Abstract
Advanced glycation end products accumulate in the ovarian granulosa-cell layer of women with polycystic ovarian syndrome. Taken that the MAPK/ERK-pathway is a key regulator of oocyte maturation and function, consisting the main pathway used by the gonadotrophic hormones (luteinizing hormone, follicle stimulating hormone) to control ovulation, the present study aims to assess advanced glycation end products' interference into luteinizing hormone-and follicle stimulating hormone-signaling via the MAPK/ERK-pathway in the human granulosa KGN cell line. KGN cells were treated with luteinizing hormone or follicle stimulating hormone in the absence or presence of human glycated albumin. The specific activation of the main components of the MAPK/ERK1/2-pathway (namely c-Raf, MEK and ERK1/2) was assessed. Treatment of KGN cells with an MEK1/2-inhibitor or a blocking anti-RAGE-antibody was also performed to shed further light on the mechanism of the involvement of advanced glycation end products in luteinizing hormone and/or follicle stimulating hormone-related signaling pathways. Luteinizing hormone treatment increased p-ERK1/2 levels in human granulosa cells, while the combined treatment of luteinizing hormone and human glycated albumin provoked a decrease of p-ERK1/2 levels. A similar reducing effect was also observed for the upstream molecule phospho-cRaf upon combined treatment, while treatment with an MEK-inhibitor confirmed that the phenomenon is MAPK/ERK-pathway-dependent. Similarly, follicle stimulating hormone treatment increased p-ERK1/2 and p-MEK1/2 levels, while the combined treatment of follicle stimulating hormone and human glycated albumin downregulated their levels. Advanced glycation end products reduce the luteinizing hormone- and follicle stimulating hormone-induced ERK1/2 activation that is critical for granulosa cell mitogenesis and proliferation. Inappropriate activation of ERK1/2 in granulosa cells may block the granulosa cell differentiation pathway and/or impair follicular responses to hormones, potentially leading to ovulation failure that characterizes polycystic ovarian syndrome.
Keywords: Advanced glycation end products, luteinizing hormone, follicle stimulating hormone, ERK1/2, MEK1/2, polycystic ovarian syndrome
Impact statement
The current study proposes a new potential mechanism for the pathogenesis of PCOS. Although previous studies have revealed that AGEs’ accumulation in serum and the ovary is highly correlated to the hormonal and metabolic dysregulation present during PCOS, the underlying mechanism was so far unclear. Herein we show for the first time a direct interference of AGEs into LH and FSH signaling of granulosa cells highlighting the potential role of AGEs in the dysregulation of the homeostasis of the ovarian and follicular microenvironment under PCOS conditions. These data support the hypothesis that AGEs are good candidates as predictive markers and therapeutic targets in new strategies for improving reproductive counselling in women with PCOS.
