Short abstract
Abnormal differentiation and growth of hematopoietic stem cells cause the development of hematopoietic diseases and hematopoietic malignancies. However, the molecular events underlying leukemia development are not well understood. In our recent study, we have demonstrated that calcium ionophore and phorbol ester force the differentiation of T lymphoblastic leukemia. The event involves a newly identified IκBα/WWOX/ERK signaling, in which WWOX is Ser14 phosphorylated. Additional evidence also reveals that pS14-WWOX is involved in enhancing cancer progression and metastasis and facilitating neurodegeneration. In this mini-review, we update the current knowledge for the functional roles of WWOX under physiological and pathological settings, and provide new insights regarding pS14-WWOX in T leukemia cell maturation, and switching the anticancer pY33-WWOX to pS14-WWOX for cancer promotion and disease progression.
Impact statement
WWOX was originally designated as a tumor suppressor. However, human newborns deficient in WWOX do not spontaneously develop tumors. Activated WWOX with Tyr33 phosphorylation is present in normal tissues and organs. However, when pY33-WWOX is overly induced under stress conditions, it becomes apoptotic to eliminate damaged cells. Notably, WWOX with Ser14 phosphorylation is upregulated in the lesions of cancer, as well as in the brain hippocampus and cortex with Alzheimer’s disease. Suppression of pS14-WWOX by Zfra reduces cancer growth and mitigates Alzheimer’s disease progression, suggesting that pS14-WWOX facilitates disease progression. pS14-WWOX can be regarded as a marker of disease progression.
Keywords: WWOX, phosphorylation, cancer, neurodegeneration, apoptosis, bubbling cell death
Introduction
The skin and immune systems are the first and second lines of defense that protect human body from microbial or viral infection.1 This highly cooperative system comprises the innate and adaptive immune systems. The mucosal system possesses the complement proteins, inflammatory cytokines, and immune cells of the innate immune system to protect from pathogen invasion. The adaptive immune system provides a long-lasting and highly specific immune reaction. In this system, T- and B-lymphocytes are activated by the antigen-presenting cells (APCs) and/or dendritic cells (DCs). Both cells recognize and process the specific foreign antigen and execute its immune functions, including T-cell mediated cytotoxicity and specific antibody-dependent elimination of the particular antigen or pathogen.
Normal and aberrant T cell development
In murine, T-cell development starts from the differentiation of the bone marrow pluripotent hematopoietic stem cells (HSCs). These unique HSCs differentiate into the precursor T-cells and migrate to the thymus for further maturation. In the special microenvironment of thymic cortex, the precursor T-cells (CD44low) differentiate into the CD4−CD8− double negative (DN) cells and then go through four stages: I, CD44+CD25−; II, CD44+CD25+; III, CD44−CD25+ with the presence of pre-T-cell receptor (pre-T-cell receptor) expression; IV, CD44−CD25−.2 Post these differentiation processes, T-cells express CD4 and CD8 molecules in the cell membrane and become CD4+CD8+ double positive (DP) cells. Later, those DP T-cells are subjected to positive selection to eliminate TCR-deficient cells in the cortex and negative selection to eliminate self-reactive cells in the medulla.3–6 The surviving T-cells become matured, including CD4+, CD8+ single positive (SP), regulatory T cells (Treg), and natural killer-like T cells (NKT). These mature T cells circulate in the blood stream or reside in the lymph nodes to execute their respective immune functions. In humans, T-cell development starts from the differentiation of the CD34+, CD7+, and CD5+ HPCs, followed by migrating to the thymus and going through the developmental processes similar to that of the murine system, thus leading to the production of functional CD4 or CD8 T-cells.7–9 However, abnormal differentiation of T-cells causes leukemia, which is an aggressive hematologic tumor. Translocation and aberrant expression of transcription factors or recombinant kinases attribute to the development of the disease.10–12 Participation of nuclear factor kappaB (NF-κB) has been implicated in the development of leukemia.13–15
Role of NF-κB in T cell development
NF-κB participates in the T-cell differentiation and positive/negative selection.3,16–18 The NF-κB protein family consists of c-Rel, p65 (RelA), RelB, p50/p105 (NF-κB1), and p52/p100 (NF-κB2). During activation, the highly conserved N-terminal Rel homology (RHD) domain mediates its binding to the κB elements in the DNA sequence. Based on the differences at the C-terminal domain, the class I NF-κB protein family includes p50/p105 and p52/p100. Each of them contains an ankyrin repeat-containing transrepression domain (TRD), and represses the transcription of κB elements. The class II members RelA, RelB, and c-Rel bear a tranactivation domain (TAD), which mediates active transcription.19 NF-κB activation is needed for cell survival and tissue inflammation and is regulated in a fine-tuned manner. IκBα is an inhibitor of NF-κB and belongs to the IκB protein family, including IκBβ, IκBγ, IκBε, and Bcl-3.20,21 IκBα participates in immune cell development. Newborn IκBa−/− mice survive for less than 10 days and possess an altered population of double-positive thymocytes and increased populations of granulocytes and macrophages.22 Thymocyte development toward maturation is severely impaired in the transgenic mice overexpressing truncated IκBα. This truncated form lacks 36 amino acids at the N-terminus, and yet possesses an increased inhibitory activity against NF-κB. Nevertheless, the CD8 lineage of thymocytes is most affected.23,24 Together, IκBα is crucial in immune cell development, whereas the molecular event needs further elucidation.
Tumor suppressor WW-domain containing oxidoreductase WWOX
The rationale for focusing on WWOX is its control of immune cell maturation,25 cancer progression,26 and neuronal injury and degeneration.27 We will update the current knowledge with immune cell maturation and mention the documented role of WWOX in cancer and neural diseases.28 WWOX/Wwox gene encodes a tumor suppressor WW domain-containing oxidoreductase, known as human WWOX or FOR and murine WOX1.29–31 Human WWOX gene is located on the common fragile site FRA16D on chromosome 16q23.2. This gene encodes approximately 1 million bases and contains nine exons and transcribes a spliced 2.2 kb mRNA. Most recently, multiple PARTICLE (Gene PARTICL ‘Promoter of MAT2A-Antisense RadiaTion Induced Circulating LncRNA) triplex clusters are found in the WWOX gene.32 PARTICLE affects remote loci in the human genome and the ability of lncRNAs to regulate the expression of numerous genes. WWOX protein possesses 414 amino acids and has two conserved N-terminal WW-domains, a C-terminal short-chain alcohol dehydrogenase/reductase (SDR) domain, and a nuclear localization signal (NLS) in between the WW domains.29–31 A proapoptotic D3 region is located at the C-terminal tail.33,34
WWOX gene alterations in cancer, metabolic disorders, and neural diseases
Null mutations in the WWOX/Wwox gene lead to complete loss of protein expression. Loss of WWOX expression has been reported in breast,35–38 esophageal,39–41 lung,42,43 ovarian,44,45 colon,46 prostate,47,48 and gastric49 carcinomas. Alternative transcripts of WWOX are found in breast cancer.50 However, truncated proteins of less than 41 kDa are not stable. They tend to be readily subjected to proteosomal degradation. Epigenetic modification of the CpG island of WWOX gene by methylation that leads to null or altered expression has been shown in breast cancer and other types of cancer.51 Loss of WWOX gene also contributes the development of osteosarcomas in Wwox heterozygous mice and lung papillary carcinoma in the Wwox knocked out mice.52 Heterozygous Wwox mice are more susceptive to chemical mutagen-induced gastric cancer and lymphoma,49,52 implying the crucial role of WWOX in the anti-tumor response. The mutagens include N-nitrosomethylbenzylamine and ethyl nitrosourea. Conditional Wwox knockout mice suffer severe metabolic defect, growth retardation, reduced bone volume, hypocapnia, impaired hematopoiesis, leukopenia, and splenic atrophy.53 Altered or loss expression of WWOX protein has been found in 51% of leukemia patients and 55% of leukemic cell lines.54
Null mutations of WWOX/Wwox in humans, mice, and rats lead to severe neural diseases (e.g. epilepsy, microcephaly, retinal degeneration, ataxia, and etc.), metabolic disorders associated with lipid, cholesterol and glucose metabolism, and early death.27,28,55,56 However, no spontaneous tumor growth is shown in the newborns of humans and rats. Studies have been limited using WWOX/Wwox-deficient animals due to death of embryos in utero. WWOX loss leads to alterations in cancer cell adhesion to the extracellular matrix, and this affects cell migration and metastasis.44 Also, under WWOX-deficiency, normal protein conformation may be subjected to changes, which results in tumor growth inhibition. Indeed, when WWOX is downregulated, a cascade protein aggregation occurs for leading to amyloid β formation and tau tangle generation.27,28,57–60 The protein cascade includes TRAPPC6AΔ (TPC6AΔ; Trafficking protein particle complex 6A delta), TIAF1 (TGFβ1-Induced Anti-Apoptotic Factor 1), and SH3GLB2 (SH3 Domain Containing GRB2 Like, Endophilin B2).57,60–62 Oddly, there is an inverse relationship between the occurrence of Alzheimer’s disease (AD) and cancer.63,64 The greater the extent of protein aggregation in AD, the less likely the incidence of cancer. Cancer cell survival is not affected by intracellular protein aggregation.61 However, cancer cells in the brain induce neuronal death due to TIAF1 aggregation in the neurons.61 We have reported cancer-induced neurodegeneration in mice.62 However, cancer patients are unlikely to survive long enough to develop neurodegeneration.
