Short abstract
Cardiac contractile function is adenosine-5'-triphosphate (ATP)-intensive, and the myocardium’s high demand for oxygen and energy substrates leaves it acutely vulnerable to interruptions in its blood supply. The myriad cardioprotective properties of the natural intermediary metabolite pyruvate make it a potentially powerful intervention against the complex injury cascade ignited by myocardial ischemia–reperfusion. A readily oxidized metabolic substrate, pyruvate augments myocardial free energy of ATP hydrolysis to a greater extent than the physiological fuels glucose, lactate and fatty acids, particularly when it is provided at supra-physiological plasma concentrations. Pyruvate also exerts antioxidant effects by detoxifying reactive oxygen and nitrogen intermediates, and by increasing nicotinamide adenine dinucleotide phosphate reduced form (NADPH) production to maintain glutathione redox state. These enhancements of free energy and antioxidant defenses combine to augment sarcoplasmic reticular Ca2+ release and re-uptake central to cardiac mechanical performance and to restore β-adrenergic signaling of ischemically stunned myocardium. By minimizing Ca2+ mismanagement and oxidative stress, pyruvate suppresses inflammation in post-ischemic myocardium. Thus, pyruvate administration stabilized cardiac performance, augmented free energy of ATP hydrolysis and glutathione redox systems, and/or quelled inflammation in a porcine model of cardiopulmonary bypass, a canine model of cardiac arrest–resuscitation, and a caprine model of hypovolemia and hindlimb ischemia–reperfusion. Pyruvate’s myriad benefits in preclinical models provide the mechanistic framework for its clinical application as metabolic support for myocardium at risk. Phase one trials have demonstrated pyruvate’s safety and efficacy for intravenous resuscitation for septic shock, intracoronary infusion for heart failure and as a component of cardioplegia for cardiopulmonary bypass. The favorable outcomes of these trials, which argue for expanded, phase three investigations of pyruvate therapy, mirror findings in isolated, perfused hearts, underscoring the pivotal role of preclinical research in identifying clinical interventions for cardiovascular diseases.
Impact statement
This article reviews pyruvate’s cardioprotective properties as an energy-yielding metabolic fuel, antioxidant and anti-inflammatory agent in mammalian myocardium. Preclinical research has shown these properties make pyruvate a powerful intervention to curb the complex injury cascade ignited by ischemia and reperfusion. In ischemically stunned isolated hearts and in large mammal models of cardiopulmonary bypass, cardiac arrest–resuscitation and hypovolemia, intracoronary pyruvate supports recovery of myocardial contractile function, intracellular Ca2+ homeostasis and free energy of ATP hydrolysis, and its antioxidant actions restore β-adrenergic signaling and suppress inflammation. The first clinical trials of pyruvate for cardiopulmonary bypass, fluid resuscitation and intracoronary intervention for congestive heart failure have been reported. Receiver operating characteristic analyses show remarkable concordance between pyruvate’s beneficial functional and metabolic effects in isolated, perfused hearts and in patients recovering from cardiopulmonary bypass in which they received pyruvate- vs. L-lactate-fortified cardioplegia. This research exemplifies the translation of mechanism-oriented preclinical studies to clinical application and outcomes.
Keywords: Anaplerosis, cardiopulmonary bypass, erythropoietin, Gibbs free energy, glutathione, reactive oxygen species, receiver operating characteristic, sarcoplasmic reticulum
Introduction
Pyruvate (2-oxopropanoate) is a natural aliphatic carbohydrate and intermediary metabolite generated in cytosol by glycolysis or lactate oxidation. At serum concentrations of approximately 0.1 mM,1,2 pyruvate is far less abundant than fatty acids, glucose and lactate, the principal blood-borne myocardial fuels, yet it is the immediate source of at least 10% of the acetyl CoA oxidized in the Krebs cycle.2 Pyruvate is freely soluble in aqueous solution, and its circulating concentrations are readily augmented by infusion of highly concentrated pyruvate solutions. The research summarized below has established that serum pyruvate concentrations approximating 3–6 mM exert energy-yielding, antioxidant and anti-inflammatory actions that collectively augment cardiac mechanical performance and protect the myocardium from ischemic injury. Pyruvate’s functional impact is especially robust in myocardium that has been reversibly injured, i.e. stunned, by ischemia and reperfusion or by oxyradical exposure.
Established cellular and molecular effects of pyruvate in mammalian myocardium
Pyruvate’s enhancements of cardiac contractile performance, Gibbs free energy of ATP hydrolysis (ΔGATP), sarcoplasmic reticular (SR) Ca2+ cycling and antioxidant defenses have been presented in earlier reviews.3–5 These mechanisms are summarized here because they likely contribute to pyruvate’s favorable clinical effects presented in later sections.
Pyruvate enhancement of contractile function and ΔGATP in pre- and post-ischemic myocardium
Pyruvate’s inotropic and lusitropic properties have been demonstrated in a variety of heart preparations. In isolated guinea-pig hearts, switching substrate supply from 16 mM glucose to 10 mM pyruvate increased left ventricular developed pressure from 97 ± 4 to 125 ± 3 mmHg while tripling phosphocreatine (PCr)/inorganic phosphate (Pi) concentration ratio, a measure of ΔGATP.6 In isolated guinea-pig hearts consuming glucose7 or octanoate,8 addition of 2–20 mM pyruvate concentration dependently increased left ventricular stroke work and power, while lactate failed to increase cardiac function.7 In in situ canine myocardium, intracoronary pyruvate infusion (0.15 mmol⋅min−1) increased systolic wall thickening by 44%.9
Pyruvate remained effective in hearts which were reversibly injured, i.e. stunned, by ischemia followed by abrupt reperfusion. In in situ canine hearts, 10 min coronary artery occlusion and 30 min reperfusion decreased systolic thickening of the post-ischemic territory by 80%, yet intracoronary pyruvate infusion restored wall thickening to 78% of the pre-ischemic value.9 In stunned guinea-pig hearts consuming 5 mM glucose and 5 mM lactate, 5–10 mM pyruvate increased left ventricular power over 10-fold.7
Pyruvate augments cardiac function by several complementary mechanisms (Figure 1). A readily oxidized metabolic fuel, pyruvate increases ΔGATP, the thermodynamic driving force for the ATP-consuming processes that orchestrate cardiac mechanical function. Although mammalian myocardium consumes fatty acids, ketone acids, glucose, lactate and amino acids as energy substrates,10–14 elevated serum pyruvate raises ΔGATP above that sustained by other fuels.3,8 In in situ canine myocardium receiving its physiological complement of blood-borne fuels, pyruvate infusion to a serum concentration of 5.3 mM increased [PCr]/[ATP], a measure of ΔGATP, by 23%, and lowered the estimated cytosolic concentration of free, i.e. unbound, adenosine-5'-bisphosphate (ADP) by 25%.15 Pyruvate augmentation of ΔGATP remained robust in stunned myocardium and paralleled the improved contractile recovery.7,16 Transient (1-2 min) decreases in contractile force at the onset of pyruvate treatment followed by sustained increases in force development were reported in rabbit right ventricular trabeculae17 and in muscle strips from failing human hearts.18 Pyruvate enters cardiomyocytes via symport with H+,19–21 and the resultant cytosolic acidification may temporarily dampen the cardiac mechanical force22,23 until surmounted by pyruvate’s enhancement of ΔGATP.
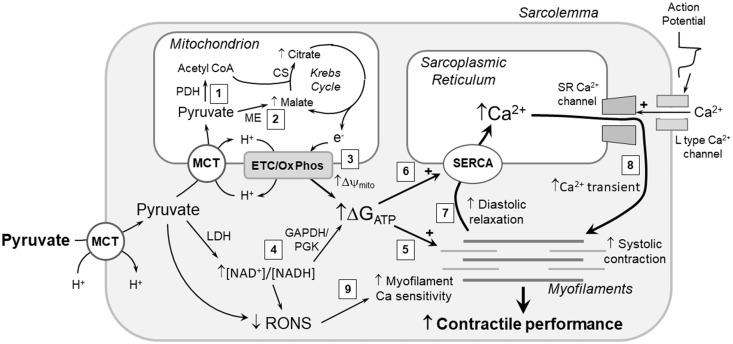
Figure 1.
Mechanisms of pyruvate enhancement of myocardial contractile function. Pyruvate’s energy-yielding and antioxidant actions increase cardiac performance by several mechanisms. Pyruvate metabolism increases ΔGATP by (1) generating Krebs cycle substrate acetyl CoA via pyruvate dehydrogenase (PDH); (2) increasing Krebs cycle intermediate pool sizes via anaplerotic pyruvate carboxylation by malic enzyme (ME); (3) augmenting mitochondrial membrane potential (Δψmito); and (4) via lactate dehydrogenase (LDH), increasing cytosolic [NAD+]/[NADH] redox state, which increases ΔGATP via the near-equilibrium glyceraldehyde 3-phosphate dehydrogenase (GAPDH)/phosphoglycerate kinase (PGK) reactions. The increased ΔGATP augments contractile function by providing more energy for (5) myofilament crossbridge cycling and (6) sarcoplasmic reticular (SR) Ca2+ ATPase, i.e. SERCA. Increased SR Ca2+ loading by SERCA facilitates diastolic relaxation of the contractile machinery (7), and, by augmenting SR Ca2+ loading, increases Ca2+ induced Ca2+ release via the ryanodine-sensitive SR Ca2+ channels, producing a greater systolic Ca2+ transient (8) and more robust activation of the myofilaments. Pyruvate’s antioxidant mechanisms, diagramed in detail in Figure 2, remove reactive O2 and N2 species (RONS), thereby augmenting myofilament Ca2+ sensitivity (9). Collectively, these mechanisms enhance both systolic contraction and diastolic relaxation, and thereby increase myocardial contractile performance.CS: citrate synthase; ETC/Ox Phos: electron transport chain/oxidative phosphorylation; MCT: monocarboxylate transporter. See text for details.
