Short abstract
Vitamin B12 is synthesized only by certain bacteria and archaeon, but not by plants. The synthesized vitamin B12 is transferred and accumulates in animal tissues, which can occur in certain plant and mushroom species through microbial interaction. In particular, the meat and milk of herbivorous ruminant animals (e.g. cattle and sheep) are good sources of vitamin B12 for humans. Ruminants acquire vitamin B12, which is considered an essential nutrient, through a symbiotic relationship with the bacteria present in their stomachs. In aquatic environments, most phytoplankton acquire vitamin B12 through a symbiotic relationship with bacteria, and they become food for larval fish and bivalves. Edible plants and mushrooms rarely contain a considerable amount of vitamin B12, mainly due to concomitant bacteria in soil and/or their aerial surfaces. Thus, humans acquire vitamin B12 formed by microbial interaction via mainly ruminants and fish (or shellfish) as food sources. In this review, up-to-date information on vitamin B12 sources and bioavailability are also discussed.
Impact statement
To prevent vitamin B12 (B12) deficiency in high-risk populations such as vegetarians and elderly subjects, it is necessary to identify foods that contain high levels of B12. B12 is synthesized by only certain bacteria and archaeon, but not by plants or animals. The synthesized B12 is transferred and accumulated in animal tissues, even in certain plant tissues via microbial interaction. Meats and milks of herbivorous ruminant animals are good sources of B12 for humans. Ruminants acquire the essential B12 through a symbiotic relationship with bacteria inside the body. Thus, we also depend on B12-producing bacteria located in ruminant stomachs. While edible plants and mushrooms rarely contain a considerable amount of B12, mainly due to concomitant bacteria in soil and/or their aerial surfaces. In this mini-review, we described up-to-date information on B12 sources and bioavailability with reference to the interaction of microbes as B12-producers.
Keywords: Bioavailability, cobalamin, food source, microbial interaction, ruminant animals, vitamin B12
Introduction
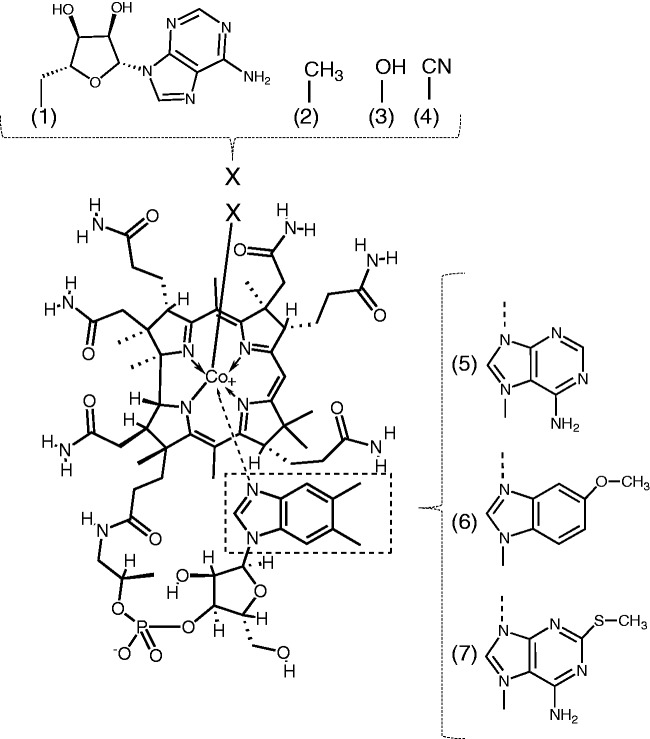
Vitamin B12 (B12) or cyanocobalamin is a member of the corrinoids that contain a corrin ring (Figure 1). Hydroxocobalamin, methylcobalamin, and 5′-deoxyadenosylcobalamin are chemically more labile than cyanocobalamin.1 In particular, methylcobalamin is the cofactor of methionine synthase (EC 2.1.1.13), and 5′-deoxyadenosylcobalamin functions as the coenzyme of methylmalonyl-CoA mutase (EC 5.4.99.2), which catalyzes the conversion of (R)-methylmalonyl-CoA to succinyl-CoA in the catabolic pathway of amino acids and odd-chain fatty acids in mammals.2,3
Figure 1.
Structural formula of vitamin B12 and partial structures of vitamin B12-related compounds. (1) 5′-Deoxyadenosylcobalamin, (2) methylcobalamin (3) hydroxocobalamin, (4) cyanocobalamin (vitamin B12), (5) pseudovitamin B12, (6) 5-methoxybenzimidazolyl cobamide, and (7) 2-methylmercaptoadenyl cobamide.
B12 is synthesized by certain bacteria and archaeon, but not by plants or animals.4 Thus, B12-synthesing bacteria (including archaeon) are sources of B12 compounds found in foods. Both aerobic5 and anaerobic6 biosynthetic pathways of B12 compounds exist. The lower ligand is attached to the cobalt-coordinated corrin ring via the nucleotide loop, and 5,6-dimethylbenzimidazole is usually found as a base. Anaerobic microorganisms can synthesize corrinoids carrying bases other than 5,6-dimethylbenzimidazole.7 Other than B12, pseudovitamin B12 (pseudoB12), which contains adenine as a base, is the only cobamide found commonly in food.8 5-Methoxybenzimidazolyl and 2-methylmercaptoadenyl cobamides are found in escargots.9
Ten years has passed since publication of my initial review concerning B12 sources and bioavailability10 in this journal. For the last 10 years, liquid chromatography/electrospray ionization–tandem mass spectrometry has been widely used to analyze B12 compounds, and various corrinoid compounds have been newly identified from food.8 In this mini-review, we describe up-to-date information on B12 sources and bioavailability with reference to the interaction of microbes as B12 producers.
Vitamin B12 in animal-derived foods
Many studies concerning the association between dietary B12 sources and serum (or plasma) B12 levels (as a marker of B12 status) indicate that meat, milk, and fish are associated with higher serum (or plasma) B12, particularly in western countries.11 Indeed, milk has been reported as the most important source of B12 for increasing serum B12 levels.11–13 Various types of animal meats (e.g. beef, veal, mutton, and lamb) are derived from the muscles of ruminant animals (e.g. cattle and sheep). The remaining major meats (pork and poultry) are derived from omnivorous animals (pig and chicken) (Figure 2). Bovine milk and fermented milk (e.g. yogurt and cheese) are widely available dairy products and good B12 sources.11
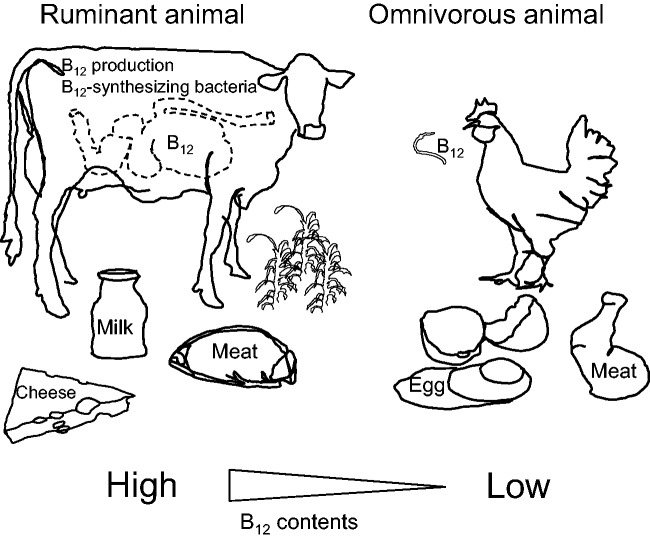
Figure 2.