In the lethal dwarfism and epilepsy lde/lde rat, there is a 13-bp deletion in the exon 9 of Wwox gene.65 The deletion causes a frameshift of the Wwox gene and the produced WWOX protein has an altered amino acid sequence at the C-terminus. Wwox knockout mice survive for three weeks,58,66 and the lde/lde rats die before maturation (within 3 to 12 weeks). No spontaneous tumor growth is shown in the lde/lde rat.65 Moreover, these rats have accumulated the extracellular vacuoles in the hippocampus,65,66 suggesting the occurrence of progressive neuronal degeneration and eventual death. WWOX is significantly downregulated in AD patients.59,67 Downregulation of WWOX in the hippocampi starts to occur in the mid-aged humans, and this may lead to slow aggregation of TRAPPC6AΔ and TIAF1 for caspase activation and mitochondrial damage, followed by degradation of amyloid precursor protein (APP), amyloid β formation, and tau tangle formation as shown in AD patients at age 70 and greater.57–59
lde/lde rats exhibit a significantly decreased number of spermatocytes, abnormal differentiation of Leydig cells, and low testosterone concentration in the plasma.65 WWOX binds sex steroid hormones such as estrogen and androgen, and may act as a cytosolic hormone receptor.68,69 Relocation of the estrogen/WWOX complex, along with p53, to the nucleus allows transcription of specific genes to support cell growth and differentiation. Without WWOX, it is not surprising to observe the defect in spermatogenesis.
Upregulation of WWOX in the early stage of cancer progression
Much less attention has been paid to the WWOX expression in the hyperplasia tissues prior to progression toward cancerous and metastatic stages. During the acute phase of UVB irradiation-induced skin squamous cell carcinoma (SCC) in hairless mice, WWOX is significantly upregulated and activated with Tyr33 phosphorylation in 24 h.70 Presumably, activated WWOX struggles to block cancerous progression, eliminate damaged cells by apoptosis, and suppress the inflammatory functions of NF-κB.70 Later, during the chronic phase, UVB-treated mice develop cutaneous SCCs in three months, with significant downregulation of WWOX and its activated form. Similarly, breast cancer progression to a premetastatic state is associated with WWOX upregulation and activation, followed by significant downregulation or absent expression during metastasis.69 Estrogen participates in the upregulation and activation of WWOX.69
WWOX structure, signaling networks, and physiological and pathological events
WW domain serves in protein/protein interactions in many signal pathways (e.g. Hippo pathway). WW domain is a compact protein module ranging from 35 to 40 amino acids and contains two conserved tryptophan (W) residues that are spaced apart by ∼20 amino acids.71–75 There are at least 52 WW domain-containing proteins identified in the human proteome (Supplementary Table 1), and more than 10,000 among all species. Both WW domain and Src homology domain 3 (SH3) recognize the proline-rich region of protein ligands. However, no consensus sequence in the binding targets is shown by both domains.72,73 There are at least four identified subgroups of WW domain. Each WW domain recognizes the specific proline-rich sequence.74,75
WWOX interacts with one or more than one proteins in each signaling paths (Figure 1). The N-terminal WW domains of WWOX, belonging to the Group I WW domain, preferably bind PPxY- or PPPY-containing proteins.26,74–77 PPxY-motif proteins include p73,66 AP-2γ,78 ErbB4,79 cJun,80 Runx2,43 Ezrin,81 TMEM207,82 and others (Figure 1). The binding interaction involves the first WW domain with each target protein, in which the secondary WW domain assists the first WW domain for the binding.83 When WWOX is transiently overexpressed in the cytoplasm, the ectopic WWOX strongly binds the aforementioned transcription factors and blocks their oncogenic activity for suppressing cancer cell survival. Unfortunately, this does not appear to be the case in vivo. Metastatic cancer cells frequently lose the expression of WWOX.26,69,77 Also, when rats are subjected to sciatic nerve dissection, endogenous WWOX is rapidly upregulated during the acute phase and binds and relocates together with transcription factors (e.g. CREB and cJUN) to the nucleus to support neuronal survival or cause death.80 WW domain-containing Yes-associated protein (YAP) interacts with tyrosine kinase ErbB4 and p73, and acts as a coactivator for transcription. Ectopic WWOX inhibits the nuclear translocation and transcriptional activity of both proteins.79 To control YAP activation, the upstream tumor suppressors LATS1/2 kinases form complexes with the WW domain-containing N-terminus of YAP.84 Together, WWOX participates in numerous physiological events due to its interactions with functional proteins.
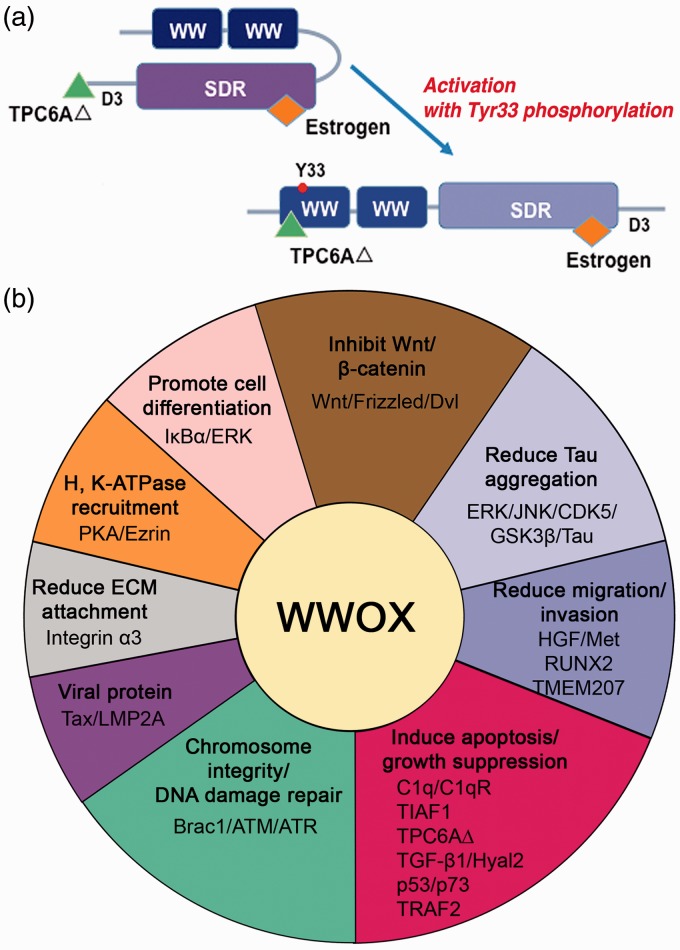
Figure 1.