Pyruvate also increased myocardial ΔGATP in large animal models of cardiopulmonary bypass (CPB)24 and cardiopulmonary resuscitation.25 Hearts of open-chest pigs were arrested with pyruvate- or lactate-fortified cardioplegia solution while the animal was maintained on a heart–lung machine. In comparison to the lactate cardioplegia, the pyruvate-enriched cardioplegia better preserved myocardial ATP content and ΔGATP.24 In a canine model of cardiac arrest–resuscitation,25 intravenous pyruvate vs. NaCl augmented the partial recovery of myocardial ΔGATP during cardiopulmonary resuscitation and increased post-arrest recovery of cardiac contractile and electrocardiographic activity and cerebral blood flow.25
Cytosolic and mitochondrial mechanisms of pyruvate enhancements of cardiac function and energy state
In liver mitochondria, ΔGATP and free [ATP]/[ADP] were directly correlated with inner membrane electrical potential (Δψmito), a major component of the protonmotive driving force for oxidative phosphorylation.26–28 In perfused rat hearts, switching fuel supply from 11 mM glucose to 5 mM pyruvate increased Δψmito by 25 mV29; thus, pyruvate, at concentrations that augment contractile function and ΔGATP in isolated and in vivo heart preparations,3,7,8,15,16,24,25 increased the electrochemical driving force for mitochondrial ATP synthesis.
Pyruvate enhancement of post-ischemic cardiac function and ΔGATP required glucose as co-substrate. In glycogen-depleted guinea-pig hearts, irreversible contractile failure ensued when glucose was withdrawn despite the presence of 5 mM pyruvate.30 Similarly, in rabbit hearts receiving 5 mM pyruvate, glycogen depletion or pharmacological inhibition of glycolysis upon reperfusion compromised recovery of left ventricular systolic and diastolic function.31 Because pyruvate is a readily oxidized substrate, the glycolytic requirement for its enhancements of post-ischemic cardiac function and ΔGATP is surprising. The proposed coupling of cytosolic [NAD+]/[NADH] concentration ratio to ATP phosphorylation potential ([ATP]/[ADP][Pi]) and, thus, ΔGATP, effected by the near-equilibrium glycolytic enzymes glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase (Figure 1), may provide an explanation for the glycolytic requirement.32 Pyruvate, in turn, increases cytosolic [NAD+]/[NADH] via the near-equilibrium lactate dehydrogenase reaction. Accordingly, in isolated rabbit hearts, 10 mM pyruvate33 increased cytosolic [ATP]/([ADP][Pi]) while raising the dihydroxyacetone phosphate/α-glycerophosphate concentration ratio, a measure of cytosolic [NAD+]/[NADH].34
The proposed [NAD+]/[NADH]:[ATP]/([ADP][Pi]) coupling32 was challenged by the finding that pharmacological inhibition of mitochondrial pyruvate uptake, which did not disrupt pyruvate’s enhancement of cytosolic [NAD+]/[NADH], prevented pyruvate-increased left ventricular power and [ATP]/([ADP][Pi]), despite provision of medium chain fatty acid octanoate as alternative fuel.8 Thus, mitochondrial metabolism of pyruvate was required for its enhancements of ΔGATP and contractile performance. Similarly, pyruvate augmentation of cell shortening and systolic [Ca2+] transient in isolated rat ventricular cardiomyocytes was blunted by pharmacological inhibition of mitochondrial pyruvate uptake.35 Unlike fatty acids, pyruvate can undergo reductive carboxylation in the mitochondrial matrix generating Krebs cycle intermediates,36–38 which augments oxidative capacity and initiates formation of cardioprotective antioxidants (Figure 2). It thus appears that pyruvate’s cytosolic and mitochondrial metabolism may act synergistically to bolster ΔGATP and antioxidant systems.
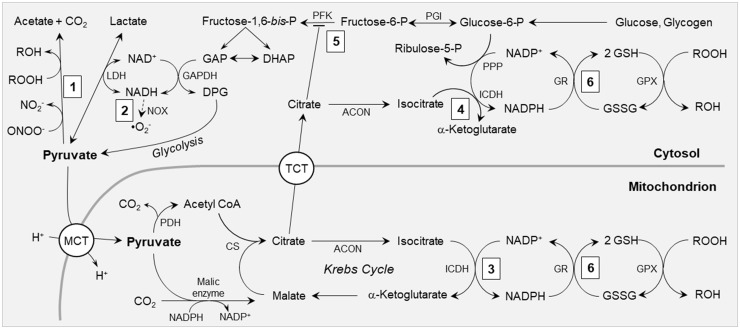
Figure 2.
Antioxidant mechanisms of pyruvate. (1) Pyruvate non-enzymatically detoxifies peroxynitrite (ONOO−) to nitrite and peroxides (ROOH) to their conjugate alcohols (ROH). (2) Via the lactate dehydrogenase (LDH) equilibrium, pyruvate oxidizes cytosolic NADH, regenerating NAD+ for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and decreasing superoxide (•O2−) formation by NAD(P)H oxidase (NOX). (3) Pyruvate carboxylation by malic enzyme increases mitochondrial concentrations of Krebs cycle intermediates, including citrate and isocitrate, thereby supporting NADPH production by mitochondrial NADP+-dependent isocitrate dehydrogenase (ICDH). (4) Citrate effluxes to the cytosol via tricarboxylate transporters (TCT), where it is converted by aconitase (ACON) to isocitrate, providing substrate for NADPH formation by cytosolic ICDH. (5) Citrate inhibits phosphofructokinase (PFK), thereby diverting glycolytic flux into the pentose phosphate pathway (PPP). (6) NADPH generated by mechanisms 3, 4 and 5 provides reducing power to reduce glutathione disulfide (GSSG) to glutathione (GSH), thereby maintaining GSH redox state for the glutathione peroxidases (GPX) and other GSH-dependent antioxidant systems. CS: citrate synthase; DAP: dihydroxyacetone phosphate; DPG: 1,3-bis-phosphoglycerate; GAP: glyceraldehyde 3-phosphate; GR: glutathione reductase; MCT: monocarboxylate transporter; PDH: pyruvate dehydrogenase; PGI: phosphoglucose isomerase. See text for details.
Pyruvate-enhanced SR Ca2+ transport
The myocardial contractile machinery is activated by Ca2+, most of which is mobilized from the SR. Mechanisms that alter SR Ca2+ uptake and release modulate the heart’s contractile performance. Diastolic relaxation requires Ca2+ reuptake by SR Ca2+ ATPase, a major ATP consumer in myocardium. The amount of Ca2+ released via the ryanodine-sensitive SR Ca2+ channels varies in proportion to SR Ca2+ loading,39 which in turn is augmented by increased ΔGATP.40 Approximately 80–85% of the free energy from ATP hydrolysis is conserved in the SR Ca2+ gradient,41,42 so even modest changes in ΔGATP will modulate SR Ca2+ uptake. In buffer-perfused rabbit hearts, 1 mM pyruvate increased left ventricular developed pressure, ΔGATP, and the free energy of the SR Ca2+ gradient,42 and in isolated guinea-pig hearts, pyruvate increased SR Ca2+ turnover, in parallel with its augmentation of contractile performance.8,43 These effects were abrogated by pharmacological blockade of mitochondrial pyruvate uptake, despite provision of an alternative fatty acid substrate, demonstrating that pyruvate enhancements of SR Ca2+ transport, and thus, cardiac function require its mitochondrial oxidation.8
In rat ventricular cardiomyocytes, 10 mM pyruvate increased the amplitude but slowed the kinetics of the systolic Ca2+ transient and increased SR Ca2+ content.44 Inhibition of mitochondrial monocarboxylate transport, adenine nucleotide translocase or the mitochondrial respiratory chain, blunted pyruvate amplification of [Ca2+] transients, implicating mitochondrial pyruvate metabolism and ATP production in pyruvate’s SR effects. Interestingly, pyruvate decreased the frequency of Ca2+ sparks and lowered the open probability of SR Ca2+ channels by approximately 60%. Thus, pyruvate-augmented ATP production and ΔGATP increased SR Ca2+ content, while pyruvate’s interaction with SR Ca2+ channels suppressed Ca2+ release.
Pyruvate-enhanced Ca2+ sensitivity of the contractile machinery
Pyruvate produced a powerful Ca2+-sensitizing effect, i.e. increased Ca2+-activated contractile force, in rabbit right ventricular trabeculae.17 Pyruvate of 10 mM doubled peak cytosolic [Ca2+] and peak contractile force, arguing that pyruvate’s inotropic actions were due to increased [Ca2+] transients, yet when SR Ca2+ cycling was pharmacologically inhibited, pyruvate increased contractile force without increasing systolic [Ca2+]. Instead, pyruvate produced a striking increase in myofilament Ca2+ sensitivity, manifest by a leftward shift in the force:[Ca2+] relationship and an increased Hill coefficient, which would augment contractile force in response to a given Ca2+ transient. When the trabeculae were chemically skinned to disrupt the sarcolemma and mitochondria, pyruvate increased neither myofilament Ca2+ sensitivity nor the Hill coefficient. It was proposed17 that pyruvate enhanced Ca2+ sensitivity by lowering Pi concentration, an effect that requires oxidative phosphorylation and, thus, intact mitochondria. This indirect myofilament Ca2+-sensitizing mechanism may complement pyruvate’s enhancement of SR Ca2+ transport to potentiate systolic contractile force. Accordingly, 20 mM pyruvate increased developed force in failing human heart muscle even after pharmacological inhibition of SR Ca2+ storage, indicating that enhanced SR Ca2+ turnover was not solely responsible for pyruvate’s inotropic effects.18
Oxido-nitrosative stress and cardiac injury
Myocardial ischemia–reperfusion provokes overproduction of toxic reactive oxygen and nitrogen species, i.e. RONS, including superoxide, H2O2 and lipid peroxides, hydroxyl radicals, singlet oxygen, hypochlorous acid and peroxynitrite. These aggressive compounds attack cellular biomolecules, compromising electrolyte homeostasis, membrane integrity, ATP production and, ultimately, cardiomyocyte survival and cardiac function. The myriad targets of RONS include polyunsaturated acyl moieties in membrane phospholipids,45 enzymes catalyzing intermediary metabolism and energy production,46–48 membrane ion transporters and channels,49,50 contractile proteins,51,52 and nuclear53,54 and mitochondrial55,56 DNA. Many of the enzymes that orchestrate fuel metabolism are potential RONS targets, including glyceraldehyde 3-phosphate dehydrogenase47 of the glycolytic pathway, aconitase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase of the Krebs cycle,46,57,58 mitochondrial respiratory complexes I and III,48,59,60 and creatine kinase,61,62 which shuttles ATP from mitochondria to myofilaments and ion pumps.63,64 Inactivation of these enzymes compromises ATP supply essential for cardiac function.