Vitamin B12 sources and microbial interactions in meat, eggs, milk, and milk products. Cattle are herbivorous ruminant animals and their stomachs contain various microorganisms including B12-synthesizing bacteria. The synthesized B12 is absorbed in the intestine and stored in the liver and muscles of cattle or secreted into milk. Bovine milk and fermented milk (yogurt and cheese) are major dairy products for humans. Chickens are omnivores and eat both plants and animals that contain considerable amounts of B12. The B12 contents of raw meats are generally higher in cattle than in chicken.
Cattle and sheep are herbivores and eat plants like grass, which is free of B12. These ruminants have stomachs consisting of four chambers that contain various microorganisms, including B12-synthesizing bacteria.14,15 The B12 synthesized in the stomach is absorbed in the intestine, transferred into the blood and stored in the liver and muscles of the animal or secreted into the milk. The cobalt content of the diet is the most important factor affecting the synthesis of B12 in ruminant microorganisms.16 Thus, cobalt-deficiency readily induces B12-deficiency in ruminants.17 To enrich the B12 content of meat and milk, various methods for increasing ruminant B12 synthesis have been investigated.18–20 Pigs and chickens are omnivores and eat both plants and animals, which are B12 sources. The B12 content of raw meat is generally higher in these ruminants than in pig or chicken,21 although the B12 content of poultry meat may be increased by the administration of lactic bacteria.22 Chicken egg consumption does not appear to significantly contribute to higher serum B12 in humans.11
Meat
Raw livers of beef, pork, and chicken contain high B12 (52.8, 25.2, and 44.4 μg/100 g wet weight, respectively)21 and are excellent sources of B12. The B12 content of raw meats (approximately 1.0–2.0 μg/100 g wet weight) is higher in beef than in pork (approximately 0.5 μg/100 g wet weight) or chicken (<0.5 μg/100 g wet weight),21,23 suggesting that the meats and livers of ruminant animals contain higher amounts of B12 relative to those of omnivorous animals. A considerable loss of B12 has been reported after cooking beef, pork, and chicken meats.23–25 The retention of B12 in vacuum-cooked meats has been reported to be 100% for veal, lamb, and pork, and 87% for beef.26 For more detailed information on animal sources of B12, such as meat and dairy products, please refer to an excellent review cited in Gille and Schmid.23
Milk
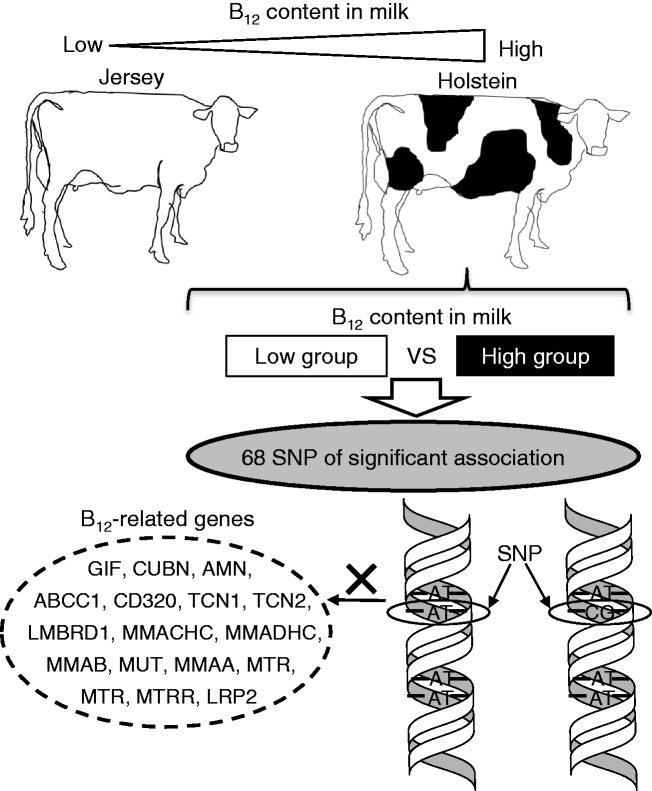
The B12 concentrations in milk from ruminants such as sheep (0.71 μg/100 g of milk), cow (0.35 μg/100 g of milk), and goat (0.06 μg/100 g of milk) are higher than those found in human milk (0.04 μg/100 g of milk).27 While the B12 content of bovine milk is not high relative to beef meats, bovine milk and fermented milk (e.g. yogurt and cheese) are major B12 sources because the intake of milk or dairy products is high in various populations.28 The B12 concentration of bovine milk varies according to many factors such as the cow type, breeding state, and milking time.29,30 B12 concentrations in milk from Holstein cows appears to be generally higher than those in milk from Jersey cows.29,30 Rutten et al.31 found that a single nucleotide polymorphism (SNP) along the cow genome affects the B12 concentration in milk (Figure 3). Although a significant association was found between 68 SNP and B12 content in the raw milk of 487 first-lactation cows, this SNP was not found in the genes known to be involved in B12 uptake or transport, implying that there are associations related to genes involved in unknown processes such as the ruminant production of B12 or the secretion of B12 by the mammary gland.31
Figure 3.
Single nucleotide polymorphism (SNP) and the vitamin B12 content of bovine milk. A significant association was found between 68 SNPs and the B12 content of raw milk in 487 first-lactation cows, but these SNPs were not in the following genes involved in B12 uptake and transport systems.
GIF: gastric intrinsic factor; CUBN: cubilin; AMN: amnionless; ABCC1: ATP-binding cassette; sub-family C; member 1; CD320: transcobalamin receptor; TCN1: transcobalaminI (haptocorin); TCN2: transcobalaminII; LMBRD1: methylmalonic aciduria cblF type 1; MMACHC: methylmalonic aciduria cblC type; MMADHC: methylmalonic aciduria cblD type; MMAB: methylmalonic aciduria cblB type; MUT: methylmalonyl CoA mutase; MMAA: methylmalonic aciduria cblA type; MTR: 5-methyltetrahydrofolate-homocysteine methyltransferase; MTRR: 5-methyltetrahydrofolate-homocysteine reductase; LRP2: low-density lipoprotein receptor-related protein 2 (megalin).
The B12 found in bovine milk mainly binds to transcobalamin, one of the mammalian B12-binding proteins located in blood,32 whereas haptocorrin is the predominant B12-binding protein in human milk.33 The bioavailability of B12 in cow’s milk appears to be higher than that of cyanocobalamin.34
When the B12 contents of 26 types of commercially available natural cheeses were determined,35 the B12 content was higher in hard and semi-hard cheeses (approximately 2.8 μg/100 g dry basis) and washed rind cheeses (approximately 4.2 μg/100 g dry basis) than in fresh (approximately 1.2 μg/100 g dry basis) or soft (approximately 1.8 μg/100 g dry basis) cheeses. Liquid chromatography/electrospray ionization—tandem mass spectrometry analysis has indicated that B12 is the predominant corrinoid compound in the tested natural cheeses, but traces of unidentified corrinoid compounds were found in some of the tested cheeses.35
An appreciable loss of B12 occurs during the storage, thermal processing, and fermentation of milk.23,25 Recently, Johns et al.36 found that the rate of B12 loss was three times greater in chocolate-flavored milk (approximately 33.5%) than in unflavored milk (approximately 15%) during heat treatment (1 h at 100°C). The increased loss of B12 in chocolate-flavored milk was attributable to cocoa polyphenols that readily form peroxides.36
The photodegradation of vitamin B2 is well known to occur in milk during light exposure.37 On exposure to light, vitamin B2 forms free radicals, which cause the color change in milk.38 A light exposure experiment of B12 indicated that B12 is decomposed by singlet oxygen formed in an aqueous solution.39 In addition, a B12 loss of 1–27% in commercially available milk products is caused by exposure to fluorescent light for 24 h at 4°C.40 These observations suggest that storage in light accelerates the degradation of both vitamin B2 and B12 in milk.