WWOX in signaling networks. (a) WWOX is composed of two N-terminal WW domains and a C-terminal SDR domain. In a native-folded conformation, the WW domains bind the SDR domain. Under stress condition, WWOX becomes Tyr33 phosphorylated, and the protein unfolded.59 An NSYK motif in the SDR domain binds estrogen or androgen.68,85 The C-terminal D3 tail is proapoptotic.33 D3 binds TRAPPC6AΔ (TPC6AΔ) to prevent aggregation in the brain cortex such as in Alzheimer’s disease.57–59 However, once activated, the unfolded WWOX utilizes its first WW domain to bind TPC6AΔ.57–59 (b) A schematic pie graph shows the representative signaling networks that WWOX is involved in. WWOX interacts with many proteins in each signaling network. See text for details. (A color version of this figure is available in the online journal.)
WWOX expression, activation and degradation
WWOX expression is induced by hyaluronidases PH-20, HYAL1 and HYAL2,31 and apoptotic stresses.31,85–88 Antisense mRNA, dominant negative, and small interfering RNA (siRNA) suppress WWOX and protect cells from apoptosis.31,70,85,88 When cells are exposed to tumor necrosis factor (TNF), anisomycin, UV irradiation, transforming growth factor β (TGF-β), and other apoptotic stresses, WWOX is upregulated and readily becomes activated via Tyr33 phosphorylation.87 Tyrosine kinase Src appears to mediate the Tyr33 phosphorylation.66 Activated pY33-WWOX is upregulated in vivo during neuronal damage,27 retinal degeneration,89 traumatic brain injury,90 initial stage of skin and breast cancer progression,69,70 and pterygium progression and recurrence.91 However, pY33-WWOX is downregulated during the progression of AD.27,67 In small cell lung cancer, Bmi1 (B Lymphoma Mo-MLV Insertion Region 1 Homolog) targets the expression of WWOX to facilitate cancer cell growth.92 Notably, a novel fusion protein of PVT1-WWOX, resulting from fusion of PVT1 exon 1 to WWOX exon 9, is present in the 8q24 rearrangement-positive multiple myeloma.93
The activated pY33-WWOX gains an increased capability in binding a broad spectrum of proteins in various signaling pathways such as Hyal-2, p53, Smad4, ERK, JNK, GSK3β, IκBα, p73, and many others.85,87,94 Anisomycin and UV light activate JNK1 for binding and functionally blocking pY33-WWOX.87 Also, UV induces the binding of pY33-WWOX with pS46-p53, and both proteins cause apoptosis in a synergistic manner.31,87 While p53 and WWOX are partners in apoptosis and cancer suppression,85–88 loss of p53 and WWOX drives the development of osteosarcoma in a double knockout mouse model.95 Estrogen also initiates the binding of pY33-WWOX with pS15-p53, and that apoptosis occurs if the concentration of estrogen is raised up to micromolar levels.68 Intracellular activated Cdc42-associated kinase (ACK1) negatively regulates WWOX by phosphorylating Tyr287 in prostate cancer, which leads to WWOX polyubiquitination and proteosomal degradation.96 Overall, activated WWOX with Tyr33 phosphorylation controls the progression of cancer and AD in vivo.
WWOX in signaling networks
Among others (Figure 1), WWOX induces human ovarian cancer cell detachment from the extracellular matrix and thus leads to apoptosis by reducing the expression of integrin α3.44 The hepatocyte growth factor (HGF)/Met pathway is deregulated in advanced breast cancer. Ectopic WWOX inhibits the nuclear translocation and transcriptional activity of Met.97 WWOX is also an inhibitor of the Wnt/β-catenin pathway. High β-catenin transcriptional activity correlates with poor prognosis of breast cancer patient. WWOX inhibits this pathway by preventing the nuclear translocation of Dishevelled and decreasing the stability of β-catenin.98 In parietal cell, binding of Ezrin with WWOX is essential for the membrane-cytoskeleton remodeling and H,K-ATPase membrane translocation and insertion during cell activation.81 The small integral membrane protein of the lysosome/late endosome (SIMPLE) binds WWOX in the Golgi apparatus, whereas the functional implication is unknown.99 Overall, the aforementioned studies showed that transiently overexpressed WWOX sequesters many transcription factors in the cytoplasm to render cancer growth inhibition.
Endogenous WWOX/MEK1 complex is a molecular switch for cancer cell death
Endogenous WWOX and its binding partners exhibit physiological effects in vivo. For example, WWOX physically binds MEK1 in the cytoplasm and, in part, in the lysosomes of leukemia T cells.34 Dissociation of the WWOX/MEK1 complex by phorbol myristate acetate (PMA) leads WWOX to relocate to the mitochondria to induce apoptosis, and MEK1 to the lipid rafts.34 Conceivably, WWOX/MEK1 is a switch for turning on/off death in cancer cells. Appropriate chemicals can be designed to dissociate the WWOX/MEK1 complex for causing cancer death in vivo.
WWOX in the bubbling cell death
We have recently discovered a novel type of cell death, termed bubbling cell death (BCD), caused by UV irradiation and cold shock.100,101 This study concerns the devastating effect of frostbite. When ambient temperature surrounding dying cells drops from 37°C to 22°C or down to 4°C, apoptosis stops. Cells choose to die by BCD. During BCD, each cell generates “a nitric oxide-containing bubble” from the nucleus. The bubble relocates to the cell surface and is then released from the cell. Ultimately, the cell dies. Unlike apoptosis, there are no phosphatidylserine flip-over, caspase activation for mitochondrial apoptosis, damage to Golgi complex, and chromosomal DNA fragmentation in BCD. Rapid release of extracellular vesicle/exosome-like materials is found in cells undergoing BCD. When cells receive UV irradiation, followed by brief cold shock at 4°C, many cytosolic and membrane proteins rapidly relocate to the nucleus. Among these, UV-induced pY33-WWOX and pS46-p53 are essential for executing BCD. As a protective mechanism, UV activates TNF receptor-associated factor-2 (TRAF2) to antagonize cell death mediated by activated WWOX and p53. Detailed interactions among WWOX, p53, TRAF2, and other proteins are being determined.28,100,101
Hyal-2/WWOX/Smad4 signaling for hyaluronan and TGF-β
WWOX is anchored, in part, in the membrane/cytoskeleton area by membrane Hyal-2,102 TMEM207,82 and Ezrin.81 WWOX binds the C-terminal PPxY motif in the transmembrane protein 207 (TMEM207) and this binding appears to nullify the tumor suppressor function of WWOX and facilitates the invasiveness of gastric signet-ring cell carcinoma. In our recent review,94 we have described that both hyaluronan and TGF-β1 utilize the non-canonical Hyal-2/WWOX/Smad4 signaling to drive the SMAD promoter activity and control cell growth and death.76,94,102 For example, during traumatic brain injury in rats, dramatic accumulation of the Hyal-2/WWOX complex in the nuclei of apoptotic neurons occurs.90 In vitro analysis revealed the indispensable role of Hyal-2 and WWOX in conferring cell death.90 Naturally occurring high molecular weight hyaluronan does not effectively induce cell death, whereas hyaluronan signals the ectopic Hyal-2/WWOX/Smad4 complex to cause BCD,90,100,101 suggesting that when the level of endogenous Hyal-2/WWOX/Smad4 complex is upregulated, extracellular matrix hyaluronan may exert cell death via the Hyal-2/WWOX/Smad4 signaling.
