition (Hedges’ g = 0.08, P = 0.33, I2 = 0%) or on individual cognitive domains. Meta-regression analyses failed to find significant relationships among participant age, baseline cognition, number of exercise sessions per wk, duration of exercise per wk, total duration of exercise during the intervention, or improvement in global cognition. Interventions combining physical with cognitive activity significantly improved global cognition (P = 0.048), whereas low-intensity interventions were also positive (P = 0.048).
Conclusions:
No impact of physical exercise was found on cognition in MDD overall. However, we found that interventions combining physical and cognitive activities had a positive impact, and that lower-intensity interventions, where adherence was improved, also impacted positively. There remains a lack of high-quality data in this population.
Keywords: physical exercise, cognitive symptoms, major depressive disorder
Abstract
Objectif:
Explorer les effets de l’exercice sur la cognition dans la dépression ainsi que l’effet des modérateurs potentiels et du type d’intervention.
Méthode:
Les études interventionnelles contrôlées et incontrôlées chez des participants souffrant du trouble dépressif majeur (TDM) qui décrivaient une intervention d’exercice et des résultats cognitifs ont été incluses après une recherche des bases de données Pubmed, Ovid Medline, PsycInfo et Embase, du début à janvier 2017. Des méta-analyses ont été menées pour calculer le g de Hedges à l’aide d’un modèle à effets aléatoires. La méta-régression a exploré la relation entre l’âge, la cognition au départ, la fréquence et la durée de l’exercice et les résultats cognitifs. Les analyses de sous-groupe ont aussi été menées selon le type et l’intensité des interventions d’exercice.
Résultats:
Sur 12 études contrôlées et 3 études incontrôlées qui satisfaisaient aux critères d’inclusion, 9 (642 patients) étaient incluses dans la méta-analyse. Aucun effet significatif de l’exercice n’a été constaté sur la cognition générale (g de Hedges = 0,08, p = 0,33, I2 = 0 %) ou sur les domaines cognitifs individuels. Les analyses de méta-régression n’ont pas réussi à trouver de relation significative entre l’âge des participants, la cognition au départ, le nombre de séances d’exercice par semaine, la durée de l’exercice par semaine, la durée totale de l’exercice durant l’intervention et l’amélioration de la cognition générale. Les interventions combinant activité physique et cognitive amélioraient significativement la cognition générale (p = 0,048) tandis que les interventions de faible intensité étaient aussi positives (p = 0,048).
Conclusions:
Aucun effet de l’exercice physique n’a été observé sur la cognition dans le TDM en général. Cependant, nous avons constaté que les interventions combinant activité physique et cognitive avaient un effet positif tandis que les interventions de faible intensité, où l’observance était meilleure, avaient aussi un effet positif. Il manque encore des données de qualité élevée dans cette population.
Depression is typically a relapsing, remitting condition, with a chronic course in many older and younger adults.1 It is frequently complicated by cognitive impairment and, although cognitive performance may improve marginally with treatments for depression, cognitive dysfunction usually persists and is associated with increased risk of persistent depression.2,3 Cognitive dysfunction is more closely associated with long-term functional outcomes than resolution of depressive symptoms, and is also associated with resistance to antidepressant treatment.4,5 Depression in later life is associated with an approximate two-fold increased risk of dementia.6 In certain instances, depression may occur as one of the earliest symptoms of an underlying cognitive disorder but depression with first onset in early life, decades before the onset of dementia, has also been associated with increased risk.7 Increased number and severity of depressive symptoms throughout life are associated with dementia risk in a dose-dependent manner, suggesting that depression may be a true risk factor for cognitive decline.8 For these reasons, it is now recognized that cognitive dysfunction is an important therapeutic target in depression. Interventions that improve underlying cognitive dysfunction could potentially modify the longitudinal course of depression with improved functional outcomes and potentially reduced resistance to treatment.
Exercise has been used to treat depression9 and is known to have cognitive benefits in the general population, with one early pivotal study showing that adults who walk on a daily basis have reduced risk of cognitive decline.10 In a recent longitudinal analysis of community dwelling adults aged 50 y and over, we found that depressive symptoms predicted accelerated cognitive decline and that physical inactivity was an important variable mediating this association.11 It is not known, however, whether exercise improves cognitive dysfunction in the context of depression. One recent systematic review of interventional studies that focused on the impact of exercise on cognitive symptoms in depression failed to find a significant benefit.12 That review included randomized controlled trials in which depression was diagnosed by diagnostic interview only. In view of the dearth of randomized controlled data in this area, we undertook a broader systematic review of the literature, including both controlled and uncontrolled studies using either diagnostic interviews or other validated measures of depression to determine whether exercise may be considered a therapeutic intervention for cognitive dysfunction in depression. We also wished to explore in greater detail several possible moderators of treatment outcome. Specifically, we assessed whether participant characteristics, such as age and baseline cognition moderated treatment outcome in addition to intervention characteristics, such as duration and dose of exercise. There is also an emerging literature to suggest that interventions combining cognitive with physical activities may be superior to those using exercise alone.13–16 We wish to determine whether such combined interventions have a positive impact on cognitive symptoms in depression.
Methods
The review protocol was registered with PROSPERO (International database of prospectively registered systematic reviews in health and social care): CRD42017069756.
Search Strategy
We conducted a systematic review of the literature regarding the impact of exercise intervention on cognition in individuals with major depressive disorder (MDD). We searched the following databases: Pubmed, Ovid Medline (1946 to present), PsycInfo (1806 to January wk 4 2017) and Embase (1947 to 26 January 2017) from inception to January 2017 using the search terms exercise, physical activity, cognition, cognitive function, depressive disorder, and depression. We searched using the search terms [(exercise OR physical activity) AND (cognition OR cognitive function) AND (depressive disorder OR depression)]. The search was limited to papers published in the English language. Reference sections from review papers were manually searched to ensure that all relevant interventional studies were included. Detailed search strategies and results for each database are reported in the supplementary materials.