The actions of RONS on myofilaments can depress Ca2+ sensitivity of the contractile machinery.65 S-nitrosylation of cysteinyl residues in sarcomeric proteins can dampen Ca2+-activation of myofibrillar ATPase and contractile force.66 Peroxynitrite has been found to depress left ventricular force development, myofilament Ca2+ sensitivity,67 and the energetic efficiency of cardiac performance, i.e. the contractile work produced at a given rate of ATP expenditure.68,69 The electrolyte transport mechanisms that orchestrate excitation–contraction coupling and diastolic relaxation also are RONS targets, including the Na+, K+ ATPase, which maintains sarcolemmal Na+ and K+ electrochemical gradients and, thus, resting membrane potential,49,70 the ryanodine-sensitive SR Ca2+ channels which release massive amounts of Ca2+ to trigger systolic contraction,71–73 the sarcolemmal Na+/Ca2+ exchangers which effect Ca2+ efflux to the extracellular space,71,74,75 and the SR Ca2+ ATPase,76,77 which sequesters Ca2+, affording diastolic relaxation and re-loading the SR for the next systole. Impairment of these processes by RONS can profoundly impact cardiac function, and the ensuing Ca2+ overload may initiate mitochondrial permeability transition and cardiomyocyte injury or death.78,79
Pyruvate’s cardioprotective antioxidant mechanisms
In addition to its ATP-yielding properties as a metabolic fuel, pyruvate also is a powerful antioxidant.80 Pyruvate exerts its antioxidant effects via several complementary mechanisms, summarized in Figure 2.4,5 Aliphatic α-keto-carboxylates, including pyruvate, undergo oxidative decarboxylation, yielding electrons to reduce and detoxify peroxides, hydroxyl radicals and peroxynitrite in direct, non-enzymatic reactions.81,82 In this fashion, pyruvate (a) reduces lipid peroxides to their conjugate alcohols, thereby interrupting propagation of membrane lipid peroxidation;24,83 (b) reduces hydrogen peroxide (H2O2) and hydroxyl radicals (⋅OH) to water81 and (c) converts peroxynitrite to a benign product, nitrite.82 Pyruvate decarboxylation also yields an oxidizable fuel, acetate.
The principal natural antioxidant in cells, the tripeptide glutathione (GSH), contains a sulfhydryl moiety capable of neutralizing peroxides, peroxynitrite and hydroxyl radicals.84,85 Massive amounts of these toxic metabolites are generated during ischemia and particularly during efforts to restore perfusion, e.g. revascularization and fluid resuscitation.86,87 This oxyradical burden depletes GSH, compromising cellular GSH-dependent antioxidant systems. Pyruvate maintains GSH in two ways (Figure 2).4,5 By directly detoxifying RONS, pyruvate unburdens GSH-dependent antioxidant enzymes, thereby preserving cellular GSH content. Secondly, pyruvate carboxylation by malic enzyme37,38,88 increases the contents of Krebs cycle intermediates, including citrate, a physiological inhibitor of the glycolytic enzyme phosphofructokinase.89,90 Thus, pyruvate administration sharply increased citrate content in isolated hearts8,91,92 and in myocardium in vivo.24,93 After its efflux into the cytosol via tricarboxylate transporters in the inner mitochondrial membrane89 (Figure 2), citrate inhibits phosphofructokinase, causing buildup of glucose 6-phosphate,91 the substrate for the hexose monophosphate pathway, a major source of NADPH which is utilized to maintain GSH via glutathione reductase.
The NADP+-dependent isocitrate dehydrogenase is another source of NADPH. Cardiomyocytes harbor distinct NADP+-isocitrate dehydrogenase isoenzymes in the cytosol and mitochondrial matrix.94,95 Because aconitase, the Krebs cycle enzyme that converts citrate to isocitrate, is near-equilibrium, pyruvate-induced citrate accumulation will, in turn, generate isocitrate, the substrate for isocitrate dehydrogenase. Thus, pyruvate carboxylation could support production of NADPH, the source of electrons to maintain GSH in its reduced, antioxidant form. It should be noted that NADPH provides electrons for reductive pyruvate carboxylation to malate by malic enzyme,36 so conversion of this malate to isocitrate, followed by isocitrate oxidation by NADP+-isocitrate dehydrogenase, does not afford net NADPH production. Instead, the sequential conversion of pyruvate to malate and then citrate, citrate efflux from the mitochondria and its conversion to isocitrate and isocitrate oxidation by cytosolic NADP+-isocitrate dehydrogenase effectively shuttles NADPH across the mitochondrial membrane (Figure 2).
In isolated working guinea-pig hearts, low-flow ischemia and reperfusion sharply lowered myocardial GSH redox state, i.e. the concentration ratio of GSH to its oxidized form, glutathione disulfide (GSSG). Post-ischemic pyruvate treatment restored GSH/GSSG and increased NADPH/NADP+ redox state and citrate content.91 Moreover, pyruvate prevented inactivation of oxyradical-sensitive enzymes in H2O2 exposed hearts,92 in situ porcine myocardium subjected to cardioplegia-induced arrest,96 and in canine myocardium subjected to cardiac arrest and cardiopulmonary resuscitation.97
Bassenge et al.98 demonstrated that shifts in cytosolic [NAD+]/[NADH] driven by pyruvate vs. lactate modulate RONS production by NADH oxidase. In cardiac homogenates, pyruvate concentration dependently suppressed NADH oxidase activity, with 55% inhibition by 5 mM pyruvate. The xanthine oxidase inhibitor oxypurinol did not alter lactate-stimulated ROS formation, and neither pyruvate nor lactate affected RONS production by an in vitro xanthine/xanthine oxidase system, excluding xanthine oxidase as the pyruvate-suppressed RONS source. In isolated guinea-pig hearts, an intense, 60 s burst of RONS production ensued upon reperfusion after 5 min severe ischemia. Pyruvate concentration dependently dampened this RONS burst, with c. 75% suppression by 5 mM pyruvate. In contrast, lactate increased post-ischemic RONS production. Notably, the monocarboxylate transport inhibitor α-cyano-3-hydroxycinnamate, at a concentration (0.5 mM) which selectively inhibits mitochondrial but not sarcolemmal pyruvate uptake,8,20 did not impede pyruvate’s suppression of RONS. Thus, pyruvate’s antioxidant actions in this model are mainly cytosolic, unlike its energy-generating and inotropic effects which require its mitochondrial metabolism.8
Pyruvate’s antioxidant capabilities likely contributed to its potentiation of β-adrenergic mechanisms in stunned myocardium.16,91 In isolated guinea-pig hearts, ischemia–reperfusion blunted, yet pyruvate treatment largely restored, β-adrenergic stimulation of contractile performance.16 Ischemia–reperfusion shifted the relationship between cardiac power and isoproterenol concentration, such that the EC50 increased from 0.3 ± 0.06 nM in pre-ischemic to 5.2 ± 1.9 nM in stunned hearts, yet 5 mM pyruvate, initiated at 15 min reperfusion, lowered the EC50 to 1.1 ± 0.3 nM isoproterenol. Consequently, 2 nM isoproterenol, which produced a robust inotropic effect in pre-ischemic hearts, was ineffective in stunned myocardium, yet 5 mM pyruvate largely restored the inotropic response to isoproterenol.16 Although pyruvate alone increased ΔGATP, during 2 nM isoproterenol stimulation, ΔGATP was similar in the absence vs. presence of pyruvate; thus, increased ΔGATP could not explain the fivefold greater power of the hearts receiving 2 nM isoproterenol + pyruvate vs. 2 nM isoproterenol alone. On the other hand, pyruvate sharply increased antioxidant GSH/GSSG and NADPH/NADP+ ratios, and the sulfhydryl antioxidant N-acetylcysteine potentiated isoproterenol-induced power and augmented GSH/GSSG to similar extents as pyruvate, without increasing ΔGATP.91 Thus, pyruvate’s antioxidant mechanisms potentiated β-adrenergic inotropism in stunned myocardium.
Recently identified cellular and molecular effects of pyruvate
Investigations conducted since the most recent comprehensive pyruvate review5 have documented several additional cardioprotective actions of this pleiotropic compound.
Anti-inflammatory actions of pyruvate in CPB and hypovolemia
Although essential for many cardiothoracic surgeries, CPB can ignite an intense systemic inflammatory response that complicates post-surgical recovery.99,100 RONS, generated in myocardium by intermittent cardioplegia infusion and abrupt reperfusion upon release of the aortic crossclamp,101,102 ignite myocardial inflammation by provoking neutrophil infiltration103 and activating metalloproteinases that degrade the extracellular matrix.104 The authors tested the hypothesis that physiological antioxidant pyruvate could quell post-bypass inflammation in pigs undergoing CPB with pyruvate-enriched vs. pyruvate-free cardioplegia.105 By 4 h reperfusion after cardiac arrest with control cardioplegia, coronary sinus [GSH]/[GSSG], a measure of [GSH]/[GSSG] within the cardiomyocytes,106 fell by 70% vs. non-arrested pigs, but in pigs receiving pyruvate-enriched cardioplegia, coronary sinus [GSH]/[GSSG] was increased nearly 10-fold vs. control cardioplegia, indicating a robust antioxidant effect still present 4 h post-CPB. Pyruvate cardioplegia also suppressed matrix metalloproteinase-9 and myeloperoxidase activation, C-reactive protein accumulation, and neutrophil infiltration in left ventricular myocardium.105 Thus, pyruvate-enriched cardioplegia exerted antioxidant and anti-inflammatory effects that persisted at least 4 h after treatment.