Introduction
Advanced glycation end products (AGEs), a group of heterogeneous compounds, are formed from non-enzymatic glycation of the amino groups of proteins, nucleic acids, and lipids, causing tissue injury either directly by trapping and cross-linking or indirectly by binding to specific receptors for AGEs (RAGE) on the surface of various cell types.1–6 Increased serum AGEs levels have been found in patients with diabetes and insulin resistance, rheumatoid arthritis, renal insufficiency, aging4,5,7,8 and polycystic ovarian syndrome (PCOS).2,9–12
We have previously demonstrated an increased deposition and differential qualitative distribution of AGE-modified proteins, their receptor RAGE and their signaling mediator NF-κB, on the granulosa cell layer of PCOS ovaries compared to controls.2 Further investigation of these observations in normal female rats fed with a high or low AGEs diet showed increased AGEs deposition and RAGE expression in the ovarian tissue of those animals fed with a high AGEs diet.2 These data were further supported by another study investigating ovulatory and anovulatory PCOS women, where the levels of anti-mullerian hormone (AMH), an indicator of disturbed ovulatory process produced by granulosa cells, were positively correlated with AGEs, follicle numbers, and the presence of anovulation.11
In addition, AGE-RAGE intracellular signaling commonly involves the MAPK/ERK pathway, predominantly ERK1/2 as well as their upstream mediators cRaf and MEK1/2, that have a key role in regulating the process of cell proliferation, cell differentiation, and oocyte maturation in vitro.13 Recent data support that the luteinizing hormone (LH) and follicle stimulating hormone (FSH) also act via activation of ERK1/2 to control ovulation and luteinization events. More specifically, disruption of ERK1/2 in mouse granulosa cells showed that these kinases are necessary for LH-induced oocyte resumption of meiosis, ovulation, and luteinization in vivo.14 In addition, it has been proposed that LH also induces expression of the epidermal growth factor (EGF) that, via activation of the EGF receptor, RAS, and ERK1/2, may act in the intrafollicular environment as a mediator to stimulate cell oocyte expansion and oocyte maturation.15–17 Similarly, FSH has been shown to activate both ERK1/2 and PI3K/Akt pathways in cultured granulosa cells and regulate cell proliferation.18 Specifically, ERK1/2 activation is essential for FSH-mediated granulosa cell mitogenesis via regulation of cyclin D2.18,19 Therefore, a potential interference of AGEs signaling with LH and FSH activity in human granulosa cells was hypothesized and was investigated in the present study.
Methods
Cells
KGN cells were established and obtained from RIKEN Bioresource center (Tsukuba, Japan) and were maintained in DMEM/F-12 medium (Cambrex, Walkerville, MD USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biochrom, Berlin Germany), 100 U/ml penicillin/streptomycin (Cambrex, Walkerville, MD, USA) at 37℃ in a humidified atmosphere of 5% CO2.
Cell treatments
KGN cells were grown up to 70–80% confluency. After 24 h, the cells were serum starved for another 24 h and treated with 100 ng/ml FSH (Follicle Stimulation Hormone) (Merck) or 200 ng/ml LH (Merck) for the indicated timepoints in the absence or presence of 0.2 mg/ml human glycated albumin (HGA) (Sigma-Aldrich St.Louis, MO). In order to evaluate specificity, KGN cells were incubated with an anti-RAGE blocking antibody (10 µg/ml) or an anti-MEK1/2 inhibitor (UO126, 20 µM) 1 h prior to treatments as indicated.14,16–19
Western blot analysis
Treated cells were harvested and cells extracts were obtained by lysis in RIPA buffer containing 0.55 Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml aprotinin, and 10 µg/ml leupeptin (all from Sigma St. Louis, USA) and phosphatase inhibitors (1 mM sodium ortovanadate, 1 mM NaF; Sigma St. Louis, USA). After 30-min incubation on ice, the lysates were cleared by centrifugation (14,000 r/min, 15 min, and 4℃). Protein concentrations were determined by Bio-Rad protein assay (BIO-RAD Laboratories, Hercules, CA, USA). Equal amount of cell lysates (20 µg) was heated at 95℃ for 5 min, electrophoresed on 12% SDS–PAGE under denaturing conditions, and transferred onto nitrocellulose membrane (BIO-RAD). The blots were blocked with TBS-T (20 mmol/L Tris–HCl, pH 7.6, 137 mmol/L NaCl, and 0.1% Tween20) containing 5% non-fat dried milk at room temperature for 1 h. The membranes were probed overnight with primary antibody against phospho-ERK1/2 (1:1000, Cell Signaling, MA, USA), phospho-MEK1/2 (1:1000, Cell Signaling), actin (1:5000, Millipore) or 2 h against phospho-c-Raf (1:1000, Cell Signaling) in TBS/T containing 5% BSA. The blots were washed and followed by incubation with a secondary goat antibody raised against rabbit IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, CA, USA). The bands were visualized by exposing the blots to X-ray film after incubation with freshly made ECL substrate for 3 min (SuperSignal, Pierce Biotechnology, Rockford, IL, USA). Relative protein amounts were evaluated by densitometric analysis using Image J software and normalized to the corresponding total ERK or actin levels. Shown are representative experiments out of two to three independent experiments.
