Abstract
Background
The CLARITY and CLARITY Extension studies demonstrated that treatment of relapsing–remitting multiple sclerosis (RRMS) with cladribine tablets (CT) results in significant clinical improvements, compared with placebo. This paper presents the key magnetic resonance imaging (MRI) findings from the CLARITY Extension study.
Methods
Patients who received a cumulative dose of either CT 3.5 or 5.25 mg/kg in CLARITY were rerandomized to either placebo or CT 3.5 mg/kg in CLARITY Extension. Patients from the arm that received placebo in CLARITY were assigned to CT 3.5 mg/kg. MRI assessments were carried out when patients entered CLARITY Extension and after Weeks 24, 48, 72 and 96, and in a supplemental follow-up period.
Results
At CLARITY Extension baseline, patients who received placebo during CLARITY had more T1 gadolinium-enhanced (Gd+) lesions than patients who received CT during CLARITY. These patients, who were then exposed to cladribine 3.5 mg/kg during the extension, experienced a 90.4% relative reduction (median difference −0.33, 97.5% confidence interval −0.33–0.00; p < 0.001) in T1 Gd+ lesions at the end of the extension compared with the end of CLARITY. Overall, the majority of patients in each treatment group remained free from T1 Gd+ lesions throughout CLARITY Extension. However, a small proportion of patients who were treated with cladribine in CLARITY and received placebo in CLARITY Extension showed evidence of increased MRI activity, and this was associated with a prolonged treatment gap between CLARITY and CLARITY Extension.
Conclusion
A 2-year treatment with CT 3.5 mg/kg has a durable effect on MRI outcomes in the majority of patients, an effect that was sustained in patients who were not retreated in the subsequent 2 years after initial treatment.
ClinicalTrials.gov identifier: NCT00641537
Keywords: cladribine, extension study, magnetic resonance imaging, relapsing–remitting multiple sclerosis
Introduction
There has been considerable interest in recent years in the potential of cladribine, an adenosine analogue synthetic prodrug,1 for the treatment of patients with multiple sclerosis (MS). In the CLARITY (CLAdRIbine tablets Treating multiple sclerosis orally) [ClinicalTrials.gov identifier: NCT00213135] study, patients with relapsing–remitting multiple sclerosis (RRMS) were randomized to receive either placebo or a cumulative dose of cladribine tablets (CT) of 3.5 or 5.25 mg/kg bodyweight over 2 years.2 Both CT doses resulted in significant clinical improvements in relapse rate, disability progression, as well as in magnetic resonance imaging (MRI) outcomes, compared with placebo.2
The significant benefits observed with respect to MRI activity outcomes for CT 3.5 and 5.25 mg/kg versus placebo in CLARITY were analyzed in greater detail:3 (1) the proportion of patients with no active T1 gadolinium-enhancing (Gd+), active T2 or combined unique (CU) lesions was significantly greater for both doses of CT than placebo; (2) the lesion-free proportion was significantly greater with CT than placebo regardless of whether patients had experienced ⩽1, 2 or ⩾3 relapses in the 12 months prior to study entry.3
Following completion of CLARITY, patients were given the option to enrol in the CLARITY Extension study (ClinicalTrials.gov identifier: NCT00641537) to investigate the safety, tolerability and efficacy of two additional years of treatment with CT or placebo beyond the initial 2-year CLARITY regimen.4 There was a variable treatment gap (median duration: 40.3 weeks) between the end of CLARITY and the start of CLARITY Extension.4 This provided a prolonged follow-up period for the assessment of efficacy, safety and tolerability of treatment with CT and is a factor for consideration in the analysis and interpretation of outcomes.
MRI measurements are an important tool in MS studies and in routine monitoring, providing important insights into disease activity, predicting clinical progression and the efficacy of treatments.5,6 Brain volume loss has been associated with T1 hypointense lesion volume, T1 Gd+ lesion count and new/enlarged T2 lesion count.6 In this paper, we present the MRI findings from the CLARITY Extension study.
Methods
The design and methodology of CLARITY Extension is described elsewhere.4 The CLARITY Extension protocol complied with the Declaration of Helsinki and standards of Good Clinical Practice according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The protocol was approved by the relevant ethics committees and institutional review boards of participating centres. Written, informed consent was obtained from all participants. After completing treatment in CLARITY, eligible patients had the option of entering the 96-week (2-year) Extension, with the blind maintained. Randomization and double blinding were performed in accordance with the procedures used in CLARITY.2
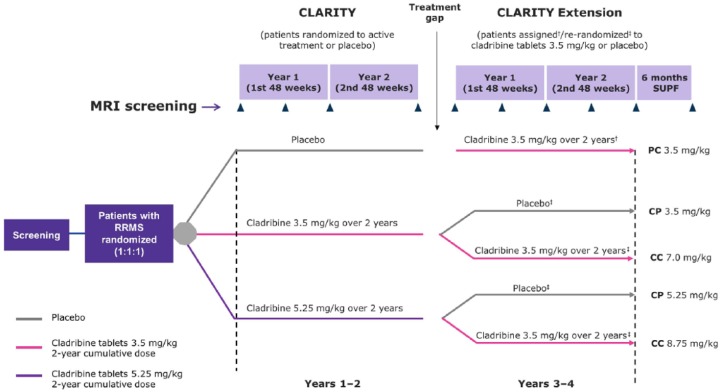
Patients treated with placebo in CLARITY were allocated to CT 3.5 mg/kg, and patients who received active treatment in CLARITY were rerandomized to receive CT 3.5 mg/kg or placebo. There were five treatment groups in CLARITY Extension (Figure 1):
Figure 1.