Inflammation is a major comorbidity and cause of death in patients suffering from hypovolemic and septic shock.107–109 Pyruvate’s antioxidant actions and its enhancement of SR Ca2+ transport suggested pyruvate-enriched resuscitation for hypovolemia may exert anti-inflammatory effects in a manner superior to lactate resuscitation. To test this hypothesis, domestic goats were exsanguinated to lower arterial pressure to a critical point approaching decompensation. A tourniquet was applied to impose hindlimb ischemia and released 90 min later during resuscitation with pyruvate- vs. lactate-enriched Ringer’s solution. Relative to non-hemorrhaged shams, in the lactate-resuscitated gastrocnemius, NADPH oxidase, a source of oxyradicals, and the pro-apoptotic enzyme poly(ADP-ribose) polymerase were activated, peroxynitrite formation and lipid peroxidation were exacerbated and the oxyradical-sensitive enzymes creatine kinase and aconitase were inactivated.93,110 In contrast, pyruvate-enriched fluid resuscitation prevented all of these untoward effects in the post-ischemic muscle.110,111 Pyruvate Ringer’s resuscitation also proved superior to lactated Ringer’s for stabilizing cardiac function. Compared to lactated Ringer’s, pyruvate Ringer’s afforded increased recovery of systemic arterial pressure, prevented pro-arrhythmic changes in the electrocardiographic QT interval, and blunted myocardial accumulation of the peroxynitrite derivative nitrotyrosine.93,112
Pyruvate induction of myocardial erythropoietin production and cytoprotective signaling
Erythropoietin is known classically as an activator of erythrocyte maturation in response to hypoxia.113 More recently, a second function of the hormone has emerged: protecting cells in heart, brain and other organs from ischemic injury and inflammation.114–116 Indeed, erythropoietin and its analogs are being developed as treatments for myocardial infarction and ischemic stroke.117–119 Furthermore, it is now becoming evident that heart120 and brain121,122 are capable of synthesizing erythropoietin as an endogenous cardio- and neuroprotectant. Treatments that induce endogenous erythropoietin hold great promise for treating ischemic syndromes of heart and brain, provided they can gain access to the target tissues.
In left ventricular myocardium of pigs subjected to cardioplegic arrest on CPB, pyruvate-enriched cardioplegia induced a nearly 1000-fold increase in the abundance of messenger RNA encoding erythropoietin, and doubled erythropoietin protein content, 4 h after pyruvate treatment.120 Pyruvate-treated myocardium secreted erythropoietin into the coronary effluent for at least 4 h post-pyruvate; thus, pyruvate-enriched cardioplegia stimulated myocardial production of the cytoprotective hormone. Phosphorylation and activation of extracellular signal-regulated kinase-1/2 (Erk-1/2), an erythropoietin-responsive signaling kinase which has been implicated in cardioprotection,123,124 and stabilization of cardiac mitochondria125 were sharply increased 4 h after pyruvate treatment. To our knowledge, this study was the first to show erythropoietin expression in myocardium, and it was pyruvate that produced this novel effect. In a subsequent study in a rat model of ischemic stroke, intravenous pyruvate infusion, for the last 60 min of 2 h middle cerebral artery occlusion and the first 30 min reperfusion, decreased lesion volume by 84% and DNA fragmentation by 77%, in a manner paralleled by increased cerebrocortical erythropoietin content.122
Clinical application of pyruvate
Although pyruvate has been repeatedly found beneficial in preclinical models of cardiovascular disease, only a limited number of clinical trials have examined pyruvate therapy for cardiovascular syndromes. Pioneering studies by Hermann et al.126 evaluated intracoronary pyruvate in patients with dilated cardiomyopathy and New York Heart Association class III heart failure. Pyruvate was infused into the left main coronary artery at 0.925 and 1.85 mmol⋅min−1 for 15 min at each dose. Both doses similarly increased stroke volume index and cardiac index, while lowering pulmonary capillary wedge pressure and heart rate. These variables returned to their respective pretreatment values within 15 min after pyruvate infusion was discontinued. In patients in cardiogenic shock after acute myocardial infarct receiving primary percutaneous coronary intervention and intra-aortic balloon pump, intracoronary pyruvate infusion (c. 0.016–0.04 mmol⋅kg−1⋅min−1) increased cardiac index, stroke volume index and mean arterial pressure.127 These effects plateaued within 30 min and again subsided by 10 min post-infusion.
In a recent clinical trial, Dong et al.128 compared 28 mM pyruvate-enriched Ringer’s to NaCl solution for intravenous resuscitation of patients in septic shock. Relative to NaCl, pyruvate Ringer’s decreased circulating activities of the pro-inflammatory cytokines tumor necrosis factor α and interleukin 6, and increased central venous O2 saturation, a measure of pulmonary function, and urine output. Most importantly, while 9 of the 45 control patients died, only 2 of the 45 pyruvate-treated patients died (P = 0.01). Thus, intravascular pyruvate treatment afforded significant clinical benefit without producing any untoward effects.
Pyruvate-fortified cardioplegia for CPB
During open-heart surgeries, CPB mechanically circulates and oxygenates blood for the body while bypassing the heart and lungs.129 CPB uses a heart–lung machine to maintain organ perfusion while the surgeon works in a bloodless surgical field. Meanwhile, the heart is arrested by intracoronary administration of a depolarizing cardioplegia solution, affording a quiescent surgical field. Afterward, the aortic cross-clamp is released, the heart is reperfused and, upon recovery of sufficient cardiac function, the patient is separated from the heart–lung machine. Although CPB permits lifesaving cardiac surgery, it depletes myocardial energy and antioxidant reserves, provokes myocardial oxyradical formation, and ignites an intense systemic inflammatory response triggered by the collective effects of anesthesia, surgical trauma, hypothermia, and the repeated passage of leukocytes and other blood components through an artificial, extracorporeal circuit.99,100
Although cardioplegic arrest interrupts coronary flow, imposing ischemia on the myocardium, it also affords opportunities for intervention with pyruvate. As an energy substrate, antioxidant and anti-inflammatory agent, pyruvate may be uniquely capable of simultaneously addressing myocardial energy depletion, oxidative stress and inflammation associated with CPB. Accordingly, the authors conducted the first clinical trial of pyruvate-enriched cardioplegia and compared it with conventional, lactate-fortified cardioplegia.130 Patients undergoing primary coronary artery grafting on CPB were randomized to receive cardioplegia containing glucose, insulin and either lactate or pyruvate. The two groups of 15 patients were well matched for age, gender and other demographic factors, and the duration of aortic crossclamp and bypass time were virtually identical in the two groups. Upon separation from bypass, both groups showed the typical post-CPB cardiodepression, a manifestation of cardiac stunning,102,131 yet within 4 h, those patients who had received the pyruvate cardioplegia experienced robust, complete recovery of left ventricular function, while those receiving the lactate cardioplegia recovered more gradually, as is typically seen following CPB.132 Relative to lactate cardioplegia, pyruvate cardioplegia also sharply lowered cardiac release of the injury marker proteins cardiac troponin I and creatine kinase MB.130 The pyruvate group required substantially less dobutamine inotropic support, and those patients met the criteria for hospital discharge on average 27 h earlier than the lactate cardioplegia cohort, at a savings of several thousand dollars per patient. Importantly, there were no adverse events associated with pyruvate treatment.
The results of this clinical trial demonstrate pyruvate’s effectiveness to improve post-cardioplegic arrest recovery of patients at risk of ischemia–reperfusion and its sequelae. Moreover, the clinical trial validates preclinical studies in a variety of animal models which demonstrated pyruvate’s multiple mechanisms that make it a potentially powerful cardioprotective intervention for ischemic and pro-inflammatory syndromes.
Receiver operating characteristic analyses of pyruvate effects in in vitro perfused hearts vs. clinical cardioplegia for CPB
A receiver operating characteristic (ROC) diagram plots the true-positive rate, i.e. sensitivity of a test or procedure outcome, on the ordinate against the false-positive rate, i.e. 100-specificity, on the abscissa, yielding an ROC curve. The area under the ROC curve, ROCAUC, is a measure of the accuracy of the test or procedure, i.e. its ability to distinguish outcomes without false negatives or false positives. At the maximum sensitivity and specificity of 100%, ROCAUC equals 1.0, and there is no overlap of the test results from, for example, the groups receiving control vs. experimental treatments. The most useful ROC information from a clinical study may come from pooling the results of several studies examining the same or comparable clinical interventions or drug tests in different situations, generating “averaged” specificity, sensitivity and ROC statistics which provide an optimum understanding of the intervention’s utility.
The ROC curve provides important information about a clinical intervention’s performance: the closer the apex of the curve approaches the upper left corner, indicating a high true-positive rate and a low false-positive rate, the greater the test’s reliability to discriminate an experimental condition from an appropriate control. Outcome or treatments are expressed as numeric categories labeled as 1 (outcome positive, diseased, treatment applied) or 0 (outcome negative, healthy, treatment not applied). Thus, positive in this context means a successful outcome, a treatment provided, an expected drug effect present, or a positive clinical test relative to control. Additionally, the ROCAUC as well as the estimates of false-positive rates (100-specificity) and false-negative rates (100-sensitivity) are of great interest to the clinician,133,134 because an ROCAUC value is an estimate of the accuracy of the results from a diagnostic test, procedure or drug test. An ROCAUC value of 1.0 indicates a perfect test/outcome where there are no overlapping data from the control and interventional states, and, therefore, no false negatives (100% sensitivity) or false positives (100% specificity). The opposite is true for an ROCAUC value of 0.5 which shows the test/procedure is no better than random chance, and therefore has no diagnostic or prognostic value. ROCAUC values between 0.9 and 1.0 are considered excellent, those between 0.8 and 0.9 are good, and those between 0.7 and 0.8 are considered fair, requiring more cases even if they are statistically significant. ROCAUC between 0.6 and 0.7 are considered to reflect poor outcome and therefore are usually clinically unacceptable; ROCAUC <0.6 should likely be considered failures.135 Another precaution is that ROCAUCs from small sample sizes may be interpreted as inherently “noisy” due to their low statistical power and should be interpreted with extreme caution136 even if the ROCAUC is high.
On the other hand, the ROC analysis offers the unique possibility for the clinician to determine, through the lens of clinical experience, the so-called cut-off value for optimizing diagnostic strategies. In other words, the clinician, evaluating the ROCAUC, must decide which cut-off value will be associated with acceptable odds ratios, likelihood ratios or confidence intervals for minimizing misclassification (e.g. diseased/healthy, test success/failure, etc.) using a sensitivity/specificity pair that promises to be maximally error-free. Thus, ROC analysis, like other statistical modeling techniques (e.g. multivariate analysis, logistic regression or non-linear regression), has specific limitations134,136,137 and is therefore probably best used as an adjunct or supplemental analysis instrument available to the practicing clinician.