Eligibility Criteria
We included interventional studies (controlled or uncontrolled) for individuals with MDD of all ages where an exercise intervention was clearly described and its impact on cognitive function measured. Our search included all studies using a validated diagnostic tool, where depression was defined either by diagnostic interview or by using a cut-off on a validated depression scale. Exercise was defined according to the American College of Sports Medicine17 as “a type of physical activity consisting of planned, structured, and repetitive bodily movement done to improve and/or maintain one or more components of physical fitness.”
Study Selection and Data Extraction
One researcher screened all titles and abstracts and obtained full texts of potentially relevant papers. Two researchers worked independently on these full texts to determine whether they met the inclusion criteria. Disagreements between the 2 reviewers were resolved by consensus. Initial agreement between the reviewers was high (k = 0.91). The 2 researchers also extracted the data separately, and all disagreements were resolved by consensus. Information was extracted regarding: 1) the trial’s inclusion and exclusion criteria; (2) the participant characteristics; (3) the type of intervention; and (4) the cognitive outcomes. The risk of bias was assessed using the Cochrane risk of bias tool. We assessed the publication bias by evaluating a funnel plot of the standard mean differences (SMD) for asymmetry, as well as Egger’s test.
Cognitive Outcomes
The primary outcome was global cognition: Global cognition was defined as the average change in all clinically validated measures of cognitive function following the exercise intervention (or control condition).18 A “mean change score” was calculated for each cognitive domain, and the average of the changes in all cognitive domains was used for global change. When multiple tasks were presented for each cognitive domain, we calculated an average score, as previously described.19 We then determined the impact on individual cognitive domains, including: processing speed, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, reasoning, and problem solving, according to the structure of the MATRICS Consensus Cognitive Battery (MCCB).20 When a task reported in studies was not part of the MCCB, we categorized the task into its respective domain according to instructions in the original study (see supplementary material).
Analyses
We conducted further analyses in studies eligible for meta-analysis. In studies with multiple treatment groups, we deleted intervention groups without physical exercise included21,22 (such as cognitive behavior treatment and antidepressants), or combined groups where different types of exercise were treated as the physical exercise group,23 according to the recommendation of the Cochrane Handbook. We used a pre–post correlation coefficient of 0.7 to calculate standard deviation (SD) of the mean change scores with the formula [SQRT(SD2 baseline+SD2 final-(2*0.7*SDbaseline*SDfinal))] when no related information was reported in the original study.24,25 SMD was calculated as effect size for each study using the mean change scores and the SDs of both exercise and control groups. A random-effects model was applied throughout the study to account for the expected heterogeneity between studies. Heterogeneity between studies was determined using Cochrane’s Q and I2 values. The above analyses were performed in Review Manager 5.3. We then conducted meta-regression analyses to determine the relationship between continuous moderators (age, baseline cognition, number of exercise sessions per wk, exercise duration per wk, and total duration of the whole intervention) and effect-size estimates. Influence plot analysis was performed by removing each study. Subgroup analyses were conducted according to the intensity of the exercise intervention and whether the interventions consisted of combined cognitive and physical activities or physical activity alone. Different exercise intensities were defined according to American College of Sports Medicine.17 These analyses were performed by STATA 12.
Results
Search Results
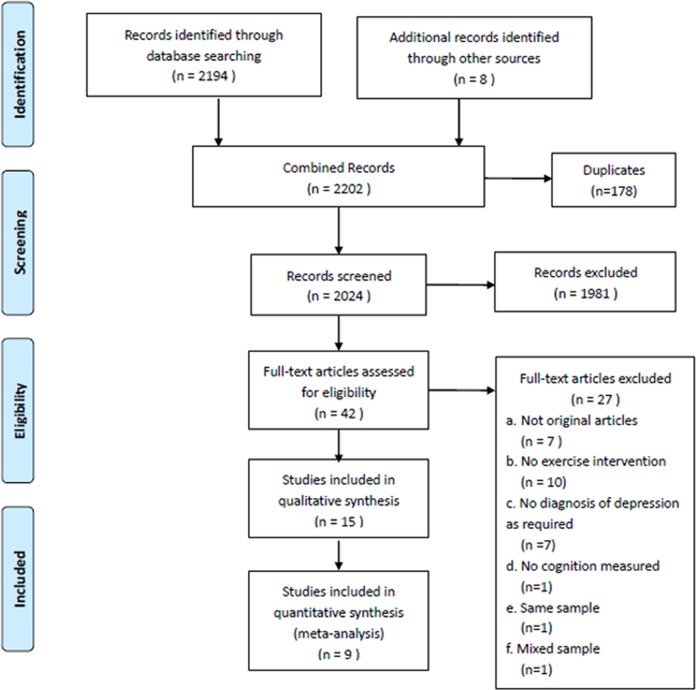
A total of 2,202 articles were identified, of which 15 were eligible for inclusion in a qualitative review. Of these 15 studies, 9 randomized controlled trials were suitable for inclusion in a quantitative meta-analysis. The full details of the search results are reported in Figure 1, and a list of the included and excluded studies is provided in Supplemental Table S1. The main characteristics of the included studies are shown in Table 1. The 9 studies in the meta-analysis included 642 study participants with depression, of whom 374 were randomized to an exercise intervention. The mean age was 46.8 y (SD, 13.8 y), and 68.2% were female (n = 438). Exercise interventions were 9.8 wk on average, with 2.1 sessions per wk (range, 1 to 3 sessions) and 131.67 min (range, 90 to 180 min) in duration per wk.
Figure 1.
PRISMA flow diagram of the selection procedure.
Table 1.