CLARITY and CLARITY Extension study plan.
Assessments of MRI activity were carried out when patients entered CLARITY Extension and after Weeks 24, 48, 72 and 96 of double-blind treatment, and at the start and end of a 6-month supplemental follow-up period (unless the final double-blind period assessment was within 4 weeks of the start of the supplemental follow up).
†patients who received placebo in CLARITY were assigned to treatment with cladribine 3.5 mg/kg in CLARITY Extension.
‡patients who received cladribine 3.5 mg/kg or 5.25 mg/kg in CLARITY were rerandomized to receive placebo or cladribine 3.5 mg/kg in CLARITY Extension.
PC 3.5 mg/kg, placebo in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CP 3.5 mg/kg, cladribine 3.5 mg/kg in CLARITY/placebo in CLARITY Extension; CC 7.0 mg/kg, cladribine 3.5 mg/kg in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg, cladribine 5.25 mg/kg in CLARITY/cladribine 3.5 mg/kg CLARITY Extension; CP 5.25 mg/kg, cladribine 5.25 mg/kg in CLARITY/placebo in CLARITY Extension; SUPF, supplemental follow up; MRI, magnetic resonance imaging; RRMS, relapsing–remitting multiple sclerosis.
CP 3.5 mg/kg (CT 3.5 mg/kg in CLARITY/placebo in CLARITY Extension)
CP 5.25 mg/kg (CT 5.25 mg/kg in CLARITY/placebo in CLARITY Extension)
CC 7.0 mg/kg (CT 3.5 mg/kg in CLARITY/CT 3.5 mg/kg in CLARITY Extension)
CC 8.75 mg/kg (CT 5.25 mg/kg in CLARITY/CT 3.5 mg/kg CLARITY Extension)
PC 3.5 mg/kg (placebo in CLARITY/CT 3.5 mg/kg in CLARITY Extension).
Since the CLARITY Extension study was not prespecified, an administrative gap period occurred between the end of CLARITY and the start of the CLARITY Extension (Figure 1). Patients who had already completed their last visit and had exited CLARITY were recontacted to be transitioned into the CLARITY Extension. Patients who had required and started treatment with interferon-β or glatiramer acetate during the gap period had to discontinue their disease-modifying drug (DMD) at least 3 months before the first day of the Extension. The number of patients (n = 6) who received a DMD between CLARITY and CLARITY Extension was low (treated with placebo during CLARITY, n = 4; treated with CT 3.5 mg/kg during CLARITY, n = 2). After CLARITY Extension, patients were eligible to enter a 6-month supplemental follow up during which they could receive DMD treatment if required, but not CT. MRI data from the supplemental follow up were included in these analyses.
MRI scans were performed using a standardized operating protocol (described in a dedicated users’ manual) to ensure consistency and quality. Operators were blinded to trial treatment and patients were scanned using the same machines throughout the trial. A central independent neuroradiology center (WorldCare Clinical LLC, Boston, MA, USA), also blinded to treatment, was used to assess MRI scans.
Assessments of MRI activity were carried out when patients entered CLARITY Extension and after Weeks 24, 48, 72 and 96 of double-blind treatment, and at the start and end of a 6-month supplemental follow-up period (unless the final double-blind period assessment was within 4 weeks of the start of the supplemental follow up). For missing baseline measurements, data could be used from a measurement if it occurred within 7 days of the first dose date or 4 weeks prior to study day 1. The prespecified endpoints assessed included:
Number of T1 Gd+ lesions.
Number of active T2 lesions (new or enlarged lesions).
Total T2 lesion volume.
Proportion of patients with no T1 Gd+ lesions.
Proportion of patients with no active T2 lesions.
The design of CLARITY Extension did not permit the treatment versus placebo comparisons used in CLARITY, and the prespecified analyses used a similar approach to assess clinical and neurological outcomes.4 Efficacy objectives were classified as exploratory; determinations of statistical significance should be regarded as nominal; a p value ⩽ 0.025 was considered nominally significant. Comparisons were made between patients treated with CT in CLARITY and placebo in CLARITY Extension versus patients who received cladribine in both CLARITY and CLARITY Extension:
CP 3.5 mg/kg was compared with CC 7.0 mg/kg, and CP 5.25 mg/kg was compared with CC 8.75 mg/kg
Also, CP 3.5 mg/kg was compared with PC 3.5 mg/kg.
A pooled comparison of all patients in the two CC groups against a pool of all patients in the CP groups was also conducted. A comparison of a pool of all patients in the two CC groups against a pool of all patients in the CP groups was also conducted. Post hoc analyses included investigation of the relationship between mean T1 Gd+ lesion numbers and treatment gap duration.