The beneficial effects of pyruvate demonstrated in clinical settings are foreshadowed by results of basic cardiovascular research on pyruvate by the authors and other investigators.5 As summarized above, these preclinical efforts demonstrated the beneficial efficacy of pyruvate administration in a variety of experimental models including in situ and ex vivo heart preparations and cultured cell lines. We focused on data obtained in isolated, working and non-working guinea-pig hearts perfused for 90–120 min, including measured variables related to the myocardial energy state, particularly ΔGATP, cytosolic [ATP]/([ADP][Pi]), intracellular Pi concentration and the PCr/creatine concentration ratio, hydraulic work output of the left ventricle, and Δψmito. The latter variable was estimated from the relationship between Δψmito and ΔGATP in non-respiring liver mitochondria,26 where Δψmito = −233 + (ΔGATP, kJ/mol)1.47. Indeed, according to this equation, the addition of 5 mM pyruvate increased Δψmito by 22 mV in perfused guinea-pig hearts receiving 5 mM glucose, in excellent agreement with the 25 mV increase reported by Wan et al.29 in isolated rat hearts. The guinea-pig hearts metabolized 5–10 mM glucose plus either 0.2–20 mM pyruvate or 5–20 mM L-lactate as the main fuels.3,7,30,43,138,139
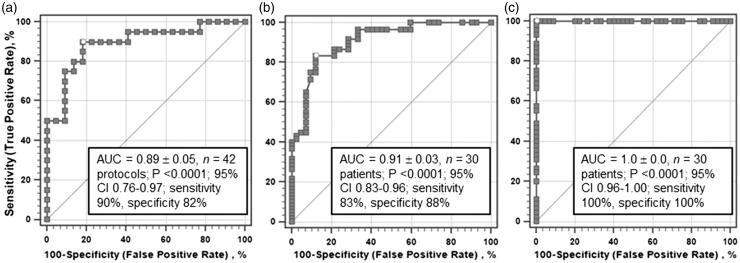
To enable ROC analysis of the isolated heart data, the variables were first analyzed in a logistic regression model with the treatment category as the dependent variable, labeled as 1 if pyruvate present (n = 20 experimental protocols) or 0 if absent (n = 22 experimental protocols) and the myocardial parameters (measures of intracellular energy state or mechanical work output) as the independent variables. This multivariant logistic model yielded coefficients ± standard errors for each independent variable with P values between 0.043 and 0.11 and Wald statistics between 5.18 and 2.55; the model, based on 20 “positive” and 22 “negative” protocols, was statistically highly significant at P = 0.0004, n = 42 protocols (5–7 experiments per protocol; c. 210 individual experiments). The Shapiro–Wilk statistic for normal distribution of the residuals approached 1.0 at W = 0.97, with a P value of 0.35, confirming normality of the residuals. The predicted pyruvate treatment categories from these logistic regressions were then subjected to standard ROC analyses (MedCalc Software v. 17.9, Ostend, Belgium) in which the actual pyruvate categories were compared with the logistically predicted categories. These ROC estimates from the pyruvate- and lactate-perfused hearts (Figure 3(a)) yielded a good to excellent ROCAUC of 0.89 ± 0.051, n = 42, P < 0.0001, with a sensitivity of 90% and specificity of 82%. This result strongly suggests that our protocols clearly distinguished the glucose plus pyruvate treated hearts from those receiving glucose plus L-lactate, examined under diverse but relatively physiological conditions. This differentiation is also evident from the classification table generated by the logistic regression, which showed an 83.3% overall correct prediction, with 19 of 22 cases (86.4%) correctly predicted in the negative (glucose ± lactate) series and 16 of 20 cases (80%) correctly predicted in the positive (pyruvate + glucose) series. Thus, there was a relatively high degree of certainty that pyruvate treatment of perfused guinea-pig hearts bolstered contractile performance and myocardial energy state in these preclinical experiments.
Figure 3.
Receiver operating characteristic (ROC) analyses of isolated, perfused guinea-pig hearts and patients recovering from coronary revascularization surgery on cardiopulmonary bypass. (a) Isolated guinea-pig hearts: Analyses of mean values of contractile function and myocardial energy state from 42 separate series3,7,30,43,138,139 comprising approximately 210 isolated hearts metabolizing glucose alone, glucose + lactate or glucose + pyruvate. Analysis of left ventricular (b: LVSWI post-bypass) and right ventricular (c: RVSWI post-bypass) stroke work indices in 30 patients (15 patients per group) at 4, 6 and 12 h recovery following cardiopulmonary bypass with lactate- vs. pyruvate-enriched cardioplegia (total 90 data points).130AUC: area under the ROC curve; CI: 95% confidence interval; LVSWI: left ventricular stroke work index; RVSWI: right ventricular stroke work index.
We then applied ROC analysis (without prior logistic regression estimates) to left and right ventricular stroke work indices from the CPB clinical trial130 to assess whether outcomes with pyruvate cardioplegia were statistically differentiated from the standard lactate cardioplegia. Specifically, ROC analyses were applied to identify whether pyruvate cardioplegia was superior to lactate cardioplegia for clinical CPB in terms of post-CPB recovery of left or right ventricular function, in this cohort of 30 patients. The categorical independent parameter was pyruvate cardioplegia = 1 vs. lactate cardioplegia = 0. ROC analysis of left ventricular function index 4, 6 and 12 h after CBP (Figure 3(b)) yielded a high ROCAUC value: 0.91 ± 0.029, n = 90 data points, P < 0.0001. The 95% confidence interval of the entire ROC curve ranged from 0.83 to 0.96, implying good to excellent precision and showing a clear statistical separation of the two patient groups. However, the estimated rate of false-negative classification (1-sensitivity) was approximately 17%, while the estimated false-positive rate (1-specificity) was approximately 12%, suggesting that this first CPB trial of pyruvate-enriched cardioplegia likely requires independent confirmation. Interestingly, ROC analysis of the right ventricular stroke work index 4–12 h post-CPB produced an estimated ROCAUC = 1.0, strongly suggesting a perfect test for that variable in these patients (Figure 3(c)).
Collectively, these first CBP trial results predicted with relatively high confidence that the pyruvate cardioplegia could have significant clinical utility, at least in the setting of cardiothoracic surgery on CPB. Schillinger et al.127 reached a similar conclusion regarding the use of intracoronary pyruvate treatment of cardiogenic shock. It is also interesting to note that pyruvate intervention was found to be effective for resuscitation for patients with septic shock,128 in which pyruvate-enriched Ringer solution decreased systemic pro-inflammatory cytokines and simultaneously improved survival rate from 80% to 95% without any signs of toxicity.
The ROCAUC estimates from the isolated guinea-pig hearts (Figure 3(a)) and the left ventricle of patients 4–12 h post-CPB (Figure 3(b)) were nearly identical: AUC 0.89 ± 0.058 and 0.91 ± 0.029, respectively; z = 0.34, P = 0.733. Thus, direct comparison of these ROCAUC estimates demonstrates remarkable concordance of the preclinical and clinical ROC estimates. The preclinical data from isolated, perfused guinea-pig hearts revealed considerable predictive power, at least with respect to the clinical post-CPB recovery trial detailed above. This robust agreement underscores the value of basic research as an essential precursor to clinical trials, both for proof of concept and for defining mechanisms responsible for therapeutic benefits.
Factors limiting the clinical application of pyruvate
Several practical considerations must be taken into account before pyruvate-enriched formulations can be introduced into general clinical practice. For instance, the shelf lives of such formulations have not been firmly established, but may be shorter than conventional Ringer’s lactate and possibly more expensive. Currently, pyruvate-enriched solutions must be prepared at or near the time and location of use and must be sterilized, e.g. by micropore filtration, as was done in the cardioplegia clinical trial.130 The source pyruvate and intravenous solutions must be tested to ensure they are endotoxin-free. These requirements may hamper clinical use of pyruvate, especially in emergency and critical care medicine where pyruvate may be particularly beneficial, yet treatments are given urgently. Another consideration is the profitability of pyruvate formulations for the pharmaceutical industry; if such formulations are unprofitable, there may be little motivation for developing and marketing them, despite their clinical efficacy.
Conclusions
The research summarized above has defined pyruvate’s unique combination of energy-yielding, antioxidant and anti-inflammatory properties, which makes it a powerful cardioprotectant against the effects of myocardial ischemia–reperfusion and oxidant stress. Thus, pyruvate, particularly at 3–6 mM, augmented post-ischemic contractile performance and myocardial ΔGATP, the energy source for myofilament crossbridge cycling and SR Ca2+ uptake. Pyruvate also increased cytosolic NAD+/NADH, thereby alleviating NADH suppression of energy-yielding glycolytic flux at glyceraldehyde 3-phosphate dehydrogenase and dampening superoxide production by NADH oxidase. By augmenting antioxidant GSH/GSSG and NADPH/NADP+ redox states, pyruvate protected RONS-vulnerable SR Ca2+ transport and myofilament Ca2+ sensitivity, and blunted myocardial inflammation. The first clinical trials of pyruvate intervention for heart failure, septic shock and CPB have demonstrated pyruvate’s safety and therapeutic efficacy. Expanded, phase three clinical trials are essential to confirm and extend the favorable outcomes of pyruvate-based interventions.
Authors' contributions
RM and RB evaluated the literature and drafted the manuscript. AY conducted the clinical trial of cardiopulmonary bypass. RB conducted receiver operating characteristic analyses of preclinical and cardiopulmonary bypass data. RM and RB prepared the figures. RM, AY and RB edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
Studies by the authors described in this article were supported by grants from the National Heart, Lung and Blood Institute (HL29060, HL36067, HL50441, HL71684), National Institute of Neurological Disorders and Stroke (NS076975), Osteopathic Heritage Foundation (OHF 02–18-522), American Heart Association Texas Affiliate (92G-155), Veterans Administration (G376CD), U.S. Department of Defense (W911NF-09–1-0086), faculty research grants from Uniformed Services University of the Health Sciences (RO-7638) and University of North Texas Health Science Center (62050, 67214, 67716, 71160, 97001).