Egg
Raw and boiled whole chicken eggs contain 0.9 µg of B12 per 100 g wet weight of the edible portion,21 and most of the B12 is located in the egg yolk.41 Although hens have been fed B12-supplemented diets to enrich B12 in eggs, egg yolk B12 levels were reportedly not changed.42 Thus, the bioavailability of B12 in egg dishes is considered very low (∼10%) due to the poor absorption of B12 of eggs.43,44 Accordingly, egg intake does not significantly contribute to higher serum B12 in humans.11
An egg product called a century egg (“Pidan” in Chinese) is an alkaline-fermented ethnic food in China. The egg yolks of these eggs contain 1.9 ± 0.6 µg of B12 per 100 g wet weight. The B12 present in the yolk of century eggs was recovered completely in macromolecular fractions.45 However, approximately 52% of the free B12 was formed from the century egg yolk during in vitro gastric digestion,45 suggesting that century eggs may be a good source of B12.
Fish and shellfish
People from Japan and France obtain most (84% and 64%, respectively) of their daily B12 intake from fish and shellfish.46,47 Scheers et al.48 indicated that serum B12 levels were significantly increased in subjects ingesting fish diets compared to meat diets, suggesting that B12 is suitable as a marker for fish intake. Several studies have also indicated that fish and shellfish are important contributors to human B12 status.11,13,48
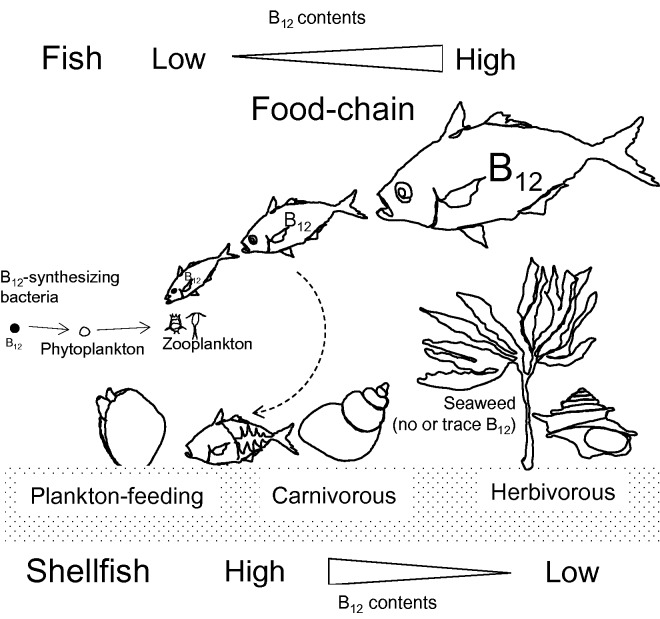
In aquatic environments, B12 produced by certain bacteria and archaea is taken up by B12-requiring bacteria, as well as eukaryotic phytoplankton,49 most of which fall as easy prey to zooplankton. Metagenomic analysis has suggested that Thaumarchaeota is the major B12 producer in aquatic environments.50 Indeed, relative to unsupplemented phytoplankton, B12-supplemented phytoplankton can significantly stimulate the growth of rotifers as a food of larval fish.51 Thus, the bacterial B12 is transferred to fish bodies via plankton and concentrated in the bodies of bigger predatory fishes in the ocean food chain (Figure 4). Thus, meat B12 content is generally higher in bigger carnivorous fish than in small body fish21: in particular, substantial amounts of B12 have been shown to be accumulated in the liver or kidney of tuna52 and salmon.53
Figure 4.
Vitamin B12 sources and microbial interactions in fish and shellfish. In aquatic environments, the B12 produced by certain bacteria and archaea is taken up by B12-requireing bacteria as well as eukaryotic phytoplankton, most of which fall as easy prey to zooplankton. The bacterial B12 is transferred to fish bodies via plankton and concentrated in the bodies of bigger predatory fishes in the ocean food chain. B12 levels were significantly higher in edible bivalves that can siphon phytoplankton than in edible snails, most of which are herbivorous, eating seaweed.
The amounts of B12 are three times greater in the viscera (approximately 37.5 μg/100 g wet weight) than in the meat (approximately 12.2 μg/100 g wet weight) of round herring.54 Approximately 73% of total B12 found in the whole fish body (except for head and bones) were recovered in the meats (approximately 5.1 μg of B12 per one body).54 Serum B12 levels of subjects consuming herring diets are significantly increased compared to meat (poultry and pork) diets48: because poultry and pork meats (less than 1.0 μg/100 g wet weight) are not high in B12.
The B12 contents of round herring and skipjack tuna meats decrease up to approximately 62% and 85% by various conventional cooking.25,48,55 However, the retention of B12 in vacuum-cooked fish has been reported to be 92% for salmon and 72% for cod.26
Shellfish, such as edible bivalves (e.g. clams, oysters, and mussels) are well known to contain substantial amounts of B12.56,57 B12 compounds have been purified from these bivalves and identified as B12.58–61 However, trace pseudoB12 and/or unidentified corrinoid compounds are rarely detected in edible bivalves61 using liquid chromatography/electrospray ionization–tandem mass spectrometry. Tanioka et al.62 have reported that B12 contents are considerably higher in edible bivalves (approximately 60 μg/100 g wet weight) than in edible snails (approximately 20 μg/100 g wet weight). There are three types of snails: sea, freshwater, and land snails.63 Most snails are herbivorous, eating plants and seaweed, while some sea snails are omnivores or carnivores. The differences in the content and B12 compounds between these edible sea snails appear to be attributable to their dietary habitats, because ivory shell (Babylonia japonica; B12 content of meat and viscera, approximately 27.2 and 92.8 μg/100 g wet weight, respectively) and turban shell (Turdo Batillus cornutus; B12 content of meat and viscera, approximately 3.0 and 15.1 μg/100 g wet weight, respectively) are carnivorous and herbivorous sea snails, respectively.64
The B12 content (0.2–0.5 μg/100 g dry weight) of seaweeds as foods of herbivorous sea snails (turban shell, T. cornutus) is very low.21 Moreover, wakame predominantly contains certain B12 analogues.65 Other herbivorous sea snails (such as abalone) mainly contain pseudoB12.66 Escargot products contain a small amount (approximately 2.2 µg/100 g wet weight) of B12 and two inactive corrinoids, which have been identified as factor IIIm (methoxymensimidazolyl cobamide) and factor S (2-methylmercaptoadenyl cobamide) using liquid chromatography/electrospray ionization–tandem mass spectrometry.67 In particular, 2-methylmercaptoadenyl cyanocobamide is reportedly the predominant corrinoid in human feces.68 These results suggest that these edible bivalves and carnivorous sea snails are good sources of B12 for humans.