Results
Patients and baseline data
Full demographic and clinical details of patients included in CLARITY and CLARITY Extension have been described.2,4 Details of patients’ MRI and neurological characteristics at entry into the CLARITY and CLARITY Extension studies are shown in Table 1. Four or more postbaseline MRI scans were taken for 703 patients (87.2%; Table 2).
Table 1.
Baseline magnetic resonance imaging and neurological assessments at entry into CLARITY and CLARITY Extension.
| CP 3.5 mg/kg (n = 98) | CP 5.25 mg/kg (n = 92) | CC 7.0 mg/kg (n = 186) | CC 8.75 mg/kg (n = 186) | PC 3.5 mg/kg (n = 244) | All (n = 806) | |
|---|---|---|---|---|---|---|
| CLARITY baseline | ||||||
| T1 hypointense lesions | ||||||
| Mean (SD) | 8.09 (9.80) | 9.41 (10.34) | 7.29 (8.18) | 8.40 (9.26) | 6.74 (8.02) | 7.72 (8.88) |
| Lesion volume (103 mm3), mean (SD) | 3.99 (5.46) | 4.97 (7.71) | 3.59 (5.92) | 4.28 (5.33) | 2.81 (3.95) | 3.73 (5.50) |
| T1 Gd+ lesions | ||||||
| Mean (SD) | 1.21 (2.59) | 1.25 (2.80) | 1.00 (3.32) | 0.90 (2.09) | 0.85 (2.38) | 0.99 (2.64) |
| Lesion volume (mm3), mean (SD) | 228.43 (540.05) | 282.49 (861.99) | 189.23 (614.75) | 217.24 (520.40) | 145.61 (404.31) | 197.85 (564.87) |
| T2 lesions | ||||||
| Lesion volume (103 mm3), mean (SD) | 18.35 (17.60) | 18.32 (18.82) | 14.34 (17.05) | 16.93 (18.09) | 13.49 (12.80) | 15.62 (16.52) |
| CLARITY Extension baseline | ||||||
| T1 hypointense lesions | ||||||
| Mean n (SD) | 13.53 (13.39) | 13.72 (15.84) | 11.46 (12.66) | 12.96 (14.66) | 13.28 (14.35) | 12.87 (14.11) |
| Lesion volume (103 mm3), mean (SD) | 2.39 (3.15) | 3.50 (7.47) | 1.95 (2.68) | 2.37 (3.66) | 2.31 (3.97) | 2.39 (4.15) |
| T1 Gd+ lesions | ||||||
| Mean n (SD) | 0.27 (0.96) | 0.10 (0.49) | 0.31 (1.56) | 0.31 (1.29) | 0.77 (1.85) | 0.42 (1.47) |
| Lesion volume (mm3), mean (SD) | 18.45 (68.14) | 19.78 (108.84) | 43.46 (245.13) | 49.19 (266.98) | 132.30 (415.18) | 65.93 (293.52) |
| T2 lesions | ||||||
| Lesion volume (103 mm3), mean (SD) | 18.57 (19.05) | 16.95 (18.17) | 13.69 (14.39) | 15.76 (14.55) | 16.43 (13.81) | 15.96 (15.40) |
CP 3.5 mg/kg, cladribine 3.5 mg/kg in CLARITY/placebo in CLARITY Extension; CP 5.25 mg/kg, cladribine 5.25 mg/kg in CLARITY/placebo in CLARITY Extension; CC 7.0 mg/kg, cladribine 3.5 mg/kg in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg, cladribine 5.25 mg/kg in CLARITY/cladribine 3.5 mg/kg CLARITY Extension; PC 3.5 mg/kg, placebo in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; Gd+, gadolinium enhanced; MRI, magnetic resonance imaging; SD, standard deviation.
Table 2.
Number of magnetic resonance imaging scans for T1 gadolinium-enhanced lesion count.
| Number of MRI scans | CP 3.5 mg/kg (n = 98) | CP 5.25 mg/kg (n = 92) | CC 7.0 mg/kg (n = 186) | CC 8.75 mg/kg (n = 186) | PC 3.5 mg/kg (n = 244) | Total (n = 806) |
|---|---|---|---|---|---|---|
| Baseline scan only, n (%) | 3 (3.1) | 2 (2.2) | 8 (4.3) | 6 (3.2) | 8 (3.3) | 27 (3.3) |
| One postbaseline scan, n (%) | 4 (4.1) | 2 (2.2) | 5 (2.7) | 5 (2.7) | 4 (1.6) | 20 (2.5) |
| Two postbaseline scans, n (%) | 2 (2.0) | 6 (6.5) | 4 (2.2) | 8 (4.3) | 9 (3.7) | 29 (3.6) |
| Three postbaseline scans, n (%) | 3 (3.1) | 2 (2.2) | 11 (5.9) | 6 (3.2) | 5 (2.0) | 27 (3.3) |
| Four postbaseline scans, n (%) | 16 (16.3) | 15 (16.3) | 28 (15.1) | 28 (15.1) | 40 (16.4) | 127 (15.8) |
| Five postbaseline scans, n (%) | 34 (34.7) | 36 (39.1) | 56 (30.1) | 66 (35.5) | 92 (37.7) | 284 (35.2) |
| Six postbaseline scans, n (%) | 26 (26.5) | 29 (31.5) | 74 (39.8) | 67 (36.0) | 86 (35.2) | 292 (36.2) |
CC 7.0 mg/kg, cladribine 3.5 mg/kg in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg, cladribine 5.25 mg/kg in CLARITY/cladribine 3.5 mg/kg CLARITY Extension; CP 3.5 mg/kg, cladribine 3.5 mg/kg in CLARITY/placebo in CLARITY Extension; CP 5.25 mg/kg, cladribine 5.25 mg/kg in CLARITY/placebo in CLARITY Extension; PC 3.5 mg/kg, placebo in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; Gd+, gadolinium enhanced; MRI, magnetic resonance imaging.