Summary of Findings.
| Author | Depression Measure | Type of Intervention | Duration | Characteristics of Groups | Cognitive Measures | Impact on Cognition | Comments (e.g. Adherence, Adverse Effect) |
|---|---|---|---|---|---|---|---|
| Controlled Studies (Exercise v. Non-exercise) | |||||||
| Chan et al. 2012 | DSM-IV | Supervised group, Dejian Mind-Body Intervention (DMBI) | 10 wk | Waiting list (N = 16, mean [SD] age: 45.44 [8.25] y, 4M/12F), no intervention; CBT group (N = 17, mean [SD] age: 46.94 [6.54] y, 4M/13F), one 90-min session/wk; DMBI (N = 17, mean [SD] age: 47.06 [9.54] y, 2M/15F), one 90-min session/wk. All remained on previous antidepressant treatment. | DVT | Significant improvement in DMBI group but no significant change in CBT group or the wait-list group. | Average attrition rate (inclusive of dropouts and participants with <70% attendance rate) of the 3 groups was 33%. |
| Heissel et al. 2015 | DSM-IV and BDI (score 13) | Supervised group, aerobic and strength exercise intervention | 4 wk | Physical exercise (N = 6, mean [SD] age: 68.7 [3.1] y, 4M/2F, drop-out = 1), two 60-min sessions/wk; Relaxation (N = 6, mean [SD] age: 64.8 [9.1] y, 3M/3F, drop-out = 1), two 60-min sessions/wk. All remained on previous antidepressant treatment. | TMT-A, TMT-B, TMT-B-A, ST-IC | No significant effects of exercise | High adherence (a completion rate of 85%) |
| Hoffman et al. 2008 | DSM-IV and HAM-D (score≥13) | Supervised group or home-based, aerobic exercise | 16 wk | Aerobic exercise (N = 104, mean [SD] age: 51.0 [7.0] y, 26M/78F), no antidepressant use, three 45-min sessions/wk; Sertraline (N = 49, mean [SD] age: 51.8 [7.7] y, 12M/37F), 50 to 200 mg of sertraline per day; Placebo (N = 49, mean [SD] age = 51.2 [7.8] y, 11M/38F), no antidepressant use. | TMT-B-A, ST-IC, R27T, DS, LM, VPA, AN, COWAT, DSF, DSB. | Exercise group had better scores compared with sertraline group on TMT B-A, R27T and DS, but was not superior to the placebo group. | Thirteen (13%) formally withdrew from the study, and another 8 (8%) remained in the study but discontinued exercise treatment among the exercise group. Seven dropouts (14%) in the sertraline condition and 14 dropouts (29%) in the placebo condition. Treatment completers attended an average of 38.8 exercise sessions. |
| Krogh et al. 2009 | ICD-10 | Supervised group, aerobic or strength exercise | 4 mo | Aerobic exercise (N = 55, mean [SD] age: 38.1 [9.0] y, 12M/43F, 4-mo drop-out = 7, 12-mo drop-out = 9), two 90-min sessions/wk; Strength exercise (N = 55, mean [SD] age: 41.9 [8.7] y, 10M/ 45F, 4-mo drop-out = 8, 12-mo drop-out =9), two 90-min sessions/wk; Relaxation training (N = 55, mean [SD] age: 36.7 [8.7] y, 21M/34F, 4-mo drop-out = 13, 12-mo drop-out =18), two 90-min sessions/wk. All remained on previous antidepressant treatment | DSFB, SS7, TMT-A, TMT-B, DS, AN, SN, BT, RCFT | No significant impact of exercise at either the 4-month or 12-month follow-up. | The mean participation was 18.0 (56.2%), 16.2 (50.6%), and 10.5 (32.8%) sessions of the 32 sessions in the strength, aerobic, and relaxation training groups, respectively. |
| Krogh et al. 2012 | DSM-IV and HAM-D17 (score 12) | Supervised group, aerobic exercise | 12 wk | Aerobic exercise (N = 56; mean [SD] age: 39.7 [11.3] y; 16M/40F), three 45-min sessions/wk; Stretch exercise (N = 59; mean [SD] age: 43.4 [11.2] y; 22M/37F), three 45-min sessions/wk. No antidepressant treatment. | BT, RCFT, DSF, DSB, ST-CC, ST-IC, DS TMT-A, TMT-B, AN, SN, SS7 | Aerobic exercise group scored significantly higher visuospatial memory compared with the stretching exercise group after the intervention. No effects were found in other cognitive domains. | 1. The mean attendance was 13.5 sessions in the aerobic exercise group v. 12.5 sessions in the stretching exercise group. 2. Adverse Events: 4 participants in the aerobic training group and 8 participants in the stretching exercise group had started antidepressant medication treatment at follow-up, and 5 patients in each group had a higher HAM-D17 rating at follow-up compared with the baseline assessment. One suicide attempt in the aerobic training group and none in the stretching exercise group. |
| Lavretsky et al. 2011 | DSM-IV and HAM-D24 (score≥16) | Supervised group, Tai Chi Chih (TCC) | 10 wk | Tai Chi (N = 36, mean [SD] age: 69.1 [7.0] y, 13M/23F, dropout = 3), one 2-hour session/wk; Health Education group (N = 37, mean [SD] age: 72.0 [7.4] y, 15M/22F, drop-out = 2), one 2-hour session/wk. All participants prescribed 10 to 20 mg of escitalopram per day | MMSE, CVLT, TMT-A, ST-CC, ST-IC | Tai Chi group yielded greater improvements in memory. | 1. Low dropout rate. Both groups did not differ by adherence to the protocol or satisfaction. 2. No serious adverse events. |
| Luttenberger et al. 2015 | A diagnosis of depression by a psychiatrist or WHO depression scale (score 13) | Supervised group, bouldering therapy | 8 wk | Climbing (N = 22, mean [SD] age: 42.71 [11.88] y, 10M/12F, 15 receiving antidepressants, drop-out = 3), one 3-hour session/wk; Waitlist (N = 25, mean [SD] age: 44.96 [12.08] y, 10M/15F, 19 receiving antidepressants, drop-out = 1), no intervention. | d2-R | No significant effects of exercise. | Dropouts had the same age and the same severity of depression. |