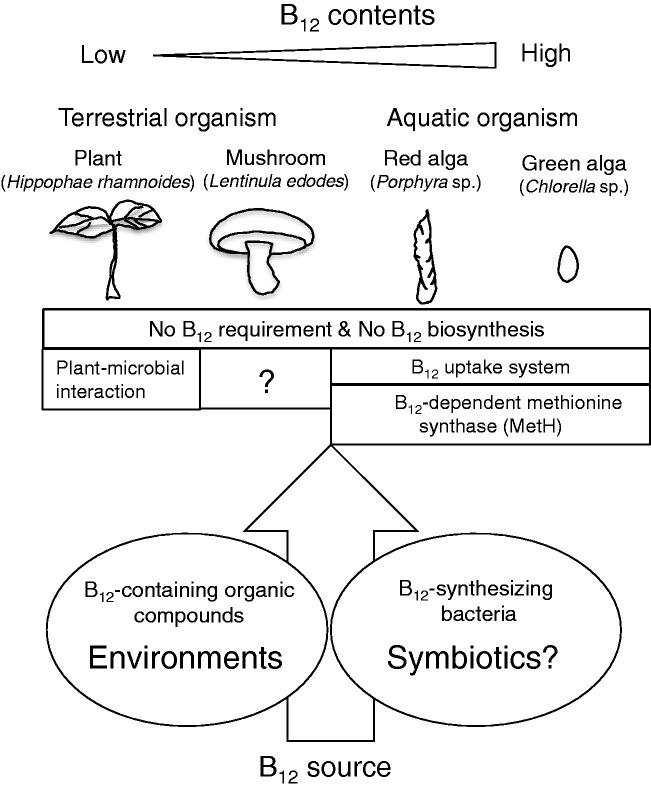
Vitamin B12 in plant-derived food
Most plants neither produce nor require B12.69 Methylotrophys inhabit soil, water, and plants70,71: in aerial surfaces of plants, Methylobacaterium sp. utilizes methanol emitted by plants; in aquatic environments, methanotrophys colonize macrophytic algae; and in soil, methonotrphs require B12 supplied from rhizobial bacteria. Furthermore, some species of Methylobacterium such as Methylobacterium extroquences NR-172 and the Methylobacterium aquaticum strain 22A73 have B12 biosynthetic pathways. Thus, plant–bacterial interactions play important roles in plant growth because B12 deficiency inhibits plant growth under nitrogen-limited conditions.70,74
B12 has also been detected in the fruiting bodies of various mushrooms that cannot synthesize B12.75 High B12 was detected in mushrooms with enhanced contact with B12-synthesizing bacteria in the soil,76 suggesting that B12 found in mushroom fruiting bodies was derived from B12 sources outside the mushrooms, such as concomitant B12-synthesizing bacteria.
As described above, in aquatic environments, phytoplankton–bacterial interactions play important roles in algal growth because half of all algae require B12.77 Even in phytoplankton or microalgae without the dependence of B12 for growth, B12 was absorbed, accumulated, and used as a cofactor of B12-dependent methionine synthase (or MetH), which has more efficient catalytic ability than B12-independent methionine synthase (or MetE)77,78 (Figure 5).
Figure 5.
Vitamin B12 sources and microbial interactions in edible plants, mushrooms, and algae. Most plants neither produce nor require B12. Methylotrophys inhabit soil, water, and plants (aerial surfaces of plants) and some of them have B12 biosynthetic pathways. Plant–bacterial interactions play important roles in plant growth. Mushrooms cannot synthesize B12, but B12 found in mushroom fruiting bodies is derived from B12 sources outside the mushrooms, including concomitant B12-synthesizing bacteria. In aquatic environments, phytoplankton–bacterial interactions play important roles in algal growth because half of all algae require B12. Even in phytoplankton or microalgae without a dependence on B12 for growth, B12 accumulates and is used as a cofactor of B12-dependent methionine synthase (MetH).
Edible plants
Sea buckthorn (Hippophae rhamnoides) berries and granulate products, sidea couch grass (Elymus repens) products (dry extract and grinded), and elecampane (Inula helenium) reportedly contain considerable amounts of B12 (approximately 11–37 μg/100 g of dry weight),79 suggesting that B12 found in these plant and plant products is due to a symbiosis with B12-synthesizing bacteria.
B12-enriched vegetables
Organic fertilizers such as cow manure appear to slightly increase the B12 content of spinach leaves (approximately 0.14 μg/100 g fresh weight).80 Our published81 and unpublished data have indicated that organic fertilizers mainly contain inactive corrinoids. B12-enriched vegetables have been prepared by treating them with a B12 solution,82,83 suggesting that free B12-supplemented vegetables may be beneficial to elderly persons because the malabsorption of protein bound B12 is most commonly seen in the elderly.84
Mushroom
Trace levels (<0.1 μg/100 g dry weight) of B12 have been found in the dried fruiting bodies of black morels, oyster mushrooms, parasol mushrooms, and porcini mushrooms.75 However, the fruiting bodies of black trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius) contain slightly higher levels of B12 (1.09–2.65 μg/100 g dry weight).75 In addition, the B12 contents of commercially available dried shiitake mushroom (Lentinula edodes) fruiting bodies significantly varied, with the average B12 value approximately 5.6 μg/100 g dry weight.85 B12 found in shiitake mushroom fruiting bodies has not been attributed to the de novo biosynthesis of B12, but appears to be derived from B12 sources outside the mushrooms, presumably concomitant B12-synthesizing bacteria or those existing in bed logs.85 Similarly cultivated white button mushroom (Agaricus bisporus) fruiting bodies contain approximately 0.2 μg of B12 per 100 g dry weight,86 with the highest B12 content found in the peel portion. B12 was also detected at similar levels in their composts. These results suggest that white button mushroom can absorb B12 from the compost or B12-synthesizing bacteria on the mushroom surface. Truffles (Tuber sp.) live in a close mycorrhizal association with the roots of specific host trees and their fruiting bodies grow underground. Indeed, B12 contents (approximately 11.5 μg of B12 per 100 g dry weight) of several truffle fruiting bodies are higher than those reported for other edible mushroom fruiting bodies.76 There is no information available on the physiological function of B12 in these mushrooms. Dried shiitake mushroom fruiting bodies rarely contain the inactive corrinoid, B12[c-lactone].85 Lion’s mane mushroom (Hericium erinaceus) fruiting bodies contain considerable amounts of B12[c-lactone].87 B12[c-lactone] binds weakly to the intrinsic factor, which is involved in the gastrointestinal absorption of B12 and inhibits the B12-dependent enzymes.88 These results suggest that these mushroom fruiting bodies are not good sources of B12 for vegetarians because of their lower B12 content and the occurrence of harmful B12[c-lactone] even in rare cases.