When patients entered CLARITY, baseline characteristics were broadly similar across groups, although there was some variation in MRI characteristics. The observed mean number of T1 Gd+ lesions, which was 0.99 across all patients at CLARITY baseline (Figure 2), was lowest in the PC 3.5 mg/kg group, which also had the smallest volume of T1 Gd+ lesions (Table 1). The observed volume of T2 lesions and the number and volume of T1 hypointense lesions were also smallest in the PC 3.5 mg/kg group. In contrast, when patients entered CLARITY Extension, the observed mean number of T1 Gd+ lesions was higher in the PC 3.5 mg/kg group than the other groups (0.77 versus 0.10–0.31), and mean T1 Gd+ lesion volume was also greater in this group (132.30 mm3) than in the others (18.45–49.19 mm3, Table 1).
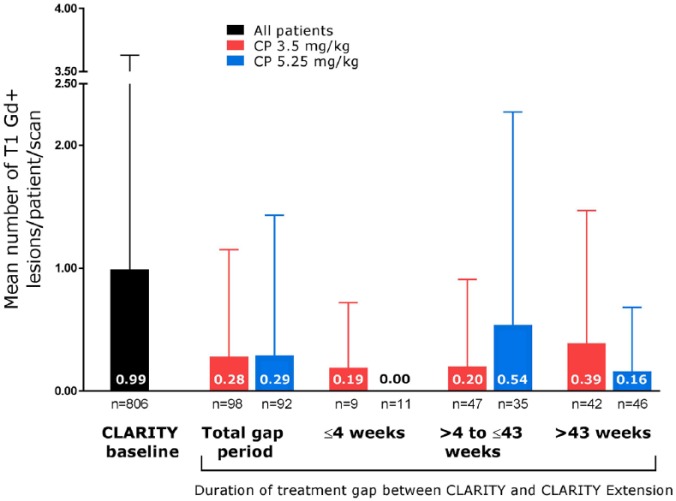
Figure 2.
Mean number of T1 gadolinium-enhanced lesions in all patients at CLARITY baseline and in the cladribine tablet 3.5 mg/kg in CLARITY/placebo in CLARITY Extension and 5.25 mg/kg groups during CLARITY Extension, according to gap duration.
Error bars indicate standard deviation.
CP, cladribine tablet in CLARITY/placebo in CLARITY Extension; Gd+, gadolinium enhanced.
Duration of gap between CLARITY and CLARITY Extension
The gap period was distributed evenly across treatment groups: the median duration was 40.3 weeks and the maximum duration was between 111.0 weeks and 118.0 weeks across the groups (range 0.1–118.0 weeks across all groups). Overall, 10.7% of patients experienced a gap duration of ⩽4 weeks; 44.8% experienced a gap >4 to ⩽43 weeks, and 44.5% had a gap >43 weeks.
T1 gadolinium-enhanced lesions
At the end of the CLARITY study, the observed mean number of T1 Gd+ lesions was lowest in the CP 3.5 and 5.25 mg/kg groups (0.07 each), followed by the CC 8.75 and 7 mg/kg groups (0.08 and 0.11, respectively) and highest in the group that received placebo in CLARITY (0.71; all 244 patients had MRI data available at the end of CLARITY). At entry to CLARITY Extension, the observed mean number of T1 Gd+ lesions in the PC 3.5 mg/kg group was still high, but then decreased following treatment with CT 3.5 mg/kg. In a longitudinal evaluation, which compared treatment groups at the end of CLARITY with the same groups of patients at the end of CLARITY Extension, a relative reduction of 90.4% [median difference −0.33 97.5% confidence interval (CI) −0.33–0.00; p < 0.001] was seen in the mean number of T1 Gd+ lesions in the PC 3.5 mg/kg group (0.07) versus the end of CLARITY (0.68; 8/244 patients did not have MRI data available both at the end of CLARITY and the end of CLARITY Extension).
Across all treatment groups, the mean number of T1 Gd+ lesions at the end of CLARITY Extension was below 0.30 (Table 3), with the highest values in the CP 3.5 and 5.25 mg/kg groups. Between-group testing showed that the mean number of T1 Gd+ lesions in CP 3.5 mg/kg was significantly higher than CC 7.0 mg/kg (treatment difference 0.00, 97.5% CI 0.00–0.00; p < 0.001), but CP 5.25 mg/kg did not reach significance compared with CC 8.75 mg/kg. The pooled group of CP 3.5 and CP 5.25 mg/kg patients also showed a significantly higher mean number of T1 Gd+ lesions compared with the pooled group of CC 7.0 and CC 8.75 mg/kg patients (treatment difference 0.00, 97.5% CI 0.00–0.00; p < 0.001).