| Oertel-Knochel et al. 2014 | DSM-IV | Supervised group, cognitive training and cardio training | 4 wk | Physical exercise (N = 8; mean [SD] age: 36.63 [12.91] y; 4M/4F), three 45-min sessions/wk; Relaxation (N = 6, mean [SD] age: 41.37 [15.69] y, 2M/4F), three 45-min sessions/wk; Waiting list (N = 8, mean [SD] age: 42.21 [8.31] y, 5M/3F) All remained on previous antidepressant treatment. | WMS-III SS, LNS. TMT-A, AN, HVLT-R, BVMT-R. | Exercise group improved more than relaxation group in speed of processing, working memory, and verbal learning. | Low adherence (high drop-out rate). |
| Sharma et al. 2006 | DSM-IV | Supervised group, Sahaj yoga | 8 wk | Yoga (N = 15, mean [SD] age: 31.87 [8.78] y, 10M/5F), three 30-min sessions/wk; Control group (N = 15, mean [SD] age: 31.67 [8.46] y, 9M/6F), three 30-min sessions/wk. All remained on previous antidepressant treatment | LCT, TMT-A and TMT-B, DSB, DSF, RFFT | Yoga group showed more significant change in LCT and DSB. | No significant clinical side effects. |
| Controlled studies (between different types of exercise) | |||||||
| Berman et al. 2012 | DSM-IV | Supervised group, aerobic exercise | 50 to 55 min | Randomly assigned to walk in nature or urban for 50 to 55 min and assigned to walk at the alternative location one wk later (N = 20, mean age: 26 y, 8M/12F, drop-out = 1) Six participants on antidepressants. | DSB | Improvement in DSB performance after the nature walk, and a trend toward decreases after the urban walk. | Same effects were found after removing the dropout. |
| Greer et al. 2015 | DSM-IV and HRSD17 (score≥14) | Combined supervised group and home-based, aerobic exercise | 12 wk | 16 KKW (N = 19, mean [SD] age: 45.4 [10.1] y, 2M/17F), 16 kcal per kg of body weight per wk [KKW] (equivalent to walking ∼6.4 kph for 210 min per wk); 4 KKW (N = 20, mean [SD] age: 48.0 [9.3] y, 3M/17F), 4 KKW exercise dose (equivalent to walking ∼4.8 kph for 75 min per wk). All remained on previous antidepressant treatment. | BLC, RTI, DMS, PAL, PRM, SWM, SOC, IED | High-dose exercise yielded more improvements in spatial working memory. Both doses improved in attention, visual memory and executive function/spatial planning. Low-dose group decreased on the PRM percent correct in visual memory. | No distinct pattern of results for adherence. |
| Kubesch et al., 2003 | DSM-IV | Supervised group, aerobic exercise | 30 min | Randomly assigned to exercise on cycle ergometer for 30 min at either 40% or 60% of the 4-mmol/L lactic acid workload level, and assigned to exercise at the alternative workload level 2 d later (N = 24, mean [SD] age: 42.2 [12.2] y, 13M/11F). All remained on previous antidepressant treatment. | Switch Task, FT-CC, FT-IC ST-CC, ST-IC, GoNogo task | 60% workload group improved significantly higher in Stroop task and GoNogo Task. Both groups improved in Stroop task and GoNogo Task. | – |
| Uncontrolled studies (comparison is with baseline condition) | |||||||
| Alderman et al. 2016 | DSM-IV-TR | Supervised group, meditation and aerobic exercise | 8 wk | N = 22, mean [SD] age: 20.7 [3.1] y, 5M/17F, two 60-min sessions/wk. No information regarding antidepressant use. | FT-CC, FT-IC | Minimal effects on accuracy and reaction time, while N2 and P3 amplitudes increased at post-intervention. | – |
| Blackwood et al., 1998 | DSM-IV | Supervised group, aerobic exercise | About 20 min (varied between subjects) | N = 10, mean [SD] age: 38.1 [10.6] y, 3M/7F. No information regarding antidepressant use. | DSF, DSB, DS, SN, Telephone search, Lottery. | Numerically improved in median for DS. | – |
| Vasques et al. 2011 | DSM-IV | Supervised group, aerobic exercise | 30 min | N = 10, mean [SD] age: 71.5 [6.0] y. No information regarding antidepressant use. | MMSE, DSF, DSB ST-CC, ST-IC | Improvement in attention and inhibitory control, but not in working memory after intervention. | – |
Note: DSM-IV, the Diagnostic and Statistic Manual of Mental Disorders-IV; BDI, Beck’s Depression Inventory II; HAM-D, Hamilton Depression Rating Scale; ICD-10, the International Classification of Disease, Tenth Revision; DMBI, Dejian Mind-Body Intervention; CBT, cognitive-behavioral treatment; DS, Digit-Symbol Coding; ST-CC, Stroop Task – congruent condition; TMT-A, Trail Making Task part A; AN, Verbal Fluency–Animal, number of words; COWAT, Controlled Oral Word Association Test; SN, Verbal Fluency–S words, number of words; DVT, Ruff Figural Fluency Test (RFFT)Digit Vigilance Test; R27T, Ruff 2 & 7 Selective Attention Test; SS7, Subtracting Serial 7s; LCT, Letter Cancellation Test; BLC, Big Circle/Little Circle; RTI, Reaction Time; FT-CC, Flanker task–congruent condition; FT-IC, Flanker task–incongruent condition; DSF, Digit Span–Forward; DSB, Digit Span–Backwards; DSFB, Digit Span–Forward and Backward; SWM, Spatial Working Memory; SOC, Stockings of Cambridge; IED, Intradimensional/Extradimensional Shift; CVLT, Task switch California Verbal Learning Test; VPA, Verbal Paired Associates Subtest of WMS-III; LM, Logical Memory subtest of WMS-III; BT, Buschke Test; RCFT, Rey-Osterrieth Complex Figure Test; VR, Visual Reproduction Subtest of VMS-III; DMS, Delayed Matching to Sample; PAL, Paired Associates Learning; PRM, Pattern Recognition Memory; ST-IC, Stroop Task–incongruent condition; TMT-B, Trail Making Test Part B; TMT-B-A, Trail Making Test Part B-A; LSP-3, Test number three of the Leistungsprüfsystem; MMSE, Mini Mental State Examination.