Red algae
The red algae Porphyra sp. is one of the most commercially available marine crops and well known as a sea vegetable.89 Various species of Porphyra are widely consumed as dried nori sheet products, which contain substantial amounts of B12 (approximately ∼77.6 μg/100 g dry weight).90 Our results91 and unpublished data have indicated that dried Chinese nori (zicai), dried New Zealand nori (karengo), dried Korean nori (kim), and canned Welsh nori (laverbread) contain approximately 60.2, 28.5, 66.8, and 2.8 μg of B12 per 100 g weight, respectively. The characterization of B12 compounds found in edible algae including Porphyra sp. have been described in the literature.10,90 Genomic analyses of Porphyra umbilicalis have suggested the physiological function of B12 as well as evolutionary insights in red algae.92 Our studies of naturally occurring plant-based foods with high B12 contents suggests that nori is the most suitable B12 source presently available for vegans.93 B12 from dried nori is significantly absorbed and functional in B12-depleted rats.94,95
Green algae
The green alga Chlorella sp. is used in human food supplements and contains biologically active B12.96–99 Recently, we analyzed B12 compounds in 19 dried Chlorella health supplements. Chlorella B12 contents varied from <0.1 μg to approximately 415 μg per 100 g of dry weight.100 Chlorella cell types of the low B12 group were aseptically grown in large culture vessels (closed culture conditions), and the other Chlorella cell types were openly grown in large culture tanks (open culture conditions). Among the Chlorella species, B12 contents were much higher in Chlorella pyrenoidosa than in Chlorella vulgaris under open culture conditions.100 Chlorella cells reportedly have an uptake system of exogenous B12.101 Thus, B12 compounds in Chlorella cells are likely derived from B12-synthesizing bacteria that are present under open culture conditions or from the addition of crystalline B12 or from B12-containing organic ingredients in the culture medium.
The coenzyme forms of B12, 5′-deoxyadenosylcobalamin (approximately 32%) and methylcobalamin (approximately 8%), were considerably present in Chlorella tablets,100 whereas cyanocobalamin was present at the lowest concentrations. Chlorella NC64A reportedly expresses homologous genes that encode B12-dependent and -independent methionine synthases and methylmalonyl-CoA mutase.102 Indeed, B12-dependent methionine synthase and methylmalonyl-CoA mutase activities were detected in cell homogenates of C. pyrenoidosa.100
We stress that if Chlorella tablets are to be consumed as a sole B12 source, Chlorella tablets with moderate or high levels of B12 must be identified using liquid chromatography/electrospray ionization–tandem mass spectrometry, because inactive corrinoid compounds (a cobalt-free corrinoid and 5-methoxybenzimidazolyl cyanocobamide) were rarely detected in some high B12-containing Chlorella tablets.100
Conclusion
B12 is synthesized by certain bacteria and archaeon, but not by plants or animals. The synthesized B12 is transferred and accumulates in animal tissues, and even in certain plant tissues via microbial interaction. Meats and milks of herbivorous ruminant animals are good sources of B12 for humans. Ruminants acquire the essential nutrient B12 through a symbiotic relationship with bacteria inside the body. In a broad sense, we (except vegetarians) also depend on B12-producing bacteria located in ruminant stomachs. In aquatic environments, most phytoplankton acquire B12 through a symbiotic relationship with bacteria. Even algae that have no requirement of B12 for growth can accumulate substantial amounts of B12 and have the ability to use B12 as a cofactor in B12-dependent methionine synthase. Then, phytoplankton becomes food for fish and bivalves in the natural food chain. Thus, humans acquire B12 formed by a microbial interaction via mainly ruminants and fish (or shellfish) as foods. Recently, it was reported that B12 is a modulator of gut microbial ecology.103 The bioavailability of food B12 is approximately 50% in healthy humans104 and unabsorbed B12 would affect intestinal microbial ecology, which is expected to have a substantial impact on human health.
Author contributions
All authors contributed equally to the preparation of this manuscript and have approved the final version.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding
This work was supported by JSPS KAKENHI Grant number 25450168 (FW) and 16K07736 (FW).
References
- 1.Watanabe F, Miyamoto E. Hydrophilic vitamins. In: Sherma J, Fried B (eds) Handbook of thin-layer chromatography, 3rd ed. revised and expanded. New York: Dekker, 2003, pp.589 − 605
- 2.Chen Z, Crippen K, Gulati S, Banerjee R. Purification and kinetic mechanism of a mammalian methionine synthase from pig liver. J Biol Chem 1994; 269:27193−7 [PubMed] [Google Scholar]
- 3.Fenton WA, Hack AM, Willard HF, Gertler A, Rosenberg LE. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Arch Biochem 1982; 228:323–9 [DOI] [PubMed] [Google Scholar]
- 4.Scheider Z, Stroiñski A. Biosynthesis of vitamin B12 In: Scheider Z, Stroiñski A. (eds) Comprehensive B12. Germany: de Gruyter, 1987, pp.93 − 110 [Google Scholar]
- 5.Heldt D, Lawrence AD, Lindenmeyer M, Deery E, Heathcote P, Rigby SE, Warren MJ. Aerobic synthesis of vitamin B12: ring contraction and cobalt chelation. Biochem Soc Trans 2005; 33:815–9 [DOI] [PubMed] [Google Scholar]
- 6.Moore SJ, Warren MJ. The anaerobic biosynthesis of vitamin B12. Biochem Soc Trans 2012; 40:581–6 [DOI] [PubMed] [Google Scholar]
- 7.Renz P. Biosynthesis of the 5,6-demethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids In: Banerjee R. (ed.) Chemistry and biochemistry of B12. New York: John-Wiley & Sons Inc, 1999, pp.557–75 [Google Scholar]
- 8.Watanabe F, Bito T. Corrinoids in food and biological samples In: Rahman A. (ed.) Frontiers in natural product chemistry ( vol.2). Sharjah: Bentham Science Publishers, 2016, pp.229–44 [Google Scholar]
- 9.Teng F, Tanioka Y, Bito T, Takenaka S, Yabuta Y, Watanabe F. Occurrence of biologically inactive corrinoid compounds in canned edible apple snail (escargots). Food Nutr Sci 2015; 6:1071–7 [Google Scholar]
- 10.Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood) 2007; 232:1266–74 [DOI] [PubMed] [Google Scholar]
- 11.Brouwer-Brolsma EM, Dhonukshe-Rutten RAM, van Wijngaarden JP, van der Zwaluw NL, van der Velde N, de Groot LCPGM. Dietary sources of vitamin B12 and their association with vitamin B12 status markers in healthy older adults in the B-PROOF study. Nutrients 2015; 7:7781–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson PW, Selhub J. Plasma vitamin B12 concentrations relate to intake source in the Framingham Offspring study. Am J Clin Nutr 2000; 71:514–22 [DOI] [PubMed] [Google Scholar]
- 13.Vogiatzoglou A, Smith AD, Nurk E, Berstad P, Crevon CA, Ueland PM, Vollset SE, Tell GS, Refsum H. Dietary sources of vitamin B12 and their association with plasma vitamin B12 concentrations in the general population: the Hordaland Homocyteine Study. Am J Clin Nutr 2009; 89:1078–97 [DOI] [PubMed] [Google Scholar]
- 14.Smith SE, Loosli JK. Cobalt and vitamin B12 in ruminant nutrition: a review. J Dairy Sci 1957; 40:1215–27 [Google Scholar]
- 15.Ortigues-Marty I, Micol D, Prache S, Dozias D, Girard CL. Nutritional value of meat: the influence of nutrition and physical activity on vitamin B12 concentrations in ruminant tissues. Reprod Nutr Dev 2005; 45:453–67 [DOI] [PubMed] [Google Scholar]
- 16.Kawashima T, Henry PR, Ammerman CB, Littell RC. Bioavailability of cobalt sources for ruminants. 2. Estimation of the relative value of reagent grade and feed grade cobalt sources from tissue cobalt accumulation and vitamin B12 concentrations. Nutr Res 1997; 17:957–74 [Google Scholar]
- 17.Stangl GI, Schwarz FJ, Jahn B, Kirchgessner M. Cobalt-deficiency-induced hyperhomocysteineaemia and oxidative status of cattle. Br J Nutr 2000; 83:3–6 [DOI] [PubMed] [Google Scholar]
- 18.Tiffany ME, Fellner V, Spears JW. Influence of cobalt concentration on vitamin B12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors. J Anim Sci 2006; 84:635–40 [DOI] [PubMed] [Google Scholar]
- 19.Kincaid RL, Lefebve LE, Cronrath JD, Socha MT, Johnson AB. Effect of dietary cobalt supplementation on cobalt metabolism and performance of dairy cattle. J Dairy Sci 2003; 86:1405–14 [DOI] [PubMed] [Google Scholar]
- 20.Bishehsari S, Tabatabaei MM, Aliarabi H, Alipour D, Zamani P, Ahmadi A. Effect of dietary cobalt supplementation on plasma and rumen metabolites in Mehraban lambs. Small Ruminant Res 2010; 90:170–3 [Google Scholar]
- 21.Standard Tables of Food Composition in Japan – 2010. The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology, Japan. Tokyo: Official Gazette Co-operation of Japan, 2010.