Table 3.
Mean number of T1 gadolinium-enhanced and active T2 lesions/patient/scan and proportion of patients with no T1 gadolinium-enhanced or active T2 lesions at the end of CLARITY Extension.
| Characteristic | CP 3.5 mg/kg (n = 98) | CP 5.25 mg/kg (n = 92) | CC 7.0 mg/kg (n = 186) | CC 8.75 mg/kg (n = 186) | PC 3.5 mg/kg (n = 244) |
|---|---|---|---|---|---|
| n (missing) | 95 (3) | 90 (2) | 178 (8) | 180 (6) | 236 (8) |
| Mean number of T1 Gd+ lesions/patient/scan, n (SD) | 0.28 (0.87) | 0.29 (1.14) | 0.03 (0.08) | 0.17 (1.04) | 0.07 (0.38) |
| Patients with no T1 Gd+ lesions, n (%) | 65 (73.0) | 65 (80.2) | 144 (88.9) | 152 (89.9) | 188 (85.1) |
| Mean number of active T2 lesions/patient/scan, n (SD) | 1.42 (3.64) | 1.44 (2.40) | 0.88 (1.63) | 1.13 (2.78) | 1.07 (1.84) |
| Patients with no active T2 lesions, n (%) | 32 (34.4) | 24 (27.6) | 64 (37.6) | 76 (43.7) | 91 (40.1) |
CC 7.0 mg/kg, cladribine 3.5 mg/kg in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg, cladribine 5.25 mg/kg in CLARITY/cladribine 3.5 mg/kg CLARITY Extension; CP 3.5 mg/kg, cladribine 3.5 mg/kg in CLARITY/placebo in CLARITY Extension; CP 5.25 mg/kg, cladribine 5.25 mg/kg in CLARITY/placebo in CLARITY Extension; PC 3.5 mg/kg, placebo in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; Gd+, gadolinium enhanced; SD, standard deviation.
The cumulative mean number of T1 Gd+ lesions was higher in the CP 3.5 mg/kg group [1.33; standard deviation (SD) 4.13] than the CC 7.0 mg/kg group [0.13 (SD 0.41); relative risk 0.10, 97.5% CI 0.03–0.29; p < 0.001]. A similar pattern was observed for the CP 5.25 and the CC 8.75 mg/kg groups (data not shown). In the pooled group of CC 7.0 and CC 8.75 mg/kg patients, the cumulative mean number of lesions was lower [0.5 (SD 4.22)] than in the pooled group of CP 3.5 and CP 5.25 mg/kg patients [1.24 (SD 4.11); relative risk 0.13, 97.5% CI 0.06–0.27; p < 0.001].
Patients with no new T1 gadolinium-enhanced lesions
At the end of CLARITY Extension, the proportion of patients with no new T1 Gd+ lesions was high across all groups and ranged from 73.0% to 89.9% (Table 3). The CP 3.5 and CP 5.25 mg/kg groups had the lowest proportions of patients without any T1 Gd+ lesions. Between-group testing showed that this proportion was significantly lower in the CP 3.5 mg/kg group than the CC 7.0 mg/kg group (73.0% and 88.9%, respectively; odds ratio 3.05, 97.5% CI 1.39–6.68; p = 0.001), but not in the CP 5.25 group compared with the 8.75 mg/kg group (80.2% and 89.9%). In the pooled group of CP 3.5 and CP 5.25 mg/kg patients, the proportion who remained free from T1 Gd+ lesions (76.5%) was significantly smaller than in the pooled group of CC 7.0 and CC 8.75 mg/kg patients (89.4%; odds ratio 2.60, 97.5% CI 1.46–4.65; p < 0.001).
New T1 hypointense lesions
During CLARITY Extension, mean numbers of new T1 hypointense lesions were low (0.58–0.73 across groups), and no significant differences were observed in between-group comparisons.
Relationship between T1 gadolinium-enhanced lesions and gap duration
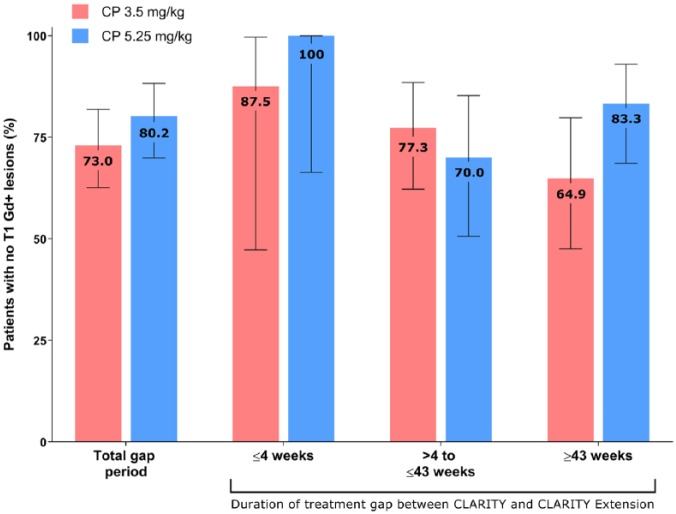
The higher observed mean T1 Gd+ counts in patients who received placebo in CLARITY Extension (CP 3.5 and CP 5.25 mg/kg) appeared to be associated with a small subgroup of patients, particularly in the CP 3.5 mg/kg group. This was analyzed by assessing the proportion of patients with a mean number of T1 Gd+ lesions ⩾ 1.0. In the CP 5.25 mg/kg group, this proportion was 6.7%, and in the CP 3.5 mg/kg group it was 11.6%. The relationship between treatment gap duration and mean T1 Gd+ lesion numbers was also assessed. Mean numbers of T1 Gd+ lesions were lowest in patients who experienced the shortest treatment gaps between CLARITY and CLARITY Extension, with a suggestion of increased MRI activity in some patients who experienced a prolonged period without active treatment. In the CP 3.5 mg/kg group, the highest mean number of T1 Gd+ lesions was seen in patients who experienced the longest treatment gap (>43 weeks, n = 42). However, this was not significantly different to the other gap durations in the CP 3.5 mg/kg group, and the mean number of T1 Gd+ lesions in this group remained <1 across all gap durations (Figure 2). Furthermore, this pattern was not observed in the CP 5.25 mg/kg group. With regard to the proportion of patients who remained free from T1 Gd+ lesions, in the CP 3.5 mg/kg group, this percentage was highest when the gap between CLARITY and CLARITY Extension was shortest and vice versa (Figure 3). Again, this pattern was not seen in the other treatment groups.