References
- 1.Meierhenrich R, Jedicke H, Voigt A, Lange H. The effect of erythropoietin on lactate, pyruvate, and excess lactate under physical exercise in dialysis patients. Clin Nephrol 1996; 45:90–6 [PubMed] [Google Scholar]
- 2.Lloyd S, Brocks C, Chatham JC. Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. Am J Physiol Heart Circ Physiol 2003; 285:H163–72 [DOI] [PubMed] [Google Scholar]
- 3.Mallet RT, Bünger R. Metabolic protection of post-ischemic phosphorylation potential and ventricular performance. Adv Exp Med Biol 1993; 346:233–41 [DOI] [PubMed] [Google Scholar]
- 4.Mallet RT, Sun J. Antioxidant properties of myocardial fuels. Mol Cell Biochem 2003; 253:103–11 [DOI] [PubMed] [Google Scholar]
- 5.Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med 2005; 230:435–43 [DOI] [PubMed] [Google Scholar]
- 6.Zweier JL, Jacobus WE. Substrate-induced alterations of high energy phosphate metabolism and contractile function in the perfused heart. J Biol Chem 1987; 262:8015–21 [PubMed] [Google Scholar]
- 7.Bünger R, Mallet RT, Hartman DA. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur J Biochem 1989; 180:221–33 [DOI] [PubMed] [Google Scholar]
- 8.Mallet RT, Sun J. Pyruvate enhancement of cardiac function and energetics requires its mitochondrial metabolism. Cardiovasc Res 1999; 42:149–61 [DOI] [PubMed] [Google Scholar]
- 9.Mentzer RM, Van Wylen DGL, Sodhi J, Weiss RJ, Lasley RD, Willis J, Bünger R, Flint LM. Effect of pyruvate on regional ventricular function in normal and stunned myocardium. Ann Surg 1989; 209:629–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svedjeholm R, Ekroth R, Joachimsson PO, Ronquist G, Svensson S, Tydén H. Myocardial uptake of amino acids and other substrates in relation to myocardial oxygen consumption four hours after cardiac operations. J Thorac Cardiovasc Surg 1991; 101:688–94 [PubMed] [Google Scholar]
- 11.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 1999; 99:578–88 [DOI] [PubMed] [Google Scholar]
- 12.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010; 90:207–58 [DOI] [PubMed] [Google Scholar]
- 13.Wentz AE, d’Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 2010; 285:24447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake KJ, Sidorov VY, McGuinness OP, Wasserman DH, Wikswo JP. Amino acids as metabolic substrates during myocardial ischemia. Exp Biol Med (Maywood) 2012; 237:1369–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laughlin MR, Taylor J, Chesnick AS, DeGroot M, Balaban RS. Pyruvate and lactate metabolism in the in vivo dog heart. Am J Physiol Heart Circ Physiol 1993; 264:H2068–79 [DOI] [PubMed] [Google Scholar]
- 16.Tejero-Taldo MI, Sun J, Caffrey JL, Mallet RT. Pyruvate potentiates β-adrenergic inotropism of stunned guinea-pig myocardium. J Mol Cell Cardiol 1998; 30:2327–39 [DOI] [PubMed] [Google Scholar]
- 17.Torres CAA, Varian KD, Canan CH, Davis JP, Janssen PML. The positive inotropic effect of pyruvate involves an increase in myofilament calcium sensitivity. PLoS One 2013;8:e63608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasenfuss G, Maier LS, Hermann H-P, Lüers C, Hünlich M, Zeitz O, Janssen PML, Pieske B. Influence of pyruvate on contractile performance and Ca2+ cycling in isolated failing human myocardium. Circulation 2002; 105:194–9 [DOI] [PubMed] [Google Scholar]
- 19.Steele RD. Blood-brain barrier transport of the alpha-keto acid analogs of amino acids. Fed Proc 1986; 45:2060–4 [PubMed] [Google Scholar]
- 20.Bünger R, Mallet RT. Mitochondrial pyruvate transport in working guinea-pig heart: work-related vs. carrier-mediated control of pyruvate oxidation. Biochim Biophys Acta 1993; 1151:223–36 [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP. Monocarboxylic acid transport. Compr Physiol 2013; 3:1611–43 [DOI] [PubMed] [Google Scholar]
- 22.Kentish JC. Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pf lugers Arch 1991; 419:310–8 [DOI] [PubMed] [Google Scholar]
- 23.Parsons B, Szczesna D, Zhao J, Van Slooten G, Kerrick WG, Putkey JA, Potter JD. The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin C in skinned muscle fibres. J Muscle Res Cell Motil 1997; 18:599–609 [DOI] [PubMed] [Google Scholar]
- 24.Knott EM, Ryou MG, Sun J, Heymann A, Sharma AB, Lei Y, Baig M, Mallet RT, Olivencia-Yurvati AH. Pyruvate-fortified cardioplegia suppresses oxidative stress and enhances phosphorylation potential of arrested myocardium. Am J Physiol Heart Circ Physiol 2005; 289:H1123–30 [DOI] [PubMed] [Google Scholar]
- 25.Sharma AB, Knott EM, Jr, Bi J, Martinez RR, Sun J, Mallet RT. Pyruvate improves cardiac electromechanical and metabolic recovery from cardiopulmonary arrest and resuscitation. Resuscitation 2005; 66:71–81 [DOI] [PubMed] [Google Scholar]
- 26.Davis EJ, Bremer J, Akerman KE. Thermodynamic aspects of translocation of reducing equivalents by mitochondria. J Biol Chem 1980; 255:2277–83 [PubMed] [Google Scholar]
- 27.Burat MK, Burat T, Davis-Van Thienen WI, Davis EJ. Control of cellular redox potential as measured in a steady-state, cell-free system. Arch Biochem Biophys 1984; 235:150–8 [DOI] [PubMed] [Google Scholar]
- 28.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. 3rd ed New York: Garland Publishing Inc, 1994, pp. 665–666 [Google Scholar]
- 29.Wan B, Doumen C, Duszynski J, Salama G, LaNoue KF. A method of determining electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Physiol Heart Circ Physiol 1993; 265:H445–52 [DOI] [PubMed] [Google Scholar]
- 30.Mallet RT, Hartman DA, Bünger R. Glucose requirement for postischemic recovery of perfused working heart. Eur J Biochem 1990; 188:481–93 [DOI] [PubMed] [Google Scholar]
- 31.Jeremy RW, Ambrosio G, Pike MM, Jacobus WE, Becker LC. The functional recovery of post-ischemic myocardium requires glycolysis during early reperfusion. J Mol Cell Cardiol 1993; 25:261–76 [DOI] [PubMed] [Google Scholar]
- 32.Veech RL, Lawson JWR, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem 1979; 254:6538–47 [PubMed] [Google Scholar]
- 33.Scholz TD, Laughlin MR, Balaban RS, Kupriyanov VV, Heineman FW. Effect of substrate on mitochondrial NADH, cytosolic redox state, and phosphorylated compounds in isolated hearts. Am J Physiol Heart Circ Physiol 1995; 268:H82–91 [DOI] [PubMed] [Google Scholar]
- 34.Opie LH, Mansford KRL. The value of lactate and pyruvate measurements in the assessment of the redox state of free nicotinamide-adenine dinucleotide in the cytoplasm of perfused rat heart. Eur J Clin Invest 1971; 1:295–306 [DOI] [PubMed] [Google Scholar]
- 35.Martin BJ, Valdivia HH, Bünger R, Lasley RD, Mentzer RM., Jr Pyruvate augments calcium transients and cell shortening in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 1998; 274:H8–17 [DOI] [PubMed] [Google Scholar]
- 36.Sundqvist KE, Heikkilä J, Hassinen IE, Hiltunen JK. Role of NADP+-linked malic enzymes as regulators of the pool size of tricarboxylic acid-cycle intermediates in the perfused rat heart. Biochem J 1987; 243:853–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell RR, 3rd, Taegtmeyer H. . Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol Heart Circ Physiol 1991; 261:H1756–62 [DOI] [PubMed] [Google Scholar]
- 38.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O-Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 2009; 104:805–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 1995; 268:C1313–9 [DOI] [PubMed] [Google Scholar]
- 40.Wimsatt DK, Hohl CM, Brierley GP, Altschuld RA. Calcium accumulation and release by the sarcoplasmic reticulum of digitonin-lysed adult mammalian ventricular cardiomyocytes. J Biol Chem 1990; 265:14849–57 [PubMed] [Google Scholar]
- 41.Kammermeier H. High energy phosphate of the myocardium: concentration versus free energy change. Basic Res Cardiol 1987; 82:31–6 [DOI] [PubMed] [Google Scholar]
- 42.Chen W, London R, Murphy E, Steenbergen C. Regulation of the Ca2+ gradient across the sarcoplasmic reticulum in perfused rabbit heart: a 19F nuclear magnetic resonance study. Circ Res 1998; 83:898–907 [DOI] [PubMed] [Google Scholar]
- 43.Mallet RT, Bünger R. Energetic modulation of cardiac inotropism and sarcoplasmic reticular Ca2+ uptake. Biochim Biophys Acta 1994; 1224:22–32 [DOI] [PubMed] [Google Scholar]
- 44.Zima AV, Kockskämper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol (Lond) 2003; 550:765–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambrosio G, Flaherty JT, Duilio C, Tritto I, Santoro G, Elia PP, Condorelli M, Chiariello M. Oxygen radicals generated at reflow induce peroxidation of membrane lipid in reperfused hearts. J Clin Invest 1991; 87:2056–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zini R, Berdeaux A, Morin D. The differential effects of superoxide anion, hydrogen peroxide and hydroxyl radical on cardiac mitochondrial oxidative phosphorylation. Free Radic Res 2007; 41:1159–66 [DOI] [PubMed] [Google Scholar]
- 47.Broniowska KA, Hogg N. Differential mechanisms of inhibition of glyceraldehde-3-phosphate dehydrogenase by S-nitrosothiols and NO in cellular and cell-free conditions. Am J Physiol Heart Circ Physiol 2010; 299:H1212–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 2014; 114:524–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplán P, Matejovičová M, Herijgers P, Flameng W. Effect of free radical scavengers on myocardial function and Na+,K+-ATPase activity in stunned rabbit myocardium. Scand Cardiovasc J 2005; 39:213–9 [DOI] [PubMed] [Google Scholar]