- 22.Al-Fataftah ARA, Herzallah SM, Alshawabkeh K, Ibrahim SA. Administration of lactic acid bacteria to enhance synthesis of vitamin B12 and B6 and lower cholesterol levels in poultry meat. J Food Agric Environ 2013; 11:604–9 [Google Scholar]
- 23.Gille D, Schmid A. Vitamin B12 in meat and dairy products. Nutr Rev 2015; 73:106–15 [DOI] [PubMed] [Google Scholar]
- 24.Ortigues-Marty I, Thomas E, Prévéraud DP, Girard CL, Bauchart D, Durand D, Peyron A. Influence of maturation and cooking treatments on the nutritional value of bovine meats: water losses and vitamin B12. Meat Sci 2006; 73:451–8 [DOI] [PubMed] [Google Scholar]
- 25.Kojima A, Ozeki A, Nakanishi T, Sato Y, Chiba T, Abe K, Umegaki K. Literature review on vitamin loss from foods during cooking (part 1) – fat soluble vitamins, and vitamin B1, B2, B6 and B12. Vitamins (in Japanese) 2017; 91:1–27 [Google Scholar]
- 26.Creed PG. The sensory and nutritional quality of ‘sous vide’ foods. Food Control 1995; 6:45–52 [Google Scholar]
- 27.Raynal-Ljutovac K, Lagriffoul G, Paccard P, Guillet I, Chilliard Y. Composition of goat and sheep milk products: an update. Small Ruminant Res 2008; 79:57–72 [Google Scholar]
- 28.Matte J, Britten M, Girard CL. The importance of milk as a source of vitamin B12 for human nutrition. Anim Front 2014; 4:32–7 [Google Scholar]
- 29.Miller J, Wentworth J, McCullogh ME. Effects of various factors on vitamin B12 content of cow’s milk. J Agric Food Chem 1966; 14:218–21 [Google Scholar]
- 30.Duplessis M, Pellerin D, Cue RI, Girard CL. Factors affecting vitamin B12 concentration in milk of commercial dairy herds: an exploratory study. J Dairy Sci 2016; 99:4886–92 [DOI] [PubMed] [Google Scholar]
- 31.Rutten MJM, Bouwman AC, Sprong RC, van Arendonk JAM, Visker MHPW. Genetic variation in vitamin B12 content of bovine milk and its association with SNP along the bovine genome. Plos One 2013; 8:e62382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedosov SN, Petersen TE, Nexø E. Transcobalamin from cow milk: isolation and physico-chemical properties. Biochim Biophys Acta 1996; 292:113–9 [DOI] [PubMed] [Google Scholar]
- 33.Burger RL, Allen RH. Characterization of vitamin B12-binding proteins isolated from human milk and saliva by affinity chromatography. J Biol Chem 1974; 249:7220–7 [PubMed] [Google Scholar]
- 34.Matte JJ, Guay F, Giard CL. Bioavailability of vitamin B12 in cow’s milk. Br J Nutr 2012; 107:61–6 [DOI] [PubMed] [Google Scholar]
- 35.Bito T, Tanabe T, Tanioka Y, Takenaka S, Yabuta Y, Watanabe F. Determination of vitamin B12 content in 26 types of natural cheeses and identification of vitamin B12 compounds in those cheeses. Vitamins (in Japanese) 2016; 90:390–4 [Google Scholar]
- 36.Johns PW, Das A, Kuil ES, Jacobs WA, Schimpf KJ, Schmitz DJ. Cocoa polyphenols accelerate vitamin B12 degradation in heated chocolate milk. Int J Food Sci Technol 2015; 50:421–30 [Google Scholar]
- 37.Allen C, Parks OW. Photodegradation of riboflavin in milks exposed to fluorescent light. J Dairy Sci 1979; 62:1377–9 [DOI] [PubMed] [Google Scholar]
- 38.Toba T, Adachi S, Arai I. Sunlight and sodium hypochloride induced color changes in milk. J Dairy Sci 1980; 63:1796–801 [Google Scholar]
- 39.Kra¨Utler B, Stepanek R. Photooxygenolysis of vitamin B12. Angew Chem Int Ed Engl 1985; 24:62–4 [Google Scholar]
- 40.Watanabe F, Katsura H, Abe K, Nakano Y. Effect of light-induced riboflavin degradation on the loss of cobalamin in milk. J Home Econ Jpn 2000; 51:231–4 [Google Scholar]
- 41.Doscherholmen A, McMahon J, Ripley D. Vitamin B12 absorption from eggs. Proc Soc Exp Biol Med 1975; 149:987–90 [DOI] [PubMed] [Google Scholar]
- 42.Bunchasak C, Kachana S. Dietary folate and vitamin B12 supplementation and consequent vitamin deposition in chicken eggs. Trop Anim Health Prod 2009; 41:1583–9 [DOI] [PubMed] [Google Scholar]
- 43.Doscherholmen A, McMahon J, Ripley D. Inhibitory effect of eggs on vitamin B12 absorption: description of a simple ovalbumin 57Co-vitamin B12 absorption test. Br J Haematol 1976; 33:261–72 [DOI] [PubMed] [Google Scholar]
- 44.Katsura H, Inui H, Doi Y. Absorption efficiency of vitamin B12 from cooked and processed foods. Its application to evaluation of vitamin B12 in the elderly. Vitamins (in Japanese) 2014; 88:267–74 [Google Scholar]
- 45.Teng F, Bito T, Takenaka S, Yabuta Y, Watanabe F. Yolk of the century egg (pidan) contains a readily digestible form of free vitamin B12. J Nutr Sci Vitaminol 2016; 62:366–71 [DOI] [PubMed] [Google Scholar]
- 46.Yoshino K, Inagawa M, Oshima M, Yokota K, Umesawa M, Endo M, Yamagashi K, Tanigawa T, Sato S, Shimamoto T, Iso H. Trends in dietary intake of folate, vitamin B6 and vitamin B12 among Japanese adults in two rural communities from 1971 through 2001. J Epidemiol 2005; 15:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourre JME, Paquotte PM. Contribution (in 2005) of marine and fresh water products (finfish and shellfish, seafood, wild and formed) to the French dietary intakes of vitamin D and B12, selenium, iodine and docosahexaenoic acid: impact on public health. Int J Food Nutr 2008; 59:491–501 [DOI] [PubMed] [Google Scholar]
- 48.Scheers N, Lindqvist H, Langkilde AM, Undeland I, Sandberg AS. Vitamin B12 as a potential compliance marker for fish intake. Eur J Nutr 2014; 53:1327–33 [DOI] [PubMed] [Google Scholar]
- 49.Bertrand EM, McCrow JP, Moustafa A, Zheng H, McQuaid JM, Delmont TO, Post AF, Sipler RE, Spackeen JL, Xu K, Bronk DA, Hutchins D. Phytoplankron-bacterial interactions mediated micronutrient colimitation at the coastal Antarctic sea ice edge. Proc Natl Acad Sci USA 2015; 112:9938–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doxey AC, Kurtz DA, Lynch MDL, Sauder LA, Neufeld JD. Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. Isme J 2015; 9:461–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi M, Yukino T, Watanabe F, Miyamoto E, Nakano Y. Effect of vitamin B12-enriched Traustochyrids on the population growth of rotifers. Biosci Biotechnol Biochem 2007; 71:222–5 [DOI] [PubMed] [Google Scholar]