Figure 3.
Proportion of patients with no T1 gadolinium-enhanced lesions in the cladribine tablet 3.5 mg/kg in CLARITY/placebo in CLARITY Extension and 5.25 mg/kg groups during CLARITY Extension according to gap duration.
Error bars indicate 95% confidence intervals.
CP, cladribine tablet in CLARITY/placebo in CLARITY Extension; Gd+, gadolinium enhanced.
T2 lesions
At the end of CLARITY Extension, mean numbers of active T2 lesions were numerically higher in groups treated with placebo in CLARITY Extension (CP 3.5 and CP 5.25 mg/kg; Table 3). These differences reached statistical significance when comparing the pooled group of CC 7.0 and CC 8.75 mg/kg patients [1.0 (SD 2.28)] versus the pooled group of CP 3.5 and 5.25 mg/kg patients [1.43 (SD 3.09); treatment difference 0.00, 97.5% CI −0.17–0.00; p = 0.019]. The other comparisons did not reach significance (data not shown).
The proportion of patients with no active T2 lesions was higher in patients treated with CT in CLARITY Extension (CC 7.0, CC 8.75 and PC 3.5 mg/kg) than in those who received placebo during CLARITY Extension (CP 3.5 and CP 5.25 mg/kg; Table 3). The proportion of patients with no active T2 lesions was significantly lower for the CP 5.25 mg/kg group than the CC 8.75 mg/kg group comparison only (27.6 and 43.7%, respectively; odds ratio 2.03, 97.5% CI 1.07–3.85; p = 0.013).
Safety
Full safety findings have been previously published.4 Rates of adverse events (AEs) were generally similar between groups, with most AEs classified as mild or moderate. There were 89 (11.0%) treatment discontinuations due to AEs and 11 patients (1.4%) withdrew from the study due to AEs.4
Rates of lymphopenia were higher in patients treated with CT during CLARITY Extension (Grade ⩾ 3 lymphocyte counts: 40.9 and 53.2% of CC 7.0 and 8.75 mg/kg, respectively) compared with placebo recipients (5.1 and 6.5% of CP 3.5 and 5.25 mg/kg, respectively). Of those patients in the CC 7.0 mg/kg group who experienced Grade ⩾ 3 lymphocyte counts, >90% recovered to Grade 0–1 by study end.4 In the CP 3.5 mg/kg group, five patients experienced Grade ⩾ 3 lymphocyte counts and all recovered to Grade 0–1 by study end. Overall, 11 patients (1.4%) developed a malignancy or unspecified tumour. There were no reports of progressive multifocal leukoencephalopathy.4
Discussion
The clinical significance of changes in MRI activity in patients with MS has not been fully characterized, although changes in MRI activity have previously been shown to correlate with relapse and disease activity.7,8 The total number and area of T1 Gd+ lesions can significantly predict the onset of clinical worsening and increasing Expanded Disability Status Scale (EDSS) score.8 A pooled analysis of 23 randomized, double-blind, placebo-controlled trials involving 6591 patients with RRMS found a strong correlation between relapses and MRI activity, with >80% of the variance in the combined treatment effects being explained by the variance in MRI activity.7 MRI lesion activity has also been suggested to predict clinical activity and conversion to MS in patients with early MS.9–11 Reflecting this, the McDonald diagnostic criteria include MRI as part of the assessment, recognizing it as a sensitive surrogate of disease activity and clinical events.12–14
Given the context of the relationship between MRI activity and MS progression, and the fact that all patients had received cladribine by the end of CLARITY Extension, it is noteworthy that MRI activity was, overall, low during CLARITY Extension. The mean number of T1 Gd+ lesions was low across all treatment groups, and although the mean values were higher in the two CP groups relative to the two CC groups, the values in each of these groups were considerably below those observed at baseline in the CLARITY study. This highlights another important aspect of these findings, that there was no evidence of rebound disease in the groups that received placebo during CLARITY Extension.