C1q/WWOX in the non-inflammatory anticancer response
WWOX is responsive to activation by serum complement C1q of the humoral immune system to limit cancer growth.103–105 The classical complement system is involved in the antibody-dependent inflammatory response against invading pathogens. When exogenous C1q interacts with its membrane receptor in prostate and neuroblastoma cancer cells, the binding transmits a signal to WWOX to carry out an untypical type of apoptosis in cancer cells in vitro.103 In vivo experiments also showed the efficacy of complement C1q in curbing the growth of breast cancer cells via WWOX activation.103–105 Presence of C1q in organs prevents them from carcinogenesis progression.103 That is, downregulation of C1q in the prostate enhances the prostate hyperplasia toward cancerous formation due to lack of WWOX activation.103 Whether C1q receptor binds Hyal-2 that triggers the downstream WWOX/Smad4 signaling for executing cancer cell death remains to be established.
WWOX in antiviral response
WWOX is involved in host defense against viral and bacterial infections.106 Human T-Lymphotropic Virus 1 (HTLV-1) contributes to the development of adult T-cell leukemia. HTLV-1-encoded oncogenic Tax protein physically binds WWOX that regulates the NF-κB signaling.107 WWOX suppresses the Tax-induced activation of canonical NF-κB signaling by blocking IKKα-mediated phosphorylation of NF-κB at the subunit RelA. However, Tax represses WWOX expression via the non-canonical NF-κB signaling mediated by p100/p52.
Epstein-Barr virus (EBV) participates in the development of nasopharyngeal carcinoma and B-cell lymphomas.108,109 Oncoprotein latent membrane protein 2A (LMP2A) encoded by EBV supports cancer growth, survival, and metastasis. The first WW domain of WWOX physically binds the PPPPY motifs in LMP2A, and the binding facilitates the ERK-Fra-1-MMP9 signaling, increases MMP9 production, and promotes cancer metastasis.110 In the absence of LMP2A, WWOX exerts negative effects on the MEK1-ERK signaling and suppresses cancer growth and invasion, suggesting that LMP2A facilitates viral proliferation via inhibition of WWOX function.110
WWOX in chromosomal stability
Loss of WWOX frequently links to tremendous chromosome instability and gene deletion, which might provide a selective advantage for neoplasm.111–113 There are at least three mechanisms that participate in repairing damaged DNA, namely non-homologous end joining (NHEJ), microhomology-mediated end joining (MMEJ), and homologous recombination (HR). NHEJ uses a short nucleotide sequence as a guide to anneal with the other strand of DNA.114 Owing to this annealing which is not specific to the homologous sequence, NHEJ frequently causes nucleotide deletion or translocation. Similar to NHEJ, MMEJ trims the breaking ends in both strands and anneals both stands through the microhomologous region present in both ends. However, this mechanism causes more nucleotides loss and contributes to extend nucleotide deletion. In contrast, HR uses the homologous sequence in the sister chromatid as the template to repair the damaged strand.
When double-strand break (DSB) occurs, WWOX associates with phosphorylated ataxia telangiectasia-mutated (ATM), triggers the downstream damage responses, and repairs damaged chromosomes. While Lys63-ubiquitination promotes the stability and nuclear translocation of WWOX, ubiquitination at Lys274 plays a critical role in the activation of WWOX to associate with ATM in the MMEJ.115,116 WWOX modulates the activity of ATM and Rad3-related protein (ATR) in response to single-strand break (SSB).117 Intriguingly, loss of WWOX enhances HR upon cisplatin and radiation treatment in breast cancer. Mechanistically, WWOX competes with Rad50, one of the components of MRN complex, for binding to Brac1 (Breast cancer 1).118 Therefore, WWOX hampers the HR as mediated by Brac1 and promotes NHEJ in WWOX-expressing cells.118
Inhibition of WWOX signaling by JNK1 and Zfra
Both JNK1 and Zfra are known inhibitors of WWOX signaling. JNK1 blocks the apoptotic function of p53 and WWOX.85,86 Zfra, known as zinc finger-like protein that regulates apoptosis, is a 31-amino-acid protein capable of counteracting apoptosis mediated by WWOX and p53.33 Synthetic Zfra peptides, Zfra1–31, and Zfra4–10, prevent and block the growth of many types of cancer cells,119 and restore memory loss in triple transgenic mice by blocking the aggregation of TRAPPC6AΔ (TPC6AΔ), SH3GLB2, tau and amyloid β, as well as inflammatory NF-κB activation.62 Zfra covalently interacts with intracellular proteins and accelerates their degradation independently of ubiquitination and proteosomal degradation.62 Zfra binds WWOX to specific sites in the WW and SDR domains at the N- and C-termini, respectively.33 Whether Zfra accelerates WWOX degradation to abolish signaling remains to be established.
WWOX in forced T-cell maturation via a novel IκBα/WWOX/ERK signal pathway
Although WWOX loss is frequently found in neoplasm, clinical findings point out that WWOX loss in leukemia is not as frequent as other non-hematopoietic neoplasms. Indeed, loss of WWOX protein in acute lymphoblastic leukemia (ALL) patients is due to promoter hypermethylation of WWOX gene.120,121 FHIT and p73 genes are also hypermethylated in ALL. Restoration of WWOX gene reduces leukemic tumorigenesis.122 Phorbol ester-mediated dissociation of WWOX/MEK1 complex leads to leukemia cell death.34 WWOX gene is located on the chromosomal fragile site FRA16D, and alterations in this site lead to loss or alterations of WWOX protein as shown in leukemia patients and leukemic cell lines.54,123 By gene trap technology, Wwox gene knockout mice are susceptible to develop B-cell lymphomas.124 While WWOX is involved in maintaining chromosomal stability, loss of WWOX may contribute to DNA breaks and fusion of chromosomes such as PML/RARα for promyelocytic leukemia (PML) and retinoic acid receptor alpha (RARα) genes in acute promyelocytic leukemia (APL).125
We have recently identified a novel IκBα/WWOX/ERK signal pathway for forced T cell maturation (Figure 2(a)).25 Upon exposure to calcium ionophore A23187 and phorbol ester (IoP), human ALL MOLT-4 cells undergo terminal maturation toward T cell phenotype via the IκBα/WWOX/ERK signaling (Figure 2(a)).25 In resting MOLT-4, an endogenous pY33-WWOX/IκBα/ERK complex is present in the cytoplasm and mitochondria. Mapping by co-immunoprecipitation and yeast two-hybrid analysis reveals that pY33-WWOX binds ERK and non-PEST (domain rich in proline, glutamate, serine and threonine) area of IκBα.25 IoP rapidly causes WWOX de-phosphorylation at Tyr33 and Tyr287. Meanwhile, phosphorylation of ERK and IκBα occurs in the complex in 5 min or less. WWOX then becomes Ser14 phosphorylated. pS14-WWOX causes instability of the complex. p-IκBα undergoes polyubiquitination/proteosomal degradation, and ERK de-phosphorylation occurs in the next 3–12 h. Later, a portion of WWOX and ERK re-associates and translocates to the nucleus to induce the expression of T cell maturation antigens CD3 and CD8 in 15–24 h. Inhibition of ERK phosphorylation by U0126 or restriction of IκBα degradation by MG132 abolishes the MOLT-4 maturation.25 In addition to WWOX, specific site phosphorylation in the WW domain (e.g. in Pin1 and YAP1) that confers biological functions has been shown.126,127 However, switches in phosphorylation and de-phosphorylation among specific sites in WW domain and non-WW domain areas that alter protein functions are largely unknown.