Results
HGA affects the LH-dependent signaling via the MAPK/ERK pathway
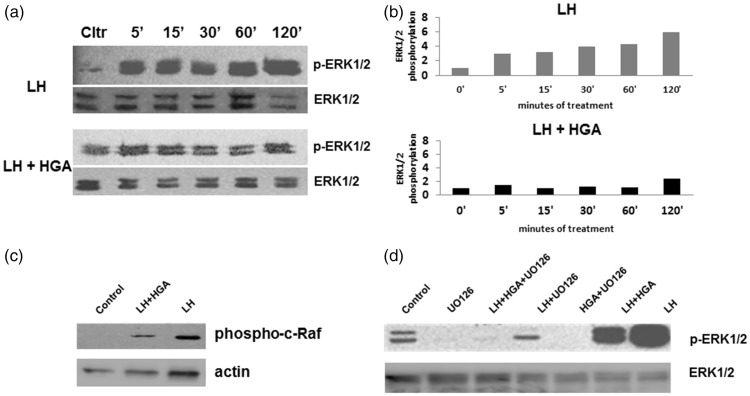
Treatment with LH increased p-ERK1/2 levels after 5- to 120-min exposure in human granulosa cells compared to basal levels. Interestingly, the combined treatment of LH and HGA decreased p-ERK1/2 levels in the indicated timepoints (5–120 min), as compared to the LH treatment (Figure 1(a) and (b)). A similar reducing effect was also observed for the upstream molecule phospho-cRaf (Figure 1(c)). To confirm whether the main upstream mediator of the MAPK/ERK pathway, namely MEK1/2, is taking part in the signaling process, a similar experiment was performed in the absence or presence of an MEK1/2 inhibitor (UO126). Indeed, pERK1/2 levels were reduced when the inhibitor was present (Figure 1(d)), indicating that the MAPK/ERK pathway is one of the main signaling cascades that are affected during the potential interference of AGEs in the LH-dependent intracellular signaling machinery of granulosa cells.
Figure 1.
(a) Western blot of phosphorylated (phospho) ERK1/2 protein expression from KGN cells starved overnight and treated with LH alone or in combination with HGA for 5–120 min. (b) Graphs of the densitometric quantification of the effect of LH and HGA exposure on activation of ERK1/2 (phospho ERK) in KGN cells after normalization to total ERK levels are shown. Data are presented as relative to control that was set as 1. (c) Western blot of phosphorylated c-Raf protein expression from KGN cells starved overnight and treated with LH alone or in combination with HGA for 30 min. Actin levels were used as loading control. (d) Western blot of p-ERK1/2 protein expression from KGN cells starved overnight, incubated with an MEK1/2 inhibitor (UO126) prior treatment with LH alone or in combination with HGA for 30 min
Effects of HGA and FSH on ERK1/2 activation in human granulosa KGN cells
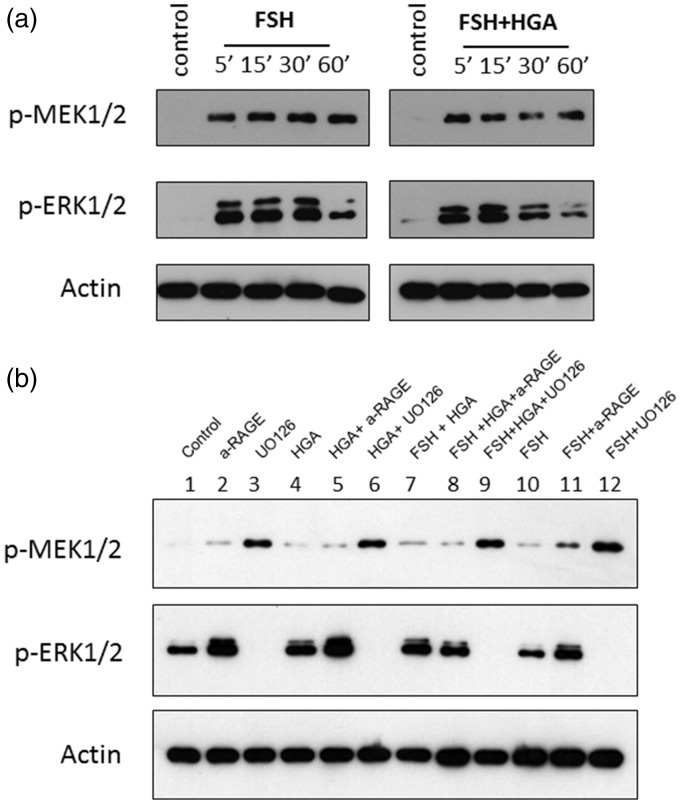
Previous studies have shown that FSH acts through ERK1/2 pathway to increase cyclin D2 expression and granulosa cell proliferation.18 In our study, we explored the effects of FSH alone or in combination with HGA on ERK1/2 phosphorylation. FSH treatment increased p-MEK1/2 levels after 5- to 60-min exposure in human granulosa cells compared to basal levels. Similarly, FSH treatment increased p-ERK1/2 levels after 5- to 30-min exposure. The combined treatment of FSH and HGA reduced p-MEK1/2 and p-ERK1/2 levels at 30 and 60 min (Figure 2(a)). Furthermore, incubation of KGN cells with an anti-RAGE blocking antibody or an MEK1/2 inhibitor (UO126) prior to treatments indicated that HGA and FSH activate specifically the MEK1/2 and ERK1/2 pathway but this activation is not mediated through RAGE (Figure 2(b)).