Among the 3 studies comparing different exercise environments, different exercise dosages and different levels of intensity, respectively, 83 patients with depression were included. The mean age was 40.4 y (12.5 y), and 68.7% were female (n = 57). Two studies used a randomized cross-over design, and one session intervention.26,27
In the 3 uncontrolled studies, 42 patients with depression were included, and mean age was 36.9 y (SD, 21.7 y). In the 2 studies that reported the gender ratio, 75% were female.13,28 In all of the above studies, 13 studies used DSM-IV for diagnosis, whereas one study used a cut-off on a validated depression scale and the other used ICD-10.
Risk of Bias in Included Studies
Bias assessments of individual studies are reported in Supplemental Figure S1. Of these, 11 studies had adequate random sequence generation,21–23,26,29–34 whereas one did not provide sufficient information,35 and there was no adequate random sequence generation in the 3 uncontrolled studies. Only 5 studies had adequate allocation concealment,21–23,29,30 and no study could achieve the blinding of participants and personnel because of the intervention type. Blinding of outcome assessment was reported in 6 studies.21–23,29–31 Seven studies were at risk of bias from incomplete outcome data with no intention-to-treat (ITT) analyses,13,21,26,28,31,32,34 whereas selective reporting was detected in 3 studies.22,28,30 According to the Cochrane Collaboration’s tool, all of these studies should be considered as having a “high risk of bias”.
Meta-analysis & Meta-regression & Subgroup Analysis
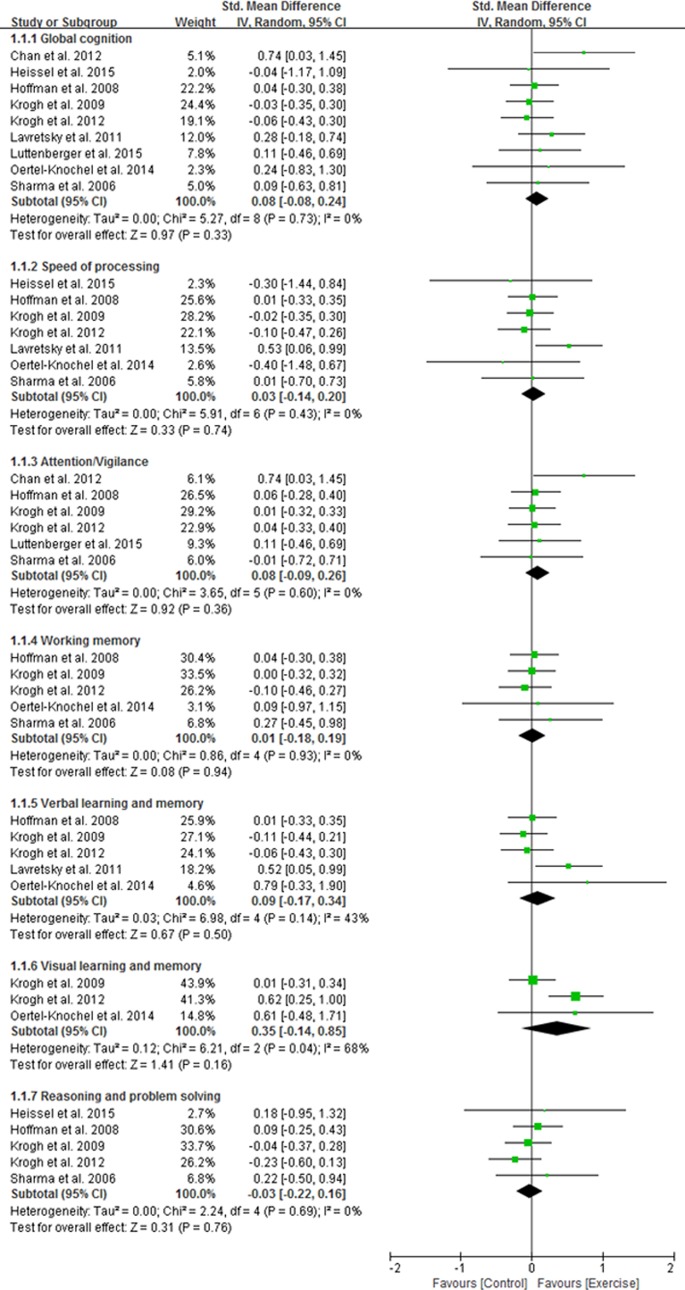
The results of meta-analysis are shown in Figure 2. No significant effect of physical exercise was found on global cognition for the study participants with depression. There was no significant effect of physical exercise in terms of speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, or reasoning and problem solving. No significant evidence of heterogeneity was observed in global cognition and among all of the individual domains, except in the visual learning and memory domain (I2 = 68%; P = 0.04).
Figure 2.
Forest plot of the effect of physical activity on cognition in depression.
Neither the funnel plot (Supplemental Figure S2) nor the Egger’s test (P = 0.188) suggested a publication bias. Removing each study from the overall effect size had no significant difference on our results (Supplemental Figure S3).
The meta-regression analyses also failed to detect a significant relationship between participant age (B = 0.0068, SE = 0.0077, t = 0.89, P = 0.401), baseline cognition (B = 0.0021, SE = 0.0012, t = 1.81, P = 0.121), sessions per wk (B = −0.1368, SE = 0.0991, t = −1.38, P = 0.210), exercise duration per wk (B = −0.0035, SE = 0.0028, t = −1.25, P = 0.253), total duration of exercise during the intervention (B = −0.0225, SE = 0.0231, t = −0.97, P = 0.364) and improvement in global cognition.