- 50.Simon JN, Duglan D, Casadei B, Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol 2014; 73:80–91 [DOI] [PubMed] [Google Scholar]
- 51.Bayeva M, Ardehali H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr Hypertens Rep 2010; 12:426–32 [DOI] [PubMed] [Google Scholar]
- 52.Ávila RA, Silva MASC, Peixoto JV, Kasouf-Silva I, Foqaça RTH, Dos Santos L. Mechanisms involved in the in vitro contractile dysfunction induced by different concentrations of ferrous iron in the rat myocardium. Toxicol in Vitro 2016; 36:38–45 [DOI] [PubMed] [Google Scholar]
- 53.Yang HY, Wang YM, Peng SQ. Metallothionein-I/II null cardiomyocytes are sensitive to Fusarium mycotoxin butenolide-induced cytotoxicity and oxidative DNA damage. Toxicon 2010; 55:1291–6 [DOI] [PubMed] [Google Scholar]
- 54.Sebai H, Sani M, Aouani E, Ghanem-Boughanmi N. Cardioprotective effect of resveratrol on lipopolysaccharide-induced oxidative stress in rat. Drug Chem Toxicol 2011; 34:146–50 [DOI] [PubMed] [Google Scholar]
- 55.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 2001; 88:529–35 [DOI] [PubMed] [Google Scholar]
- 56.Yue R, Xia X, Jiang J, Yang D, Han Y, Chen X, Cai Y, Li L, Wang WE, Zeng C. Mitochondrial DNA oxidative damage contributes to cardiomyocyte ischemia/reperfusion-injury in rats: cardioprotective role of lycopene. J Cell Physiol 2015; 230:2128–41 [DOI] [PubMed] [Google Scholar]
- 57.Batinic-Haberle I, Benov LT. An SOD mimic protects NADP+-dependent isocitrate dehydrogenase against oxidative inactivation. Free Radic Res 2008; 42:618–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan PE, Sheahan PJ, Pattison DI, Davies MJ. Methylglyoxal-induced modification of arginine residues decreases the activity of NADPH-generating enzymes. Free Radic Biol Med 2013; 61:229–42 [DOI] [PubMed] [Google Scholar]
- 59.Pearce LL, Kanai AJ, Epperly MW, Peterson J. Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide 2005; 13:254–63 [DOI] [PubMed] [Google Scholar]
- 60.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res 2007; 75:283–90 [DOI] [PubMed] [Google Scholar]
- 61.Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res 2011; 49:798–807 [DOI] [PubMed] [Google Scholar]
- 62.Brioschi M, Polvani G, Fratto P, Parolari A, Agostoni P, Tremoli E, Banfi C. Redox proteomics identification of oxidatively modified myocardial proteins in human heart failure: implications for protein function. PLoS One 2012; 7:e35841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000; 80:1107–213 [DOI] [PubMed] [Google Scholar]
- 64.Joubert F, Mazet JL, Mateo P, Hoerter JA. 31P NMR detection of subcellular creatine kinase fluxes in the perfused rat heart: contractility modifies energy transfer pathways. J Biol Chem 2002; 277:18469–76 [DOI] [PubMed] [Google Scholar]
- 65.Maron BA, Tang SS, Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal 2013; 18:270–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Figueiredo-Freitas C, Dulce RA, Foster MW, Liang J, Yamashita AM, Lima-Rosa FL, Thompson JW, Moseley MA, Hare JM, Nogueira L, Sorenson MM, Pinto JR. S-nitrosylation of sarcomeric proteins depresses myofilament Ca2+ sensitivity in intact cardiomyocytes. Antioxid Redox Signal 2015; 23:1017–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunner F, Wölkart G. Peroxynitrite-induced cardiac depression: role of myofilament desensitization and cGMP pathway. Cardiovasc Res 2003; 60:355–64 [DOI] [PubMed] [Google Scholar]
- 68.Ferdinandy P, Panas D, Schulz R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working hearts. Am J Physiol Heart Circ Physiol 1999; 276:H1861–7 [DOI] [PubMed] [Google Scholar]
- 69.Lee WH, Gounarides JS, Roos ES, Wolin MS. Influence of peroxynitrite on energy metabolism and cardiac function in a rat ischemia-reperfusion model. Am J Physiol Heart Circ Physiol 2003; 285:H1385–95 [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Zhang Z, Guo H, Wang Y. Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol 2008; 22:615–21 [DOI] [PubMed] [Google Scholar]
- 71.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 2006; 71:310–21 [DOI] [PubMed] [Google Scholar]
- 72.Donoso P, Sanchez G, Bull R, Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci (Landmark Ed) 2011; 16:553–67 [DOI] [PubMed] [Google Scholar]
- 73.Cooper LL, Li W, Lu Y, Centracchio J, Terentyeva R, Koren G, Terentyev D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J Physiol 2013; 591:5895–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soliman D, Hamming KS, Matemisz LC, Light PE. Reactive oxygen species directly modify sodium-calcium exchanger activity in a splice variant-dependent manner. J Mol Cell Cardiol 2009; 47:595–602 [DOI] [PubMed] [Google Scholar]
- 75.Liu T, O’Rourke B. Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J Biol Chem 2013; 288:31984–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balderas-Villalobos J, Molina-Muñoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gómez-Viquez NL. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 2013; 305:H1344–53 [DOI] [PubMed] [Google Scholar]
- 77.Plummer BN, Liu H, Wan X, Deschênes I, Laurita KR. Targeted antioxidant treatment decreases cardiac alternans associated with chronic myocardial infarction. Circ Arrhythm Electrophysiol 2015;8:165–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruiz-Meana M, Abellán A, Miró-Casas E, Agulló E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol 2009; 297:H1281–9 [DOI] [PubMed] [Google Scholar]
- 79.Abdallah Y, Kasseckert SA, Iraqi W, Said M, Shahzad T, Ergogan A, Neuhof C, Gündüz D, Schlüter KD, Tillmanns H, Piper HM, Reusch HP, Ladilov Y. Interplay between Ca2+ cycling and mitochondrial permeability transition pores promotes reperfusion-induced injury of cardiac myocytes. J Cell Mol Med 2011; 15:2478–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bünger R, Kang YH, Lee YJ, Mongan P, Sharma P. Redox and antioxidant mechanisms of pyruvate in cellular oxidative stress: enhanced survival signaling and mitochondrial stability. Recent Res Devel Physiol 2006; 4:1–25 [Google Scholar]
- 81.Constantopoulos G, Barranger JA. Nonenzymatic decarboxylation of pyruvate. Anal Biochem 1984; 139:353–8 [DOI] [PubMed] [Google Scholar]
- 82.Vásquez-Vivar J, Denicola A, Radi R, Augusto O. Peroxynitrite-mediated decarboxylation of pyruvate to both carbon dioxide and carbon dioxide radical anion. Chem Res Toxicol 1997; 10:786–94 [DOI] [PubMed] [Google Scholar]
- 83.Raghavamenon A, Garelnabi M, Babu S, Aldrich A, Litvinov D, Parthasarathy S. α- Tocopherol is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes: a potential explanation for the failure of antioxidants to affect human atherosclerosis. Antioxid Redox Signal 2009; 11:1237–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molina H, Garcia M. Enzymatic defenses of the rat heart against lipid peroxidation. Mech Ageing Dev 1997; 97:1–7 [DOI] [PubMed] [Google Scholar]
- 85.Cheung PY, Wang W, Schulz R. Glutathione protects against myocardial ischemia-reperfusion injury by detoxifying peroxynitrite. J Mol Cell Cardiol 2000; 32:1669–78 [DOI] [PubMed] [Google Scholar]
- 86.García-de-la-Asuncíon J, Pastor E, Perez-Griera J, Belda FJ, Moreno T, García-del-Olmo E, Martí F. Oxidative stress injury after on-pump cardiac surgery: effects of aortic cross clamp time and type of surgery. Redox Rep 2013; 18:193–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zakkar M, Guída G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev 2015; 2015. Article ID 189863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hiltunen JK, Davis EJ. The disposition of citric acid cycle intermediates by isolated rat heart mitochondria. Biochim Biophys Acta 1981; 678:115–21 [DOI] [PubMed] [Google Scholar]
- 89.Cheema-Dhadli S, Robinson BH, Halperin ML. Properties of the citrate transporter in rat heart: implications for regulation of glycolysis by cytosolic citrate. Can J Biochem 1976; 54:561–5 [DOI] [PubMed] [Google Scholar]
- 90.Nielsen TT, Henningsen P, Bagger JP, Thomsen PE, Eyjolfsson K. Myocardial citrate metabolism in control subjects and patients with coronary artery disease. Scand J Clin Lab Invest 1980; 40:575–80 [DOI] [PubMed] [Google Scholar]
- 91.Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of β-adrenergic inotropism in stunned myocardium. J Mol Cell Cardiol 1999; 31:1863–72 [DOI] [PubMed] [Google Scholar]
- 92.Mallet RT, Squires JE, Bhatia S, Sun J. Pyruvate restores contractile function and antioxidant defenses of hydrogen peroxide-challenged myocardium. J Mol Cell Cardiol 2002; 34:1173–84 [DOI] [PubMed] [Google Scholar]
- 93.Gurji HA, White DW, Hoxha B, Sun J, Olivencia-Yurvati AH, Mallet RT. Pyruvate-fortified resuscitation stabilizes cardiac electrical activity and energy metabolism during hypovolemia. World J Crit Care Med 2013; 2:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benderdour M, Charron G, Comte B, Ayoub R, Beaudry D, Foisy S, Deblois D, Des Rosiers C. Decreased cardiac mitochondrial NADP+-isocitrate dehydrogenase activity and expression: a marker of oxidative stress in hypertrophy development. Am J Physiol Heart Circ Physiol 2004; 287:H2122–31 [DOI] [PubMed] [Google Scholar]