Figure 2.
(a) Western blot of p-MEK1/2 and p-ERK1/2 protein expression from KGN cells starved overnight and treated with FSH alone or in combination with HSA or HGA for 5–60 min. (b) Western blot of p-MEK1/2 and p-ERK1/2 protein expression after the effect of anti-RAGE antibody and an MEK1/2 inhibitor (UO126) on HGA and FSH treatments of KGN cells for 30 min
Discussion
We have shown for the first time a direct interaction of AGEs (HGA) with steroidogenic enzyme activity in human ovarian granulosa cells, highlighting the potential role of AGEs in the homeostasis of the ovarian and follicular microenvironment. This is especially relevant to pathological states of disturbed ovulation such as PCOS that is characterized by elevated circulating AGE levels.11,12 With their prolonged half-life and ability to act as signaling molecules, AGEs may gradually accumulate in the ovary. Previous studies from our group have shown increased AGEs deposition in ovarian granulosa cell layer in rodents treated with high AGE diet.2 Relevant data support the hypothesis that AGEs are good candidates as predictive markers and therapeutic targets in new strategies for improving reproductive counselling in specific populations, i.e. women with failed assisted reproductive techniques.20
In this study, it was clearly demonstrated that AGEs presence in the ovary interferes with LH-induced MAPK/ERK signaling pathway in granulosa cells. A direct and specific effect of AGEs was observed over this pathway leading to reduced activation of ERK1/2. Since activation of ERK1/2 has been implicated to the regulation of cell proliferation and differentiation, but also the maturation of oocytes in culture, this effect can prove fundamental in normal follicle development and proper initiation of the ovulation process.13 Furthermore, FSH-induced phosphorylation of MEK1/2 and ERK1/2 was also affected by the presence of AGEs leading to reduced activation of these molecules at 30 and 60 min. FSH has been shown to act through ERK pathway to increase cyclin D2 expression and granulosa cell proliferation.18 Since ERK1/2 activation is critical for FSH-mediated granulosa cell mitogenesis, the reducing effect of AGEs presence will disturb their physiology and ovarian function overall.