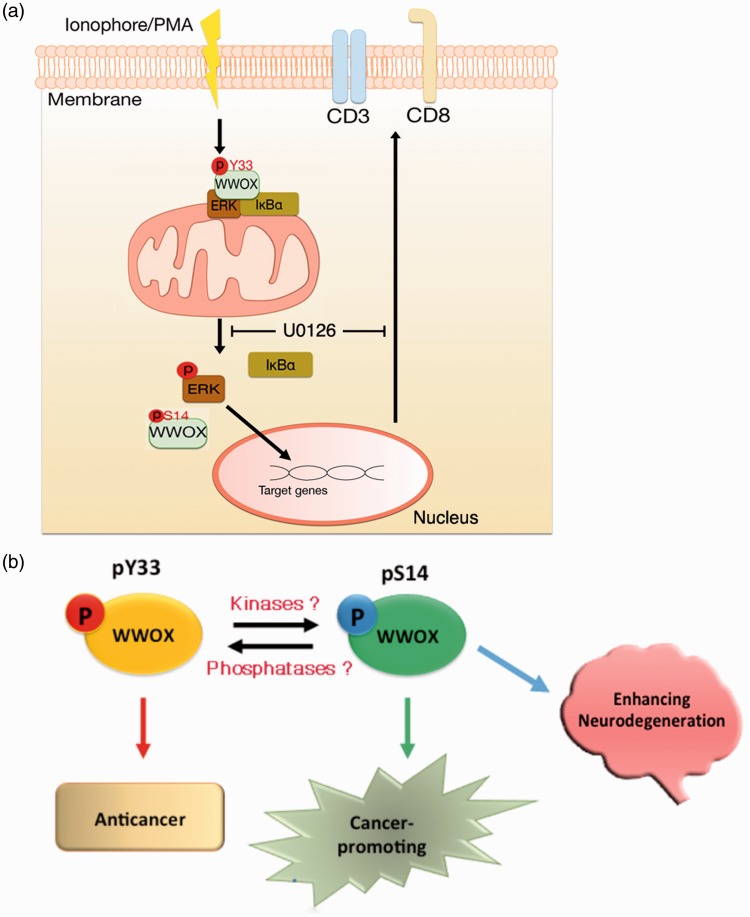
Figure 2.
pS14-WWOX in promoting T cell differentiation and cancer progression and neurodegeneration. (a) During forced leukemia T cell maturation by calcium ionophore and phorbol ester, WWOX becomes dephosphorylated at Tyr33 in the IκBα/WWOX/ERK complex, followed by phosphorylation at Ser14.25 Translocation of WWOX and ERK to the nucleus results in induction of leukemia T cell maturation in 16–24 h.25 (b) Activated WWOX with Tyr33 phosphorylation is proapoptotic.85,87,88 WWOX with Ser14 phosphorylation can be found in the lesions of cancer and brain hippocampus and cortex with Alzheimer’s disease.62 Suppression of pS14-WWOX by Zfra reduces cancer growth and mitigates AD symptoms,62,119 suggesting that pS14-WWOX facilitates disease progression. (A color version of this figure is available in the online journal.)
Potential role of pS14-WWOX in disease progression
Whether pS14-WWOX drives normal T cell differentiation and maturation is unknown. We have shown that pS14-WWOX is involved in disease progression. For example, nude mice receive subcutaneous inoculation with melanoma B16F10 cells in both flanks. Two months later, tumors grow up to 2000–3000 mm3 in the mice. There are occurrences of neurodegeneration in the hippocampus, amyloid plaque formation in the cortex, and melanoma infiltration in the lung.62 However, when nude mice receive Zfra peptide injections first, followed by inoculating B16F10 cells, cancer cell growth and metastasis are blocked.62 In the brain, no neurodegeneration and plaque formation are found. Zfra inhibits pS14-WWOX expression, thus resulting in suppression of cancer growth and neurodegeneration. That is, pS14-WWOX expression positively correlates with the melanoma metastasis to the lung and neurodegeneration in the brain. Zfra does not suppress the protein level of WWOX and its Tyr33 phosphorylation in control and Zfra-treated mice. Clearly, there is a positive correlation between the expression of pS14-WWOX and the progression of cancer growth and neurodegeneration in the hippocampus and plaque formation in the cortex.
Concluding remarks
When intracellular WWOX is Tyr33 phosphorylated, cells are self-protected. pY33-WWOX functions in blocking cancer growth and supporting normal neuronal physiology (Figure 2(b)).86 Substantial evidence shows that pY33-WWOX inhibits tumor growth in vitro and in vivo. However, down-regulation of pY33-WWOX occurs in the hippocampi in AD patients.67 This downregulation may start to occur in the mid-aged humans, which facilitates the activation of a cascade of protein aggregation that allows generation of amyloid β plaques and tau tangles in the AD brains.27,57–60 Thus, pY33-WWOX runs against neurodegeneration. In stark contrast, pS14-WWOX supports cancer growth and AD progression (Figure 2). Whether pS14-WWOX works alone or together with other proteins to promote disease progression remains to be established. Overall, the tumor suppressor function of endogenous WWOX is abolished upon acquiring Ser14 phosphorylation. Suppression of endogenous pS14-WWOX expression by Zfra results in inhibition of tumor growth and restoration of memory loss in triple transgenic mice for AD.62 That is, one of the mechanisms by which Zfra prevents and blocks cancer growth is due to its suppression of WWOX phosphorylation at Ser14. Nevertheless, endogenous pS14-WWOX is needed for forcing maturation of T cell leukemia,25 which is a conceptual advance for leukemia therapy.
Supplementary Material
Authors’ contributions
The manuscript supported, in part, SSH graduation for his Master degree. Both SSH and NSC contributed to manuscript writing and discussion and proof reading.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research was supported by the Ministry of Science and Technology, Taiwan (MOST 105–2320-B-006–046, 105–2320-B-006–036, 106–2320-B-006 –017, and 106–2320-B-006 –061 for NSC), and the Department of Defense, USA (W81XWH-08–1–0682 for NSC). Publication cost was supported in part by the China Medical University.