There were some increases in lesion volume and fewer patients remained free from new lesions in patients who switched from CT in CLARITY to placebo in CLARITY Extension, particularly the CP 3.5 mg/kg group, compared with patients who received CT in both studies. Compared with patients randomized to placebo in the CLARITY study, those treated with CT had a considerably lower mean number and smaller volume of T1 Gd+ lesions at CLARITY Extension baseline (Table 1). Furthermore, patients who received placebo in CLARITY followed by CT 3.5 mg/kg in CLARITY Extension went on to experience a relative reduction in the mean number of T1 Gd+ lesions of 90.4% (median difference −0.33, 97.5% CI −0.33–0.00; p < 0.001) compared with the end of CLARITY. This observation provides further support for the findings of the original CLARITY study.2
Patients in both groups that were switched from CT in CLARITY to placebo in CLARITY Extension (CP 3.5 and 5.25 mg/kg) showed a relatively small increase in mean numbers of T1 Gd+ or active T2 lesions at the end of the study compared with the groups that continued to receive treatment with CT (CC 7.0 and 8.75 mg/kg). This increase in MRI activity was nominally significant for the CP 3.5 mg comparison, but not the 5.25 mg/kg group, possibly reflecting an effect of the dosage administered during CLARITY, although the study was not powered to compare treatment effects between groups in this way. Despite these minor increases, the mean numbers of T1 Gd+ lesions in the CP 3.5 and 5.25 mg/kg groups remained low, and below the values observed at CLARITY baseline. Furthermore, a high proportion of patients remained free from T1 Gd+ lesions in the CP 3.5 and 5.25 mg/kg groups during CLARITY Extension (CP 3.5 and 5.25 mg/kg, 73.0 and 80.2%, respectively). This indicates that in the majority of patients, 2 years of treatment with CT in CLARITY resulted in a sustained absence of MRI activity. The uptick in the proportion of patients with an increased number of T1 Gd+ lesions in the CP 3.5 and 5.25 mg/kg groups may have been seen in patients who experienced a prolonged treatment gap between CLARITY and CLARITY Extension. This suggests that CT has a durable impact that prevents or slows MRI activity in patients with RRMS.
Patients with a longer treatment gap between CLARITY and CLARITY Extension were more likely to experience MRI activity than patients with a shorter treatment gap. Post hoc analyses indicated that patients in the CP 3.5 mg/kg group who had experienced prolonged treatment gaps between CLARITY and CLARITY Extension were more likely to experience T1 Gd+ lesions than patients who had experienced shorter gaps between CLARITY and CLARITY Extension. The patients who experienced prolonged treatment gaps finished CLARITY Extension between 5.5 years and 6.5 years after entering CLARITY. Therefore, patients receiving placebo in CLARITY Extension received the last dose of CT at the start of year 2 in CLARITY, approximately 4.5–5.5 years before the end of CLARITY Extension. These circumstances may explain some of the 11.6% of patients who had a mean number of T1 Gd+ lesions ⩾ 1.0 in the CP 3.5 mg/kg group.
The patterns of observed low-level MRI activity did not translate into a detectable clinical activity within the study period. The safety and efficacy outcomes from CLARITY Extension have been described in a separate publication.4 The efficacy outcomes showed that there were no significant differences in the annualized relapse rates or EDSS scores of patients who received CT in CLARITY and placebo in CLARITY Extension compared with patients who received CT in both phases of the study.4 Given the previous observations of a relationship between MRI activity and disease progression, it is possible that the minor differences observed in MRI activity during CLARITY Extension may ultimately translate into clinical differences over much longer timescales (i.e. spanning decades). Further studies to provide insights into the long-term efficacy of treatment with CT are needed, including assessment of the effects of disease activity detectable by MRI measurements. Such studies will help to determine whether some patients may benefit from new treatment with CT after a prolonged period without active treatment, such as 4–5 years.
A limitation of the study is that the baseline characteristics of patients at the start of CLARITY Extension may not match the characteristics of patients in the original CLARITY study. This may have resulted in some selection bias during CLARITY Extension. In addition, the variable treatment gap was not preplanned and must be considered when interpreting the findings of this study. The design of CLARITY Extension was not finalized until after the CLARITY study had started. Delays also occurred as a result of administrative issues related to obtaining the necessary approvals from the study centres. Although not prespecified, the gap does substantially increase the overall length of follow up, thus providing insights into the long-term effects of treatment with CT on safety and efficacy. Other limitations that affect these results include inequalities in the distribution of patient numbers in the treatment arms, which is expected because patients receiving CT in CLARITY were rerandomized to CT 3.5 mg/kg or placebo in a 2:1 ratio in CLARITY Extension.
In summary, the findings from CLARITY Extension suggest that treatment with CT 3.5 mg/kg has a durable effect on MRI outcomes. The mean numbers of T1 Gd+ lesions remained well below CLARITY baseline across all treatment groups, including the CP groups, which had a higher mean number of T1 Gd+ lesions than the CC groups. Furthermore, the majority of patients in each treatment group remained free from T1 Gd+ lesions, even without retreatment after 2 years.