- 52.Ishiharam Y, Imai E, Takenaka S, Fujita T, Yabuta Y, Watanabe F. Characterization of a corrinoid compound from pacific bluefin tuna (Thunnus orientalis) liver. Food Sci Technol Res 2011; 17:589–94 [Google Scholar]
- 53.Adachi S, Miyamoto E, Watanabe F, Enomoto T, Kuda T, Hayashi M, Nakano Y. Purification and characterization of a corrinoid compound from Japanese salted and fermented salmon kidney ‘Mefun’. J Liq Chrom Rel Technol 2005; 28:2561–9 [Google Scholar]
- 54.Nishioka M, Kanosue F, Yabuta Y, Watanabe F. Loss of vitamin B12 in fish (round herring) meats during various cooking treatments. J Nutr Sci Vitaminol 2011; 57:432–6 [DOI] [PubMed] [Google Scholar]
- 55.Nishioka M, Kanosue F, Tanioka Y, Miyamoto E, Watanabe F. Characterization of vitamin B12 in skipjack meats and loss of the vitamin from the fish meats by various cooking conditions. Vitamins (in Japanese) 2006; 80:507–11 [Google Scholar]
- 56.Watanabe F, Tanioka Y. Characterization of corrinoid compounds in edible shellfish. In: Hay RM (ed) Shellfish: human consumptions, health implications and conservation concerns New York: Nova Science Publishers, 2014, pp.413 − 20.
- 57.Watanabe F, Katsura H, Takenaka S, Enomoto E, Miyatomo E, Nakatsuka T, Nakano Y. Characterization of vitamin B12 compounds from edible shellfish, clam, oyster, and mussel. Int J Food Sci Nutr 2001; 52:263–8 [DOI] [PubMed] [Google Scholar]
- 58.Ueta K, Nishioka M, Yabuta Y, Watanabe F. TLC-B. analysis of vitamin B12 compound from the short-necked clam (Ruditapes Philippinarum) extract used as a flavoring. J Liq Chrom Rel Technol 2010; 33:972–9 [Google Scholar]
- 59.Ueta K, Takenaka S, Yabuta Y, Watanabe F. Broth from canned clams is suitable for use as an excellent source of free vitamin B12. J Agric Food Chem 2011; 59:12054–8 [DOI] [PubMed] [Google Scholar]
- 60.Ueta K, Ishihara Y, Yabuta Y, Masuda S, Watanabe F. TLC-A. of a corrinoid compound from Japanease rock-oyster “Iwa-gaki” (Crassostrea Nippona). J. Liq Chrom Rel Technol 2011; 34:928–35 [Google Scholar]
- 61.Ishihara Y, Ueta K, Bito T, Takenaka S, Yabuta Y, Watanabe F. Characterization of vitamin B12 compounds from the brackish-water bivalve Corbicula japonica. Fish Sci 2013; 79:321–6 [Google Scholar]
- 62.Tanioka Y, Takenaka S, Furusho T, Yabuta Y, Nakano Y, Watanabe F. Identification of vitamin B12 and pseudovitamin B12 from various edible shellfish using liquid chromatography-electrospray ionization/tandem mass spectrometry. Fish Sci 2013; 80:1065–71 [Google Scholar]
- 63.Dang V, Benkendorff K, Green T, Speck P. Marine snails and slugs: a great place to look for antiviral drugs. J Virol 2015; 89:8114–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teng F, Tanioka Y, Hamaguchi N, Bito T, Takenaka S, Yabuta Y, Watanabe F. Determination and chacterization of vitamin B12 compounds in edible sea snails, ivory shell Babylonia japonica and turban shell Turdo Batillus cornutus. Fish Sci 2015; 81:1105–11 [Google Scholar]
- 65.Yamada S, Shibata Y, Takeyama M, Narita Y, Sugawara K, Fukuda M. Content and characterization of vitamin B12 in some seaweeds. J Nutr Sci Vitaminol 1996; 42:497–505 [DOI] [PubMed] [Google Scholar]
- 66.Tanioka Y, Takenaka S, Furusho T, Yabuta Y, Nakano Y, Watanabe F. Characterization of vitamin B12-related compounds isolated from edible portion of abalone. Vitamins (in Japanese) 2012; 86:390–4 [Google Scholar]
- 67.Teng F, Tanioka Y, Bito T, Takenaka S, Yabuta Y, Watanabe F. Occurrence of biologically inactive corrinoid compounds in canned edible apple snail (escargots). Food Nutr Sci 2015; 6:1071–7 [Google Scholar]
- 68.Allen RH, Stabler SP. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am J Clin Nutr 2008; 87:1324–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roth JR, Lawrence JG, Bobik TA. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol 1996; 50:137–81 [DOI] [PubMed] [Google Scholar]
- 70.Iguchi H, Yurimoto H, Sakai Y. Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganims 2015; 3:137–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minamisawa K, Imaizumi-Anraku H, Bao Z, Shinoda R, Okubo T. Are symbiotic methanotrophs key microbes for N acquisition in paddy rice root? Microbes Environ 2016; 31:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyamoto E, Watanabe F, Yamaji R, Inui H, Sato K, Nakano Y. Purification and characterization of methylmalonyl-CoA mutase from a methanol-utilizing bacterium, methylobacterium extrorquens NR-1. J Nutr Sci Vitaminol 2002; 48:242–6 [DOI] [PubMed] [Google Scholar]
- 73.Tani A, Ogura Y, Hayashi T, Kimbara K. Complete genome sequence of methylobacterium aquaticum strain 22A, isolated from Racomitrium japonicum moss. Gen Ann 2015; 3:e00266–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iguchi H, Yurimkoto H, Sakai Y. Stimulation of methanotrophic growth in cocultures by cobalamin excreted by rhizobia. Appl Environ Microbiol 2011; 77:8509–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe F, Schwarz J, Takenaka S, Miyamoto T, Ohishi N, Nelle E, Hochstrasser R, Yabuta Y. Characterization of vitamin B12 compounds in the wild edible mushrooms black trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius). J Nutr Sci Vitaminol 2012; 58:50–3 [DOI] [PubMed] [Google Scholar]
- 76.Teng F, Bito T, Takenaka S, Yabuta Y, Shimomura N, Watanabe F. Determination and characterization of corrinoid compounds in truffle (Tuber spp.) and shoro (Rhizopogon rubescens) fruiting bodies. Mushroom Sci Biotechnol 2015; 22:159–64 [Google Scholar]
- 77.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005; 438:90–3 [DOI] [PubMed] [Google Scholar]