References
- 1.Kountouras J, Deretzi G, Gavalas E, Zavos C, Polyzos SA, Kazakos E, Giartza-Taxidou E, Vardaka E, Kountouras C, Katsinelos P, Boziki M, Giouleme O. A proposed role of human defensins in Helicobacter pylori-related neurodegenerative disorders. Med Hypotheses 2014; 82:368–73 [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003; 21:139–76 [DOI] [PubMed] [Google Scholar]
- 3.Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Differ 2006; 13:785–97 [DOI] [PubMed] [Google Scholar]
- 4.Franek KJ, Chervenak R. T cell protocols: development and activation In: Kearse KP. (ed) Methods in molecular biology. Totowa, N.J: Humana Press, 2000, pp.103–16 [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med 1997; 185:263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 2014; 14:377–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado MD, Leon J. Myc roles in hematopoiesis and leukemia. Genes Cancer 2010; 1:605–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauwerky CE, Croce CM. Chromosomal translocations in leukaemia. Semin Cancer Biol 1993; 4:333–40 [PubMed] [Google Scholar]
- 9.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer 2003; 3:639–49 [DOI] [PubMed] [Google Scholar]
- 10.La Starza R, Borga C, Barba G, Pierini V, Schwab C, Matteucci C, Lema Fernandez AG, Leszl A, Cazzaniga G, Chiaretti S, Basso G, Harrison CJ, Te Kronnie G, Mecucci C. Genetic profile of T-cell acute lymphoblastic leukemias with MYC translocations. Blood 2014; 124:3577–82 [DOI] [PubMed] [Google Scholar]
- 11.Nowell PC. The minute chromosome (Phl) in chronic granulocytic leukemia. Blut 1962; 8:65–6 [DOI] [PubMed] [Google Scholar]
- 12.Salesse S, Verfaillie CM. BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia. Oncogene 2002; 21:8547–59 [DOI] [PubMed] [Google Scholar]
- 13.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood 2007; 109:2700. [DOI] [PubMed] [Google Scholar]
- 14.Kordes U, Krappmann D, Heissmeyer V, Ludwig WD, Scheidereit C. Transcription factor NF-kappaB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000; 14:399–402 [DOI] [PubMed] [Google Scholar]
- 15.Packham G. The role of NF-kappaB in lymphoid malignancies. Br J Haematol 2008; 143:3–15 [DOI] [PubMed] [Google Scholar]
- 16.Bakker TR, Renno T, Jongeneel CV. Impaired fetal thymocyte development after efficient adenovirus-mediated inhibition of NF-kappa B activation. J Immunol 1999; 162:3456–62 [PubMed] [Google Scholar]
- 17.Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF-kappaB control of T cell development. Nat Immunol 2014; 15:15–25 [DOI] [PubMed] [Google Scholar]
- 18.Hettmann T, Leiden JM. NF-kappa B is required for the positive selection of CD8+ thymocytes. J Immunol 2000; 165:5004–10 [DOI] [PubMed] [Google Scholar]
- 19.Kaltschmidt B, Kaltschmidt C. NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front Mol Neurosci 2015; 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16:225–60 [DOI] [PubMed] [Google Scholar]
- 21.Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell 1998; 95:749–58 [DOI] [PubMed] [Google Scholar]
- 22.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev 1995; 9:2736–46 [DOI] [PubMed] [Google Scholar]
- 23.Esslinger CW, Wilson A, Sordat B, Beermann F, Jongeneel CV. Abnormal T lymphocyte development induced by targeted overexpression of IkappaB alpha. J Immunol 1997; 158:5075–8 [PubMed] [Google Scholar]
- 24.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med 1997; 185:1897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SS, Su WP, Lin HP, Kuo HL, Wei HL, Chang NS. Role of WW domain-containing oxidoreductase WWOX in driving T cell acute lymphoblastic leukemia maturation. J Biol Chem 2016; 291:17319–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen SJ, Huang SS, Chang NS. Role of WWOX and NF-kappaB in lung cancer progression. Transl Respir Med 2013; 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang HT, Liu CC, Chen ST, Yap YV, Chang NS, Sze CI. WW domain-containing oxidoreductase in neuronal injury and neurological diseases. Oncotarget 2014; 5:11792–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang NS. Introduction to a thematic issue for WWOX. Exp Biol Med 2015; 240:281–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 2000; 60:2140–5 [PubMed] [Google Scholar]
- 30.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 2000; 9:1651–63 [DOI] [PubMed] [Google Scholar]
- 31.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276:3361–70 [DOI] [PubMed] [Google Scholar]
- 32.O'Leary VB, Smida J, Buske FA, Carrascosa LG, Azimzadeh O, Maugg D, Hain S, Tapio S, Heidenreich W, Kerr J, Trau M, Ovsepian SV, Atkinson MJ. PARTICLE triplexes cluster in the tumor suppressor WWOX and may extend throughout the human genome. Sci Rep 2017; 7:7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong Q, Hsu LJ, Schultz L, Pratt N, Mattison J, Chang NS. Zfra affects TNF-mediated cell death by interacting with death domain protein TRADD and negatively regulates the activation of NF-kappaB, JNK1, p53 and WOX1 during stress response. BMC Mol Biol 2007; 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HP, Chang JY, Lin SR, Lee MH, Huang SS, Hsu LJ, Chang NS. Identification of an In Vivo MEK/WOX1 Complex as a Master Switch for Apoptosis in T Cell Leukemia. Genes Cancer 2011; 2:550–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat 2005; 89:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Sahin A, Aldaz CM. Deletion map of chromosome 16q in ductal carcinoma in situ of the breast: refining a putative tumor suppressor gene region. Cancer Res 1996; 56:5605–9 [PubMed] [Google Scholar]
- 37.Iliopoulos D, Fabbri M, Druck T, Qin HR, Han SY, Huebner K. Inhibition of breast cancer cell growth in vitro and in vivo: effect of restoration of Wwox expression. Clin Cancer Res 2007; 13:268–74 [DOI] [PubMed] [Google Scholar]
- 38.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX–the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 2006; 32:153–7 [DOI] [PubMed] [Google Scholar]
- 39.Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res 2002; 62:2258–60 [PubMed] [Google Scholar]
- 40.Pimenta FJ, Gomes DA, Perdigao PF, Barbosa AA, Romano-Silva MA, Gomez MV, Aldaz CM, De Marco L, Gomez RS. Characterization of the tumor suppressor gene WWOX in primary human oral squamous cell carcinomas. Int J Cancer 2006; 118:1154–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo W, Wang G, Dong Y, Guo Y, Kuang G, Dong Z. Decreased expression of WWOX in the development of esophageal squamous cell carcinoma. Mol Carcinog 2013; 52:265–74 [DOI] [PubMed] [Google Scholar]
- 42.Singla S, Chen J, Sethuraman S, Sysol JR, Gampa A, Zhao S, Machado RF. Loss of lung WWOX expression causes neutrophilic inflammation. Am J Physiol Lung Cell Mol Physiol 2017; 312:L903–L11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng QW, Zhou YL, You QJ, Shou F, Pang QF, Chen JL. WWOX inhibits the invasion of lung cancer cells by downregulating RUNX2. Cancer Gene Ther 2016; 23:433–8 [DOI] [PubMed] [Google Scholar]
- 44.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 2009; 69:4835–42 [DOI] [PubMed] [Google Scholar]
- 45.Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, Aldaz CM. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 2005; 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zelazowski MJ, Pluciennik E, Pasz-Walczak G, Potemski P, Kordek R, Bednarek AK. WWOX expression in colorectal cancer–a real-time quantitative RT-PCR study. Tumour Biol 2011; 32:551–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin HR, Iliopoulos D, Nakamura T, Costinean S, Volinia S, Druck T, Sun J, Okumura H, Huebner K. Wwox suppresses prostate cancer cell growth through modulation of ErbB2-mediated androgen receptor signaling. Mol Cancer Res 2007; 5:957–65 [DOI] [PubMed] [Google Scholar]