In conclusion, we have shown that AGEs are capable to interfere with steroidogenic enzyme activity. Inappropriate AGEs-induced activation of ERK1/2 in granulosa cells may derange their differentiation pathway and /or the follicular responses to hormones. Therefore, these findings support our hypothesis that intraovarian AGEs accumulation, from endogenous or exogenous sources, via interference with LH and FSH action may contribute to the pathophysiology of conditions characterized with anovulation and insulin resistance such as PCOS. Nevertheless, among the limitations of the current study is predominantly the usage of the KGN cell line and not primary granulosa cells, as well as the usage of HGA as an AGE type and not specific AGEs such as carboxymethyllysine (CML) or methylglyoxal. Therefore, further studies are needed to highlight the aforementioned molecular mechanism and confirm the possible clinical involvements and implications.
Authors’ contributions
EAK designed and performed experiments, analyzed and interpreted data and edited the paper, AC wrote the paper, EP and CA performed experiments, analyzed and interpreted data, CP, AP and MK participated in experimental design and discussion, EDK designed the study and wrote the paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Chatzigeorgiou A, Kandaraki E, Piperi C, Livadas S, Papavassiliou AG, Koutsilieris M, Papalois A, Diamanti-Kandarakis E. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J Endocrinol 2013; 218: 331–7. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E, Piperi C, Korkolopoulou P, Kandaraki E, Levidou G, Papalois A, Patsouris E, Papavassiliou AG. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J Mol Med 2007; 85: 1413–20. [DOI] [PubMed] [Google Scholar]
- 3.Kandaraki E, Chatzigeorgiou A, Piperi C, Palioura E, Palimeri S, Korkolopoulou P, Koutsilieris M, Papavassiliou AG. Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: a causative link to polycystic ovarian syndrome. Mol Med 2012; 18: 1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001; 44: 129–46. [DOI] [PubMed] [Google Scholar]
- 5.Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update 2008; 14: 131–42. [DOI] [PubMed] [Google Scholar]
- 6.Tatone C, Heizenrieder T, Di Emidio G, Treffon P, Amicarelli F, Seidel T, Eichenlaub-Ritter U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum Reprod 2011; 26: 1843–59. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001; 56: 1–21. [DOI] [PubMed] [Google Scholar]
- 8.Chatzigeorgiou A, Kandaraki E, Papavassiliou AG, Koutsilieris M. Peripheral targets in obesity treatment: a comprehensive update. Obes Rev 2014; 15: 487–503. [DOI] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and enviromental factors: their influence in PCOS. Curr Pharm Des 2012; 18: 270–82. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, Panidis D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol 2008; 69: 634–41. [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E, Piouka A, Livadas S, Piperi C, Katsikis I, Papavassiliou AG, Panidis D. Anti-mullerian hormone is associated with advanced glycosylated end products in lean women with polycystic ovary syndrome. Eur J Endocrinol 2009; 160: 847–53. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol 2005; 62: 37–43. [DOI] [PubMed] [Google Scholar]
- 13.Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002; 143: 2221–32. [DOI] [PubMed] [Google Scholar]
- 14.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009; 324: 938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 2006; 20: 1300–21. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 2007; 27: 1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004; 303: 682–4. [DOI] [PubMed] [Google Scholar]
- 18.Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5'-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology 2009; 150: 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayampilly PP, Menon KM. AMPK activation by dihydrotestosterone reduces FSH-stimulated cell proliferation in rat granulosa cells by inhibiting ERK signaling pathway. Endocrinology 2012; 153: 2831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinno M, Takeuchi M, Watanabe A, Teruya K, Hirohama J, Eguchi N, Miyazaki A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum Reprod 2011; 26: 604–10. [DOI] [PubMed] [Google Scholar]