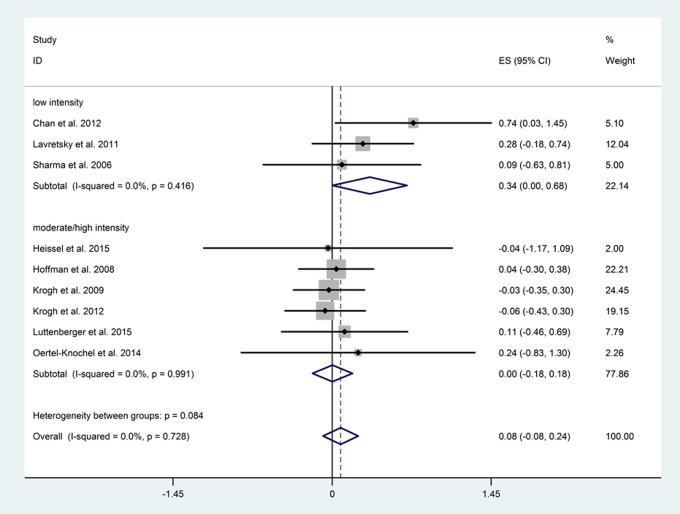
Adherence was determined according to the mean number of completed sessions for all participants, which was reported in 4 studies. Low-intensity exercise interventions had a high rate of adherence (≥70%, N = 1) and significantly improved global cognition (N = 3, n = 136, P = 0.048), whereas moderate- to high-intensity interventions generally had a lower rate of adherence (mean, 55.17%; range, 37.5% to 74.61%, N = 3) and did not impact significantly on global cognition (N = 6, n = 506, P = 0.969). No significant difference in cognition was detected between the 2 groups (P = 0.084; Figure 3).
Figure 3.
Subgroup analysis between different exercise intensity groups.
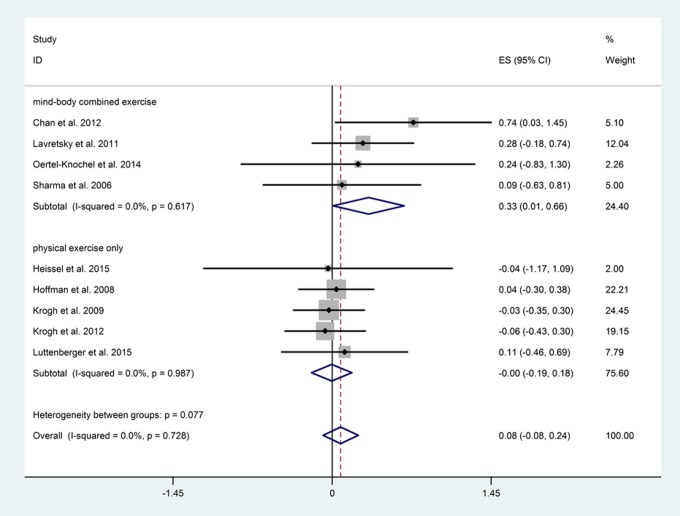
Exercise interventions that combined physical exercise with cognitive activity (including Dejian Mind-Body Intervention, Tai-chi, cognitive training and cardio training, and Sahaj yoga) significantly improved global cognition (N = 4, n = 150, P = 0.048), whereas those with physical exercise alone did not (N = 5, n = 492, P = 0.971); although the differences between the subgroups did not reach significance (P = 0.077; Figure 4).
Figure 4.
Subgroup analysis between different exercise types.
Discussion
In this meta-analysis, we failed to find a significant effect of physical exercise either on global cognition or on individual cognitive domains in study participants with depression. The impact was not influenced by the age of participants, baseline cognition, or the time spent exercising. However, interventions that combined physical exercise with cognitive activity significantly improved global cognition, and we also report positive findings for low-intensity interventions.
The lack of an overall positive impact of physical exercise on cognition in this meta-analysis is consistent with previously reported findings in this population.12 However, this contrasts with findings in other populations, including those with dementia,36 mild cognitive impairment,37 and schizophrenia.18 In some studies of healthy adults, positive findings have also been reported.38 This may reflect the overall low dosage of physical exercise in the studies included (mean duration of 131 min), which is lower than the 150 min of moderate intensity per wk recommended by the World Health Organization.39 Another variable, which may explain the generally negative findings in this meta-analysis, may be the lack of cognitive impairment at baseline in the samples included. Cognitive improvement may be greater in patients with more cognitive impairment at baseline.38 None of the studies included in this meta-analysis required the presence of cognitive impairment at baseline, which may have resulted in an underestimation of the impact of physical exercise on cognition, particularly among those with cognitive impairment. An overall high risk of bias was noted among the included studies, which may have also impacted on the findings. All 3 uncontrolled studies found a positive effect for physical exercise on either speed of processing28 or attention.13,40 However, it is not possible to determine whether this represents a true effect of the intervention or simply reflects practice effects given the lack of a control group.
In the meta-regression analyses, we found that the impact of exercise on cognition was not influenced by the characteristics of participants, such as age and cognitive function at baseline. However, the under-representation of older participants and those with cognitive impairment at baseline could have resulted in reduced power to detect the impact of these factors. Some studies have suggested that physical exercise may have a greater impact on cognition in older persons and in those with cognitive impairment at baseline.37,41 One study, published following the completion of this meta-analysis, reported a significant positive impact of progressive aerobic exercise on the cognition of older adults with depression, whereas those randomised to the non-progressive exercise group did not achieve significant cognitive benefits.42 It is noteworthy that in that study, exercise was combined with antidepressant treatment, whereas, in many of the other studies, exercise was simply added to the existing treatment plan, which may or may not have included antidepressant medication. It is not possible, therefore, to determine whether the added use of antidepressant medication significantly moderated the effect of exercise on cognition in these subjects with depression.