- 48.Qin HR, Iliopoulos D, Semba S, Fabbri M, Druck T, Volinia S, Croce CM, Morrison CD, Klein RD, Huebner K. A role for the WWOX gene in prostate cancer. Cancer Res 2006; 66:6477–81 [DOI] [PubMed] [Google Scholar]
- 49.Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM. Cancer Res Inactivation of the Wwox gene accelerates forestomach tumor progression in vivo. Cancer Res 2007; 67:5606–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene 2002; 21:1832–40 [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Chao L, Jin G, Ma G, Zang Y, Sun J. Association between CpG island methylation of the WWOX gene and its expression in breast cancers. Tumour Biol 2009; 30:8–14 [DOI] [PubMed] [Google Scholar]
- 52.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A 2007; 104:3949–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 2009; 4:e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishii H, Vecchione A, Furukawa Y, Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M, Saito Y, Ozawa K, Croce CM, Huebner K, Furukawa Y. Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic malignancies. Mol Cancer Res 2003; 1:940–7 [PubMed] [Google Scholar]
- 55.Aldaz CM, Ferguson BW, Abba MC. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim Biophys Acta 2014; 1846:188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabarki B, Al Mutairi F, Al Hashem A. The fragile site WWOX gene and the developing brain. Exp Biol Med 2015; 240:400–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sze CI, Kuo YM, Hsu LJ, Fu TF, Chiang MF, Chang JY, Chang NS. A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency. Cell Death Dis 2015; 6:e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang JY, Lee MH, Lin SR, Yang LY, Sun HS, Sze CI, Hong Q, Lin YS, Chou YT, Hsu LJ, Jan MS, Gong CX, Chang NS. Trafficking protein particle complex 6A delta (TRAPPC6ADelta) is an extracellular plaque-forming protein in the brain. Oncotarget 2015; 6:3578–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JY, Chang NS. WWOX dysfunction induces sequential aggregation of TRAPPC6ADelta, TIAF1, tau and amyloid beta, and causes apoptosis. Cell Death Discov 2015; 1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MH, Lin SR, Chang JY, Schultz L, Heath J, Hsu LJ, Kuo YM, Hong Q, Chiang MF, Gong CX, Sze CI, Chang NS. TGF-beta induces TIAF1 self-aggregation via type II receptor-independent signaling that leads to generation of amyloid beta plaques in Alzheimer's disease. Cell Death Dis 2010; 1:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang JY, Chiang MF, Lin SR, Lee MH, He H, Chou PY, Chen SJ, Chen YA, Yang LY, Lai FJ, Hsieh CC, Hsieh TH, Sheu HM, Sze CI, Chang NS. TIAF1 self-aggregation in peritumor capsule formation, spontaneous activation of SMAD-responsive promoter in p53-deficient environment, and cell death. Cell Death Dis 2012; 3:e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee MH, Shih YH, Lin SR, Chang JY, Lin YH, Sze CI, Kuo YM, Chang NS. Zfra restores memory deficits in Alzheimer's disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation. Alzheimers Dement 2017; 3:189–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, Palmer K, Russo A. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology 2013; 81:322–8 [DOI] [PubMed] [Google Scholar]
- 64.Shafi O. Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review. BMC Neurol 2016; 16:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki H, Katayama K, Takenaka M, Amakasu K, Saito K, Suzuki K. A spontaneous mutation of the Wwox gene and audiogenic seizures in rats with lethal dwarfism and epilepsy. Genes Brain Behav 2009; 8:650–60 [DOI] [PubMed] [Google Scholar]
- 66.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A 2004; 101:4401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sze CI, Su M, Pugazhenthi S, Jambal P, Hsu LJ, Heath J, Schultz L, Chang NS. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. J Biol Chem 2004; 279:30498–506 [DOI] [PubMed] [Google Scholar]
- 68.Su WP, Chen SH, Chen SJ, Chou PY, Huang CC, Chang NS. WW Domain-containing oxidoreductase is a potential receptor for sex steroid hormones In: Raghvendra D. (ed) Sex hormones. London: Intech, 2012, pp.333–51 [Google Scholar]
- 69.Chang NS, Schultz L, Hsu LJ, Lewis J, Su M, Sze CI. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005; 24:714–23 [DOI] [PubMed] [Google Scholar]
- 70.Lai FJ, Cheng CL, Chen ST, Wu CH, Hsu LJ, Lee JY, Chao SC, Sheen MC, Shen CL, Chang NS, Sheu HM. WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin Cancer Res 2005; 11:5769–77 [DOI] [PubMed] [Google Scholar]
- 71.Bork P, Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci 1994; 19:531–3 [DOI] [PubMed] [Google Scholar]
- 72.Sudol M, Hunter T. NeW wrinkles for an old domain. Cell 2000; 103:1001–4 [DOI] [PubMed] [Google Scholar]
- 73.Hu H, Columbus J, Zhang Y, Wu D, Lian L, Yang S, Goodwin J, Luczak C, Carter M, Chen L, James M, Davis R, Sudol M, Rodwell J, Herrero JJ. A map of WW domain family interactions. Proteomics 2004; 4:643–55 [DOI] [PubMed] [Google Scholar]
- 74.Sudol M, Recinos CC, Abraczinskas J, Humbert J, Farooq A. WW or WoW: the WW domains in a union of bliss. IUBMB Life 2005; 57:773–8 [DOI] [PubMed] [Google Scholar]
- 75.Salah Z, Alian A, Aqeilan RI. WW domain-containing proteins: retrospectives and the future. Front Biosci 2012; 17:331–48 [DOI] [PubMed] [Google Scholar]
- 76.Chang JY, He RY, Lin HP, Hsu LJ, Lai FJ, Hong Q, Chen SJ, Chang NS. Signaling from membrane receptors to tumor suppressor WW domain-containing oxidoreductase. Exp Biol Med 2010; 235:796–804 [DOI] [PubMed] [Google Scholar]
- 77.Abu-Remaileh M, Joy-Dodson E, Schueler-Furman O, Aqeilan RI. Pleiotropic functions of tumor suppressor WWOX in normal and cancer cells. J Biol Chem 2015; 290:30728–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64:8256–61 [DOI] [PubMed] [Google Scholar]
- 79.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res 2005; 65:6764–72 [DOI] [PubMed] [Google Scholar]
- 80.Li MY, Lai FJ, Hsu LJ, Lo CP, Cheng CL, Lin SR, Lee MH, Chang JY, Subhan D, Tsai MS, Sze CI, Pugazhenthi S, Chang NS, Chen ST. Dramatic co-activation of WWOX/WOX1 with CREB and NF-kappaB in delayed loss of small dorsal root ganglion neurons upon sciatic nerve transection in rats. PLoS One 2009; 4:e7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin C, Ge L, Ding X, Chen Y, Zhu H, Ward T, Wu F, Cao X, Wang Q, Yao X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochem Biophys Res Commun 2006; 341:784–91 [DOI] [PubMed] [Google Scholar]
- 82.Takeuchi T, Adachi Y, Nagayama T. A WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis 2012; 33:548–54 [DOI] [PubMed] [Google Scholar]
- 83.Farooq A. Structural insights into the functional versatility of WW domain-containing oxidoreductase tumor suppressor. Exp Biol Med 2015; 240:361–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014; 94:1287–312 [DOI] [PubMed] [Google Scholar]
- 85.Chang NS, Doherty J, Ensign A, Schultz L, Hsu LJ, Hong Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J Biol Chem 2005; 280:43100–8 [DOI] [PubMed] [Google Scholar]
- 86.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13:12–22 [DOI] [PubMed] [Google Scholar]
- 87.Chang NS, Doherty J, Ensign A, Lewis J, Heath J, Schultz L, Chen ST, Oppermann U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem Pharmacol 2003; 66:1347–54 [DOI] [PubMed] [Google Scholar]
- 88.Chang NS, Doherty J, Ensign A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J Biol Chem 2003; 278:9195–202 [DOI] [PubMed] [Google Scholar]
- 89.Chen ST, Chuang JI, Cheng CL, Hsu LJ, Chang NS. Light-induced retinal damage involves tyrosine 33 phosphorylation, mitochondrial and nuclear translocation of WW domain-containing oxidoreductase in vivo. Neuroscience 2005; 130:397–407 [DOI] [PubMed] [Google Scholar]