- 95.Murakami K, Haneda M, Iwata S, Yoshino M. Differential effects of polyamine on the cytosolic and mitocondrial NADP-isocitrate dehydrogenases. Biofactors 2012; 38:365–71 [DOI] [PubMed] [Google Scholar]
- 96.Knott EM, Sun J, Lei Y, Ryou MG, Olivencia-Yurvati AH, Mallet RT. Pyruvate mitigates oxidative stress during reperfusion of cardioplegia-arrested myocardium. Ann Thorac Surg 2006; 81:928–34 [DOI] [PubMed] [Google Scholar]
- 97.Sharma AB, Sun J, Howard LL, Williams AG, Jr, Mallet RT. Oxidative stress reversibly inactivates myocardial enzymes during cardiac arrest. Am J Physiol Heart Circ Physiol 2007; 292:H198–206 [DOI] [PubMed] [Google Scholar]
- 98.Bassenge E, Sommer O, Schwemmer M, Bünger R. Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am J Physiol Heart Circ Physiol 2000; 279:H2431–8 [DOI] [PubMed] [Google Scholar]
- 99.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003; 75:S715–20 [DOI] [PubMed] [Google Scholar]
- 100.Warren OJ, Smith AJ, Alexiou C, Rogers PLB, Jawad N, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 1 – mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23:223–31 [DOI] [PubMed] [Google Scholar]
- 101.Hayashi Y, Sawa Y, Ohtake S, Fukuyama N, Nakazawa H, Matsuda H. Peroxynitrite formation from human myocardium after ischemia-reperfusion during open heart operation. Ann Thorac Surg 2001; 72:571–6 [DOI] [PubMed] [Google Scholar]
- 102.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Guadino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg 2004; 25:304–11 [DOI] [PubMed] [Google Scholar]
- 103.Moro MA, Darley-Usmar V, Goodwin DA, Read NG, Zamora-Pino R, Feelisch M, Radomski MW, Moncada S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci USA 1994; 91:6702–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okamoto T, Akuta T, Tamura F, van der Vleit A, Akaike T. Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem 2004; 385:997–1006 [DOI] [PubMed] [Google Scholar]
- 105.Ryou MG, Flaherty DC, Hoxha B, Gurji H, Sun J, Hodge LM, Olivencia-Yurvati AH, Mallet RT. Pyruvate-enriched cardioplegia suppresses cardiopulmonary bypass-induced myocardial inflammation. Ann Thorac Surg 2010; 90:1529–36 [DOI] [PubMed] [Google Scholar]
- 106.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res 2006; 9:169–81 [DOI] [PubMed] [Google Scholar]
- 107.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007; 38:1336–45 [DOI] [PubMed] [Google Scholar]
- 108.De Backer D, Scolletta S. Clinical management of the cardiovascular failure in sepsis. Curr Vasc Pharmacol 2013; 11:222–42 [PubMed] [Google Scholar]
- 109.Gomez HG, Gonzalez SM, Londoño JM, Hoyos NA, Niño CD, Leon AL, Rugeles MT, Jaimes FA. Immunological characterization of compensatory anti-inflammatory response syndrome in patients with severe sepsis: a longitudinal study. Crit Care Med 2014; 42:771–80 [DOI] [PubMed] [Google Scholar]
- 110.Flaherty DC, Hoxha B, Sun J, Gurji H, Simecka JW, Olivencia-Yurvati AH, Mallet RT. Pyruvate-enriched resuscitation protects hindlimb muscle from ischemia-reperfusion injury. Mil Med 2010; 175:1020–6 [DOI] [PubMed] [Google Scholar]
- 111.Gurji HA, White DW, Hoxha B, Sun J, Harbor JP, Schulz DR, Williams AG, Jr, Olivencia-Yurvati AH, Mallet RT. Pyruvate-enriched resuscitation: antioxidant and energetic support for post-ischemic hindlimb muscle during hemorrhagic shock. Exp Biol Med 2014; 239:241–50 [DOI] [PubMed] [Google Scholar]
- 112.Flaherty DC, Hoxha B, Sun J, Gurji H, Simecka JW, Mallet RT, Olivencia-Yurvati AH. Pyruvate-fortified fluid resuscitation improves hemodynamic stability while suppressing systemic inflammation and myocardial oxidative stress after hemorrhagic shock. Mil Med 2010; 175:166–72 [DOI] [PubMed] [Google Scholar]
- 113.Fisher JW. Landmark advances in the development of erythropoietin. Exp Biol Med (Maywood) 2010; 235:1398–411 [DOI] [PubMed] [Google Scholar]
- 114.Cai Z, Monalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion. Circulation 2003; 108:79–85 [DOI] [PubMed] [Google Scholar]
- 115.Hasselblatt M, Ehrenreich H, Sirén A-L. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol 2006; 18:132–8 [DOI] [PubMed] [Google Scholar]
- 116.Mammis A, McIntosh TK, Maniker AH. Erythropoietin as a neuroprotective agent in traumatic brain injury. Surg Neurol 2009; 71:527–31 [DOI] [PubMed] [Google Scholar]
- 117.Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, van Zonneveld AJ, Schoemaker RG, van Gilst WH, Zijlstra F, van Veldhuisen DJ. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther 2006; 20:135–41 [DOI] [PubMed] [Google Scholar]
- 118.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C; EPO Stroke Trial Group Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009; 40:e647–56 [DOI] [PubMed] [Google Scholar]
- 119.Suh JW, Yoon YE, Oh IY, Yoon CH, Cho YS, Youn TJ, Chae IH, Choi DJ. A single-center prospective randomized controlled trial evaluating the safety and efficacy of IntraCoronary Erythropoietin delivery Before Reperfusion: gauging infarct size in patients with acute ST-segment elevation myocardial infarction. Study design and rationale of the ‘ICEBEG Trial’. Contemp Clin Trials 2013; 35:145–50 [DOI] [PubMed] [Google Scholar]
- 120.Ryou MG, Flaherty DC, Hoxha B, Sun J, Gurji H, Rodriguez S, Bell G, Olivencia-Yurvati AH, Mallet RT. Pyruvate-fortified cardioplegia evokes myocardial erythropoietin signaling in swine undergoing cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2009; 297:H1914–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol 2004; 207:3233–42 [DOI] [PubMed] [Google Scholar]
- 122.Ryou MG, Liu R, Ren M, Sun J, Mallet RT, Yang S-H. Pyruvate protects brain against ischemia-reperfusion injury by activating erythropoietin signaling. Stroke 2012; 43:1101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahasi A, Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 2007; 102:163–70 [DOI] [PubMed] [Google Scholar]
- 124.Grisanti LA, Talarico JA, Carter RL, Yu JE, Repas AA, Radcliffe SW, Tang HA, Makarewich CA, Houser SR, Tilley DG. β-Adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential activation of ERK1/2 and Akt. J Mol Cell Cardiol 2014; 72:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei C, Li H, Wang Y, Peng X, Shao H, Li H, Bai S, Xu C. Exogenous spermine inhibits hypoxia/ischemia-induced myocardial apoptosis via regulation of mitochondrial permeability transition pore and associated pathways. Exp Biol Med 2016; 241:1505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hermann HP, Pieske B, Schwartzmüller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 1999; 353:1321–3 [DOI] [PubMed] [Google Scholar]
- 127.Schillinger W, Hünlich M, Sossalla S, Hermann H-P, Hasenfuss G. Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. Clin Res Cardiol 2011; 100:433–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong W, Zhang G, Qu F. Effects of Ringer’s sodium pyruvate solution on serum tumor necrosis factor-α and interleukin-6 upon septic shock. Pak J Med Sci 2015; 31:672–7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Gunaydin S. Cardiopulmonary bypass technologies In: Gourlay T, Black RA. (eds) Biomaterials and devices for the circulatory system. Oxford: Woodhead Publishing, 2010, pp. 144–172 [Google Scholar]
- 130.Olivencia-Yurvati AH, Blair JL, Baig M, Mallet RT. Pyruvate-enhanced cardioprotection during surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2003; 17:715–20 [DOI] [PubMed] [Google Scholar]
- 131.Schulze CJ, Castro MM, Kandasamy AD, Cena J, Bryden C, Wang SH, Koshal A, Tsuyuki RT, Finegan BA, Schulz R. Doxycycline reduced matrix metalloproteinase-2 activity but does not ameliorate myocardial dysfunction during reperfusion in coronary artery bypass patients undergoing cardiopulmonary bypass. Crit Care Med 2013; 41:2512–20 [DOI] [PubMed] [Google Scholar]
- 132.Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, Hardesty RL. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol 1990; 15:1261–9 [DOI] [PubMed] [Google Scholar]
- 133.Greiner M. Two-graph receiver operating characteristic (TG-ROC): update version supports optimisation of cut-off values that minimize overall misclassification costs. J Immunol Methods 1996; 191:93–4 [DOI] [PubMed] [Google Scholar]
- 134.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 2000; 45:23–41 [DOI] [PubMed] [Google Scholar]
- 135.Tape TG. Interpreting diagnostic tests, http://gim.unmc.edu/dxtests/Default.htm (accessed 11 November 2017).
- 136.Singh S, Chatterji T, Sen M, Dhayal IR, Mishra S, Husain N, Goel A, Roy R. Serum procalcitonin levels in combination with 1H NMR spectroscopy: a rapid indicator for differentiation of urosepsis. Clin Chim Acta 2016; 453:205–14 [DOI] [PubMed] [Google Scholar]
- 137.To KK, Lee KC, Wong SS, Lo KC, Lui YM, Jahan AS, Wu AL, Ke YH, Law CY, Sze KH, Lau SK, Woo PC, Lam CW, Yuen KY. Lipid mediators of inflammation as novel plasma biomarkers to identify patients with bacteremia. J Infect 2015; 70:433–44 [DOI] [PubMed] [Google Scholar]
- 138.Bünger R, Haddy FJ, Querengässer A, Gerlach E. An isolated guinea pig heart preparation with in vivo like features. Pf lugers Arch 1975; 353:317–26 [DOI] [PubMed] [Google Scholar]
- 139.Bünger R, Sommer O, Walter G, Stiegler H, Gerlach E. Functional and metabolic features of an isolated perfused guinea pig heart performing pressure-volume work. Pf lugers Arch 1979; 380:259–66 [DOI] [PubMed] [Google Scholar]