The impact of exercise was not associated with time spent on physical exercise per wk or with the frequency or total duration of the exercise over the whole intervention, which is consistent with the only other meta-analysis published on this topic.12 This may reflect the overall low dose of exercise in the included studies. We note that one study comparing different doses of exercise found a greater impact on cognition with higher doses of exercise, suggesting a positive relationship between dose and impact of exercise.32
Significant improvement was found in the low-intensity group, whereas no effect was found in the moderate- to high-intensity groups. This contrasts with previous findings in the general population,43 and may be explained by the reduced motivation and energy in depressed patients as well as the higher rate of attrition in studies with a longer duration of follow-up and higher intensity of exercise. We note that studies with a moderate- or high-intensity intervention generally had a lower rate of completion (mean of 55%), whereas lower-intensity interventions reported more favourable adherence (70%). One study, comparing the effects of different levels of exercise in depression, revealed greater cognitive benefits with higher levels of exercise.27 However, both workload levels in this meta-analysis could be considered low intensity according to their defined lactic acid workload.39
In subgroup analyses, we found that interventions combining physical exercise with cognitive activity—so called, mind-body interventions—significantly improved cognition. This finding is supported by a recent meta-analysis in healthy adults that found a significant benefit for interventions combining exercise with cognitive activity over exercise alone.44 However, a recent randomized, controlled trial45 in older adults with cognitive complaints failed to find significant differences between the combined effects of physical plus mental activity compared with that of physical or mental activity alone. The factorial design in that study may have resulted in reduced power to detect the combined impact on cognitive function. In this meta-analysis, yoga, tai chi, a Chan-based, mind-body intervention, and cognitive training combined with aerobic exercise were included in the combined group. The positive effects for yoga, tai chi, and cognitive training have been reported in previous studies15,16,46,47 but their impact on cognition in depressed patients remains relatively underexplored. Both cognitive training and physical exercise are known to have positive effects on several mechanisms underlying neural plasticity and cognitive functioning.48–51 The interventions may have independent or additive effects on cognition and further investigation of the underlying mechanisms and predictors of response is required.52 One study reported a greater benefit for walking in a natural compared to an urban environment.26 Previous studies have reported that environmental enrichment may have beneficial effects upon cognition; although, it was not possible to definitively address this question here.53
This systematic review has several strengths and limitations. The main limitation is lack of rigorous studies in this field, meaning that the findings reported here should be interpreted with caution. Another limitation is that different cognitive outcomes were used. This may have led to variability in estimates of the cognitive effects of exercise. However, we did not find significant evidence of heterogeneity for global cognition among the included studies. Individual cognitive domains were measured in only a small number of studies, and most studies assessed cognition as a secondary outcome or were part of larger trials. Most studies used an aerobic exercise intervention, which made it difficult to compare the relative impact of aerobic exercise v. resistance training. We conducted a series of meta-regression and subgroup analyses but note that some of these analyses may have been underpowered because of the limited number of studies. Finally, as all of these studies were conducted over a relatively short duration (range from 4 to 16 wk), it is not possible to determine the effects of exercise over a longer duration.
Conclusion
In conclusion, our meta-analysis failed to find a significant overall beneficial effect of exercise on cognitive symptoms in MDD. However, there is a relative lack of high-quality data in this population and negative findings may be attributable to methodological limitations among the included studies. In subgroup analyses, we found that interventions combining physical with cognitive activity had a positive impact upon cognition, whereas lower-intensity interventions, where adherence was improved, also had a positive impact. Future studies should determine how cognitive outcomes in depression vary according to the type of exercise intervention and the baseline characteristics of the study participants.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Authors declared that there is no conflict of interest in this systematic review. For included studies: Krogh et al (2012) reported two funders were conmmercial funders, and one co-author was an editor at PLOS ONE in their study; Authors had received grants and honoraria from several pharmaceutical companies in studies of Geer et al (2015) and Hoffman et al (2008); No declaration of conflicting interests were made in the study of Sharma et al (2006); All the other included studies declared no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Beekman AT, Geerlings SW, Deeg DJ, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59(7):605–611. [DOI] [PubMed] [Google Scholar]
- 2. Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14(5):419–427. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher D, Savva GM, Kenny R, et al. What predicts persistent depression in older adults across Europe? Utility of clinical and neuropsychological predictors from the SHARE study. J Affect Disord. 2013;147(1-3):192–197. [DOI] [PubMed] [Google Scholar]
- 4. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–527. [DOI] [PubMed] [Google Scholar]
- 5. Pimontel MA, Culang-Reinlieb ME, Morimoto SS, et al. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry. 2012;27(9):893–899. [DOI] [PubMed] [Google Scholar]
- 6. Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belvederi MM, Amore M, Menchetti M, et al. Physical exercise for late-life major depression. Br J Psychiatry. 2015;207(3):235–242. [DOI] [PubMed] [Google Scholar]
- 10. Yaffe K, Barnes D, Nevitt M, et al. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. [DOI] [PubMed] [Google Scholar]
- 11. Gallagher D, Kiss A, Lanctot K, et al. Depressive symptoms and cognitive decline: A longitudinal analysis of potentially modifiable risk factors in community dwelling older adults. J Affect Disord. 2016;190:235–240. [DOI] [PubMed] [Google Scholar]
- 12. Brondino N, Rocchetti M, Fusar-Poli L, et al. A systematic review of cognitive effects of exercise in depression. Acta Psychiatr Scand. 2017;135(4):285–295. [DOI] [PubMed] [Google Scholar]
- 13. Alderman BL, Olson RL, Brush CJ, et al. MAP training: combining meditation and aerobic exercise reduces depression and rumination while enhancing synchronized brain activity. Transl Psychiatry. 2016;6(2):e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacQueen GM, Memedovich KA. Cognitive dysfunction in major depression and bipolar disorder: assessment and treatment options. Psychiatry Clin Neurosci. 2017;71(1):18–27. [DOI] [PubMed] [Google Scholar]
- 15. Field T. Yoga research review. Complement Ther Clin Pract. 2016;24:145–161. [DOI] [PubMed] [Google Scholar]
- 16. Schitter AM, Nedeljkovic M, Ausfeld-Hafter B, et al. Changes in self-reported symptoms of depression and physical well-being in healthy individuals following a Taiji beginner course - Results of a randomized controlled trial. Brain Behav. 2016;6(4):e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American College Of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Philadelphia (PA): Wolters Kluwer Health; 2013. [DOI] [PubMed] [Google Scholar]
- 18. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. [DOI] [PubMed] [Google Scholar]
- 20. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 21. Chan AS, Wong QY, Sze SL, et al. A Chinese Chan-based mind-body intervention for patients with depression. J Affect Disord. 2012;142(1-3):283–289. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman BM, Blumenthal JA, Babyak MA, et al. Exercise fails to improve neurocognition in depressed middle-aged and older adults. Med Sci Sports Exerc. 2008;40(7):1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogh J, Saltin B, Gluud C, et al. The DEMO trial: a randomized, parallel-group, observer-blinded clinical trial of strength versus aerobic versus relaxation training for patients with mild to moderate depression. J Clin Psychiatry. 2009;70(6):790–800. [DOI] [PubMed] [Google Scholar]
- 24. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions: Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org.