- 90.Hsu LJ, Hong Q, Chen ST, Kuo HL, Schultz L, Heath J, Lin SR, Lee MH, Li DZ, Li ZL, Cheng HC, Armand G, Chang NS. Hyaluronan activates Hyal-2/WWOX/Smad4 signaling and causes bubbling cell death when the signaling complex is overexpressed. Oncotarget 2017; 8:19137–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang YH, Chang NS, Tseng SH. Expression of WW domain-containing oxidoreductase WWOX in pterygium. Mol Vis 2015; 21:711–7 [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura M, Takenobu H, Akita N, Nakazawa A, Ochiai H, Shimozato O, Fujimura Y, Koseki H, Yoshino I, Kimura H, Nakagawara A, Kamijo T. Bmi1 regulates cell fate via tumor suppressor WWOX repression in small-cell lung cancer cells. Cancer Sci 2011; 102:983–90 [DOI] [PubMed] [Google Scholar]
- 93.Nagoshi H, Taki T, Hanamura I, Nitta M, Otsuki T, Nishida K, Okuda K, Sakamoto N, Kobayashi S, Yamamoto-Sugitani M, Tsutsumi Y, Kobayashi T, Matsumoto Y, Horiike S, Kuroda J, Taniwaki M. Frequent PVT1 rearrangement and novel chimeric genes PVT1-NBEA and PVT1-WWOX occur in multiple myeloma with 8q24 abnormality. Cancer Res 2012; 72:4954–62 [DOI] [PubMed] [Google Scholar]
- 94.Hsu LJ, Chiang MF, Sze CI, Su WP, Yap YV, Lee IT, Kuo HL, Chang NS. HYAL-2-WWOX-SMAD4 Signaling in cell death and anticancer response. Front Cell Dev Biol 2016; 4:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Del Mare S, Husanie H, Iancu O, Abu-Odeh M, Evangelou K, Lovat F, Volinia S, Gordon J, Amir G, Stein J, Stein GS, Croce CM, Gorgoulis V, Lian JB, Aqeilan RI. WWOX and p53 dysregulation synergize to drive the development of osteosarcoma. Cancer Res 2016; 76:6107–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res 2005; 65:10514–23 [DOI] [PubMed] [Google Scholar]
- 97.Matteucci E, Bendinelli P, Desiderio MA. Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis 2009; 30:937–45 [DOI] [PubMed] [Google Scholar]
- 98.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009; 28:2569–80 [DOI] [PubMed] [Google Scholar]
- 99.Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene 2004; 23:5049–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen SJ, Lin PW, Lin HP, Huang SS, Lai FJ, Sheu HM, Hsu LJ, Chang NS. UV irradiation/cold shock-mediated apoptosis is switched to bubbling cell death at low temperatures. Oncotarget 2015; 6:8007–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang NS. Bubbling cell death: a hot air balloon released from the nucleus in the cold. Exp Biol Med 2016; 241:1306–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsu LJ, Schultz L, Hong Q, Van Moer K, Heath J, Li MY, Lai FJ, Lin SR, Lee MH, Lo CP, Lin YS, Chen ST, Chang NS. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J Biol Chem 2009; 284:16049–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong Q, Sze CI, Lin SR, Lee MH, He RY, Schultz L, Chang JY, Chen SJ, Boackle RJ, Hsu LJ, Chang NS. Complement C1q activates tumor suppressor WWOX to induce apoptosis in prostate cancer cells. PLoS One 2009; 4:e5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bandini S, Macagno M, Hysi A, Lanzardo S, Conti L, Bello A, Riccardo F, Ruiu R, Merighi IF, Forni G, Iezzi M, Quaglino E, Cavallo F. The non-inflammatory role of C1q during Her2/neu-driven mammary carcinogenesis. Oncoimmunology 2016; 5:e1253653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghebrehiwet B, Hosszu KK, Valentino A, Peerschke EI. The C1q family of proteins: insights into the emerging non-traditional functions. Front Immunol 2012; 3: pii: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang Y, Lan YY, Hsiao JR, Chang NS. Strategies of oncogenic microbes to deal with WW domain-containing oxidoreductase. Exp Biol Med 2015; 240:329–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, Xiao G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-kappaB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 2011; 117:1652–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu J, Lin WH, Chen SY, Longnecker R, Tsai SC, Chen CL, Tsai CH. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem 2006; 281:8806–14 [DOI] [PubMed] [Google Scholar]
- 109.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol 2000; 74:10681–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lan YY, Wu SY, Lai HC, Chang NS, Chang FH, Tsai MH, Su IJ, Chang Y. WW domain-containing oxidoreductase is involved in upregulation of matrix metalloproteinase 9 by Epstein-Barr virus latent membrane protein 2A. Biochem Biophys Res Commun 2013; 436:672–6 [DOI] [PubMed] [Google Scholar]
- 111.Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res 2010; 70:5577–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hazan I, Hofmann TG, Aqeilan RI. Tumor suppressor genes within common fragile sites are active players in the DNA damage response. PLoS Genet 2016; 12:e1006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schrock MS, Karras JR, Guggenbiller MJ, Druck T, Batar B, Huebner K. Fhit and Wwox loss-associated genome instability: a genome caretaker one-two punch. Adv Biol Regul 2017; 63:167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 1996; 16:2164–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abu-Odeh M, Salah Z, Herbel C, Hofmann TG, Aqeilan RI. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc Natl Acad Sci U S A 2014; 111:E4716–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hazan I, Abu-Odeh M, Hofmann TG, Aqeilan RI. WWOX guards genome stability by activating ATM. Mol Cell Oncol 2015; 2:e1008288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abu-Odeh M, Hereema NA, Aqeilan RI. WWOX modulates the ATR-mediated DNA damage checkpoint response. Oncotarget 2016; 7:4344–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schrock MS, Batar B, Lee J, Druck T, Ferguson B, Cho JH, Akakpo K, Hagrass H, Heerema NA, Xia F, Parvin JD, Aldaz CM, Huebner K. Wwox-Brca1 interaction: role in DNA repair pathway choice. Oncogene 2017; 36:2215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee MH, Su WP, Wang WJ, Lin SR, Lu CY, Chen YA, Chang JY, Huang SS, Chou PY, Ye SR, Chen SJ, He H, Liu TH, Chou YT, Hsu LJ, Lai FJ, Chen SJ, Lee HC, Kakhniashvili D, Goodman SR, Chang NS. Zfra activates memory Hyal-2+ CD3- CD19- spleen cells to block cancer growth, stemness, and metastasis in vivo. Oncotarget 2015; 6:3737–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen X, Zhang H, Li P, Yang Z, Qin L, Mo W. Gene expression of WWOX, FHIT and p73 in acute lymphoblastic leukemia. Oncol Lett 2013; 6:963–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elbossaty WF, Malak CEDM. Prognostic relevance of Ww-oxidoreductase gene expression in patients with acute lymphoblastic leukemia. J Cancer Sci Ther 2015; 7:302–7 [Google Scholar]
- 122.Cui Z, Lin D, Cheng F, Luo L, Kong L, Xu J, Hu J, Lan F. The role of the WWOX gene in leukemia and its mechanisms of action. Oncol Rep 2013; 29:2154–62 [DOI] [PubMed] [Google Scholar]
- 123.Ishii H, Furukawa Y. Alterations of common chromosome fragile sites in hematopoietic malignancies. Int J Hematol 2004; 79:238–42 [DOI] [PubMed] [Google Scholar]
- 124.Ludes-Meyers JH, Kil H, Nunez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosom Cancer 2007; 46:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang NS. WWOX drives T leukemia cell maturation via IκBα/WWOX/ERK signal pathway. J Tumor Res 2016; 2:e101 [Google Scholar]
- 126.Lu PJ, Zhou XZ, Liou YC, Noel JP, Lu KP. Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J Biol Chem 2002; 277:2381–4 [DOI] [PubMed] [Google Scholar]
- 127.Li YW, Guo J, Shen H, Li J, Yang N, Frangou C, Wilson KE, Zhang Y, Mussell AL, Sudol M, Farooq A, Qu J, Zhang J. Phosphorylation of Tyr188 in the WW domain of YAP1 plays an essential role in YAP1-induced cellular transformation. Cell Cycle 2016; 15:2497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.