- 78.Helliwell KE. The roles of B vitamins in phytoplankton nutrition: new perspectives and prospects. New Phytol 2017; 216:62–8 [DOI] [PubMed] [Google Scholar]
- 79.Nakos M, Pepelanova I, Beutel S, Krings U, Berger RG, Scheper T. Isolation and analysis of vitamin B12 from plant samples. Food Chem 2017; 216:301–8 [DOI] [PubMed] [Google Scholar]
- 80.Mozafar A. Enrichment of some B-vitamins in plants with application of organic fertilizers. Plant Soil 1994; 167:305–11 [Google Scholar]
- 81.Bito T, Ohishi N, Takenaka S, Yabuta Y, Miyamoto E, Nishihara E, Watanabe F. Characterization of vitamin B12 compounds in biofertilizers containing purple photosynthetic bacteria. Trends Chromatogr 2012; 7:23–8 [Google Scholar]
- 82.Sato K, Kudo Y, Muramatsu K. Incorporation of a high level of vitamin B12 into a vegetable, kaiware daikon (Japanese radish sprout), by the absorption from its seeds. Biochim Biophys Acta 2004; 1672:135–7 [DOI] [PubMed] [Google Scholar]
- 83.Bito T, Ohishi N, Hatanaka Y, Takenaka S, Nishihara E, Yabuta Y, Watanabe F. Production and characterization of cyanocobalamin-enriched lettuce (Lactuca sativa L.) grown using hydroponics. J Agric Food Chem 2013; 61:3852–8 [DOI] [PubMed] [Google Scholar]
- 84.Shipton MJ, Thachil J. Vitamin B12 deficiency-A 21st century perspective. Clin Med (Lond) 2015; 15:145–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bito T, Teng F, Ohishi N, Takenaka S, Miyamoto E, Sakuno E, Terashima K, Yabuta Y, Watanabe F. Characterization of vitamin B12 compounds in the fruiting bodies of shiitake mushroom (Lentinula edodes) and bed logs after fruiting of the mushroom. Mycoscience 2014; 55:464–8 [Google Scholar]
- 86.Koyyalamudi SR, Jeong SC, Cho KY, Pang G. Vitamin B12 is the active corrinoid produced in cultivated white button mushrooms (Agaricus bisporus). J Agric Food Chem 2009; 57:6327–33 [DOI] [PubMed] [Google Scholar]
- 87.Teng F, Bito T, Takenaka S, Yabuta Y, Watanabe F. Vitamin B12[c-lactone], a biologically inactive corrinoid compound, occurs in cultured and dried lion’s mane mushroom (Hericium erinaceus) fruiting bodies. J Agric Food Chem 2014; 62:1726–32 [DOI] [PubMed] [Google Scholar]
- 88.Stabler SP, Brass EP, Marcell PD, Allen RH. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J Clin Invest 1991; 87:1422–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH. Porphyra: a marine crop shaped by stress. Trends Plant Sci 2011; 16:29–37 [DOI] [PubMed] [Google Scholar]
- 90.Watanabe F, Takenaka S, Kittaka-Katsura H, Ebara S, Miyamoto E. Characterization and bioavailability of vitamin B12 – compounds from edible algae. J Nutr Sci Vitaminol 2002; 48:325–31 [DOI] [PubMed] [Google Scholar]
- 91.Miyamoto E, Yabuta Y, Kwak CS, Enomoto T, Watanabe F. Characterization of vitamin B12 compounds from Korean purple laver (Porphyra sp.) products. J Agric Food Chem 2009; 57:2793–6 [DOI] [PubMed] [Google Scholar]
- 92.Brawley SH, Blouin NA, Kicko-Blean E, Wheeler G, Lohr M, Goodson HV, Jenkins JW, Blaby-Haas CE, Helliwell KH, Chan CX, Marriage TN, Bhattacharya D, Klein AS, Badis Y, Brodie J, Cao Y, Collen J, Dittami SM, Gachon CMM, Green BR, Karpowicz SJ, Kim JW, Kudahl UJ, Lin S, Michel G, Mittag M, Olson BJSC, Pangilinan JL, Peng Y, Qiu H, Shu S, Singer J, Smith AG, Sprecher BN, Wagner V, Wang W, Wang W, Wang ZY, Yan J, Yarish C, Zauner-Riek S, Zhuang Y, Zou Y, Lindquist EA, Grimwood J, Barry KW, Rokhsar DS, Schmutz J, Stiller JW, Grossman AR, Prochnik S. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc Natl Acad Sci USA 2017; 114:E6361−70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014; 6:1861–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takenaka S, Sugiyama S, Ebara S, Miyamoto E, Abe K, Tamura Y, Watanabe F, Tsuyama S, Nakano Y. Feeding dried purple laver (nori) to vitamin B12-deficient rats significantly improves vitamin B12 status. Brit J Nutr 2001; 85:699–703 [DOI] [PubMed] [Google Scholar]
- 95.van den Berg H, Brandsen L, Sinkeldam BJ. Vitamin B12 content and bioavailability of spirulina and nori in rats. J Nutr Biochem 1991; 2:314–8 [Google Scholar]
- 96.Kittaka-Katsura H, Fujita T, Watanabe F, Nakano Y. Purification and characterization of a corrinoid compound from Chlorella tablets as an algal health food. J Agric Food Chem 2002; 50:4994–7 [DOI] [PubMed] [Google Scholar]
- 97.Yang SD, Feng YJ, Fu W. Determination of cobalamin in Chlorella food by cation exchange column and graphite furnace atomic absorption spectrometry. J Food Drug Anal 2006; 14:50–3 [Google Scholar]
- 98.Chen JH, Jiang SJ. Determination of cobalamin in nutritive supplements and chlorella foods by capillary electrophoresis-inductively coupled plasma mass spectrometry. J Agric Food Chem 2008; 56:1210–5 [DOI] [PubMed] [Google Scholar]
- 99.Kumudha A, Selvakumar S, Dilshad P, Vaidyanathan G, Thakur MS, Sarada R. Methylcobalamin-A form of vitamin B12 identified and characterized in Chlorella vulgaris. Food Chem 2015; 170:316–20 [DOI] [PubMed] [Google Scholar]
- 100.Bito T, Bito M, Asai Y, Takenaka S, Yabuta Y, Tago K, Ohnishi M, Mizoguchi T, Watanabe F. Characterization and quantitation of vitamin B12 compounds in various Chlorella supplements. J Agric Food Chem 2016; 64:8516–24 [DOI] [PubMed] [Google Scholar]
- 101.Watanabe F, Abe K, Takenaka S, Tamura Y, Maruyama I, Nakano Y. Occurrence of cobalamin coenzymes in the photosynthetic green alga, Chlorella vulgaris. Biosci Biotechnol Biochem 1997; 61:896–7 [DOI] [PubMed] [Google Scholar]
- 102.Helliwell KH, Wheeler GL, Leptos KC, Goldstein RE, Mith AG. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol Biol Evol 2011; 28:2921–33 [DOI] [PubMed] [Google Scholar]
- 103.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab 2014; 20:769–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Institute of Medicine. Vitamin B12 In: Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: Institute of Medicine, National Academy Press, 1998, pp.306–56 [PubMed]