Acknowledgments
Medical writing assistance was provided by Phil Jones and Ash Dunne of inScience Communi-cations, Chester, UK, and supported by Merck KGaA, Darmstadt, Germany.
Footnotes
Funding: This study was sponsored by EMD Serono, Inc., a business of Merck KGaA, Darmstadt, Germany (in the USA), and Merck Serono SA, Geneva, an affiliate of Merck KGaA Darmstadt, Germany (ROW).
Conflict of interest statement: GC has received consulting fees from Novartis, Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck, Receptos, Biogen Idec, Genentech-Roche, and Bayer Schering; lecture fees from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi-Aventis, Merck, Biogen Dompè, Bayer Schering, and Serono Symposia International Foundation; and trial grant support from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi-Aventis, Receptos, Biogen Idec, Genentech-Roche, Merck, Biogen Dompè, and Bayer Schering.
SC has received honoraria for lectures/consultations from Merck, Bayer HealthCare, Sanofi-Aventis, Neurology Reviews, Biogen Idec, Teva Pharmaceuticals and Actinobac Biomed Inc.; has served on advisory boards for Bayer HealthCare, Merck, Actinobac Biomed, Teva Pharmaceuticals, and Biogen Idec; and received grant support from Bayer HealthCare.
KR has received honoraria for lectures and steering committee meetings from EMD Serono, Biogen Idec, Sanofi-Aventis, Genzyme, Novartis, Teva Neurosciences, Acorda and Roche/Genentech.
PS-S has served on advisory boards for Biogen, Merck, Novartis, Teva, MedDay Pharmaceuticals, and GSK; on steering committees or independent data monitoring boards in trials sponsored by Merck, Teva, GSK, and Novartis; has received speaker honoraria from Biogen Idec, Merck, Teva, Sanofi-Aventis, Genzyme, and Novartis. His department has received research support from Biogen, Merck, Teva, Novartis, Roche, and Genzyme.
PV has received honoraria or consulting fees from Biogen, Sanofi-Genzyme, Bayer, Novartis, Merck, Celgen, Roche and Almirall; and research support from Biogen, Sanofi-Genzyme, Bayer, and Merck.
AA and FD are employees of EMD Serono, Inc., a business of Merck KGaA, Darmstadt, Germany.
GG has received speaker honoraria and consulting fees from Abbvie, Atara Bio, Almirall, Bayer Schering Pharma, Biogen Idec FivePrime, GlaxoSmithKline, GW Pharma, Merck, Pfizer Inc., Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd., Sanofi-Genzyme, UCB, Vertex Pharmaceuticals, Ironwood, and Novartis; and has received research support unrelated to this study from Biogen Idec, Merck, Novartis and Ironwood.
Contributor Information
Giancarlo Comi, Department of Neurology and Institute of Experimental Neurology, Università Vita-Salute San Raffaele, Ospedale San Raffaele, Milan 20132, Italy.
Stuart Cook, Rutgers, The State University of New Jersey, New Jersey Medical School, Newark, NJ, USA.
Kottil Rammohan, Depatment of Neurology, University of Miami School of Medicine, Miami, FL, USA.
Per Soelberg Sorensen, Department of Neurology, University of Copenhagen, Copenhagen, Denmark.
Patrick Vermersch, University of Lille, Lille, France.
Abidemi K. Adeniji, EMD Serono, Inc., Billerica, MA, USA
Fernando Dangond, EMD Serono, Inc., Billerica, MA, USA.
Gavin Giovannoni, Queen Mary University of London, Barts and The London School of Medicine and Dentistry, London, UK.
References
- 1. Beutler E. Cladribine (2-chlorodeoxyadenosine). Lancet (London, England) 1992; 340: 952–956. [DOI] [PubMed] [Google Scholar]
- 2. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 3. Comi G, Cook SD, Giovannoni G, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol 2013; 260: 1136–1146. [DOI] [PubMed] [Google Scholar]
- 4. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. Epub ahead of print 1 August 2017. DOI: 10.1177/1352458517727603. [DOI] [PubMed] [Google Scholar]
- 5. Simon JH. MRI outcomes in the diagnosis and disease course of multiple sclerosis. Handb Clin Neurol 2014; 122: 405–425. [DOI] [PubMed] [Google Scholar]
- 6. Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sormani MP, Bonzano L, Roccatagliata L, et al. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 2009; 65: 268–275. [DOI] [PubMed] [Google Scholar]
- 8. Smith ME, Stone LA, Albert PS, et al. Clinical worsening in multiple sclerosis is associated with increased frequency and area of gadopentetate dimeglumine-enhancing magnetic resonance imaging lesions. Ann Neurol 1993; 33: 480–489. [DOI] [PubMed] [Google Scholar]
- 9. Freedman MS, Comi G, De Stefano N, et al. Moving toward earlier treatment of multiple sclerosis: findings from a decade of clinical trials and implications for clinical practice. Mult Scler Relat Disord 2014; 3: 147–155. [DOI] [PubMed] [Google Scholar]
- 10. Moraal B, Pohl C, Uitdehaag BM, et al. Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Arch Neurol 2009; 66: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 11. Barkhof F, Rocca M, Francis G, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta1a. Ann Neurol 2003; 53: 718–724. [DOI] [PubMed] [Google Scholar]
- 12. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 13. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 14. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]