- 25. Rosenthal R. Meta-analysis precedure for social research. Newbury Park (CA): SAGE Publications; 1991. [Google Scholar]
- 26. Berman MG, Kross E, Krpan KM, et al. Interacting with nature improves cognition and affect for individuals with depression. J Affect Disord. 2012;140(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubesch S, Bretschneider V, Freudenmann R, et al. Aerobic endurance exercise improves executive functions in depressed patients. J Clin Psychiatry. 2003;64(9):1005–1012. [DOI] [PubMed] [Google Scholar]
- 28. Blackwood SK, MacHale SM, Power MJ, et al. Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression. J Neurol Neurosur Ps. 1998;65(4):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krogh J, Videbech P, Thomsen C, et al. DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS One. 2012;7(10):e48316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oertel-Knochel V, Mehler P, Thiel C, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2014;264(7):589–604. [DOI] [PubMed] [Google Scholar]
- 32. Greer TL, Grannemann BD, Chansard M, et al. Dose-dependent changes in cognitive function with exercise augmentation for major depression: results from the TREAD study. Eur Neuropsychopharmacol. 2015;25(2):248–256. [DOI] [PubMed] [Google Scholar]
- 33. Sharma VK, Das S, Mondal S, et al. Effect of Sahaj Yoga on neuro-cognitive functions in patients suffering from major depression. Indian J Physiol Pharmacol. 2006;50(4):375–383. [PubMed] [Google Scholar]
- 34. Luttenberger K, Stelzer E, Först S, et al. Indoor rock climbing (bouldering) as a new treatment for depression: study design of a waitlist-controlled randomized group pilot study and the first results. BMC Psychiatry. 2015;15(2015):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heissel A, Vesterling A, White SA, et al. Feasibility of an exercise program for older depressive inpatients: a pilot study. GeroPsych. 2015;28(4):163–171. [Google Scholar]
- 36. Groot C, Hooghiemstra AM, Raijmakers PG, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25(2016):13–23. [DOI] [PubMed] [Google Scholar]
- 37. Zheng G, Xia R, Zhou W, et al. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50(23):1443–1450. [DOI] [PubMed] [Google Scholar]
- 38. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization. Recommended population levels of physical activity for health. Global recommendations on physical activity for health. Geneva (CH): WHO; 2010. [Google Scholar]
- 40. Vasques PE, Moraes H, Silveira H, et al. Acute exercise improves cognition in the depressed elderly: the effect of dual-tasks. Clinics (Sao Paulo). 2011;66(9):1553–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kelly ME, Loughrey D, Lawlor BA, et al. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31. [DOI] [PubMed] [Google Scholar]
- 42. Neviani F, Belvederi MM, Mussi C, et al. Physical exercise for late life depression: effects on cognition and disability. Int Psychogeriatr. 2017;29(7):1105–1112. [DOI] [PubMed] [Google Scholar]
- 43. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2017:1–9. doi:10.1136/bjsports-2016-096587 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44. Zhu X, Yin S, Lang M, et al. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res Rev. 2016;31:67–79. [DOI] [PubMed] [Google Scholar]
- 45. Barnes DE, Santos-Modesitt W, Poelke G, et al. The mental activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173(9):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koenig AM, Butters MA. Cognition in late life depression: treatment considerations. Curr Treat Options Psychiatry. 2014;1(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oh CU, Kim NC. Effects of T’ai Chi on Serotonin, Nicotine Dependency, Depression, and Anger in Hospitalized Alcohol-Dependent Patients. J Altern Complement Med. 2016;22(12):957–963. [DOI] [PubMed] [Google Scholar]
- 48. Penades R, Gonzalez-Rodriguez A, Catalan R, et al. Neuroimaging studies of cognitive remediation in schizophrenia: a systematic and critical review. World J Psychiatry. 2017;7(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li BY, Wang Y, Tang HD, et al. The role of cognitive activity in cognition protection: from Bedside to Bench. Transl Neurodegener. 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chirles TJ, Reiter K, Weiss LR, et al. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aguiar AJ, Castro AA, Moreira EL, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132(11-12):560–567. [DOI] [PubMed] [Google Scholar]
- 52. Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–1087. [DOI] [PubMed] [Google Scholar]
- 53. Brenes JC, Lackinger M, Hoglinger GU, et al. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2016;524(8):1586–1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.