Short abstract
Orientin (luteolin-8-C-glucoside) is a phenolic compound found abundantly in millet, juice, and peel of passion fruit and has been shown to have antioxidant properties. In the present study, we explored the effects of orientin on oxygen-glucose deprivation/reperfusion (OGD/RP)-induced cell injury in primary culture of rat cortical neurons using an in vitro model of neonatal ischemic brain injury. The reduced cell viability and elevated lactate dehydrogenase leakage were observed after OGD/RP exposure, which were then reversed by orientin (10, 20, and 30 µM) pretreatment in a dose-dependent manner. Additionally, OGD/RP treatment resulted in significant oxidative stress, accompanied by enhanced intracellular reactive oxygen species (ROS) generation, and obvious depletion in the activities of intracellular Mn-superoxide dismutase, catalase, and glutathione peroxidase antioxidases. However, these effects were dose dependently restored by orientin pretreatment. We also found that orientin pretreatment dose dependently suppressed [Ca2+]i increase and mitochondrial membrane potential dissipation caused by OGD/RP in primary culture of rat cortical neurons. Western blot analysis showed that OGD/RP exposure induced a distinct decrease of Bcl-2 protein and a marked elevation of Bax, caspase-3, and cleaved caspase-3 proteins; whereas these effects were dose dependently reversed by orientin incubation. Both the caspase-3 activity and the apoptosis rate were increased under OGD/RP treatment, but was then dose dependently down-regulated by orientin (10, 20, and 30 µM) incubation. Moreover, orientin pretreatment dose dependently inhibited OGD/RP-induced phosphorylation of JNK and ERK1/2. Notably, JNK inhibitor SP600125 and ERK1/2 inhibitor PD98059 also dramatically attenuated OGD/RP-induced cell viability loss and ROS generation, and further, orientin failed to protect cortical neurons with the interference of JNK activator anisomycin or ERK1/2 activator FGF-2. Taken together, these results demonstrated that orientin has significant neuroprotective effects against OGD/RP-induced cell injury via JNK and ERK1/2 signaling pathways in primary culture of rat cortical neurons.
Impact statement
Orientin has been used in traditional eastern medicine and reported to possess antioxidant properties. However, the effects of orientin on neonatal ischemic brain injury and the underlying mechanisms involved have not been studied. Our results showed that orientin exerts significant neuroprotective effects on cell injury caused by oxygen-glucose deprivation/reperfusion via the JNK and ERK1/2 signaling pathways in primary culture of rat cortical neurons, implying the potential therapeutic application of orientin via the suppression of oxidative stress and cell apoptosis. This research suggested that orientin may be used as a therapeutic and preventive option for newborn cerebral ischemia/reperfusion injury.
Keywords: Orientin, oxygen-glucose deprivation/reperfusion, primary rat cortical neurons, oxidative stress, cell injury
Introduction
Perinatal/neonatal hypoxic–ischemic brain injury is a leading cause of mortality and morbidity in infants and children1 and occurs at a rate of three per thousand in live-born infants.2 However, there is no safe and effective pharmacotherapy for ischemic brain injury at present. As neuroprotection remains the central focus of the treatment of ischemic brain injury after reperfusion, it is necessary to find new suitable agent for neuroprotective therapies. In particular, protection against neuronal injury has become an important strategy in the development of novel neuroprotective therapies for neonatal ischemic brain injury.
Oxidative stress has been implicated in the mechanism through which ischemia/reperfusion induces brain injury.3 Oxidative injury to brain tissues manifests itself predominantly as lipid peroxidation. Lipid peroxidation generates many reactive electrophiles, some of which react with protein and DNA, resulting in toxic and mutagenic effects.4,5 Furthermore, oxidative stress is an early event of brain injury and has been reported to play an active role in the pathogenesis of brain injury, especially neurodegenerative diseases.6 It is well known that neurons are especially vulnerable to oxidative stress because of the limited antioxidant capacity.7 Oxidative stress is a pathological condition with overproduction of reactive oxygen species (ROS), which implies the cell’s antioxidant defense system becomes non-effective, ultimately resulting in neuronal cell death. It has also been proven that several kinds of antioxidants can reduce cerebral ischemia/reperfusion injury.8,9 Thus, the application of exogenous antioxidants and antiapoptotic agents may be used as an effective therapeutic intervention in ischemic brain injury. Additionally, an improved understanding of the underlying mechanisms is also essential for developing applicable treatment strategies.
Flavonoids are found ubiquitously in plants and recognized as the pigments responsible for the colors of leaves, especially during autumn. Certain plants and spices containing flavonoids have been used for thousands of years in traditional eastern medicine.10 Orientin (luteolin-8-C-glucoside) is a phenolic compound found abundantly in millet and the juice and peel of passion fruit (Passiflora tripartita Breiter, Passifloraceae)11,12 and has been reported to possess antioxidant properties.13 Previous work suggests that orientin is capable of crossing the blood–brain barrier.14 Isoorientin and orientin, as major compounds present in the crude aqueous extract of Cecropia pachystachya leaves, can cross the blood–brain barrier and act on central nervous system.15 Orientin attenuates cerebral ischemia/reperfusion injury in middle cerebral artery occlusion rat model.16 Pharmacokinetic studies in rabbits indicated that distribution and elimination of orientin were fastest with no accumulative toxication.17 Besides, orientin engenders antiaging effects through their antioxidant capacities18 and protects myocardial cells against hypoxia–reoxygenation injury.19 Orientin-2″-O-galactopyranoside also elicits neuroprotective effects in lipopolysaccharide-stimulated microglia via the induction of HO-1-mediated inhibition of ERK pathways.20 However, the effects of orientin on cell injury caused by oxygen-glucose deprivation/reperfusion (OGD/RP) in primary culture of rat cortical neurons and the underlying mechanisms involved have not been studied.
Nimodipine is a calcium antagonist and has the ability to cross the blood–brain barrier and enter the brain. It has been demonstrated that nimodipine has the potent cerebrovascular activity in vitro,21 and nimodipine has also been used as a positive control in several experiments against OGD/RP injury.22,23 In the present study, we aimed to investigate the possible neuroprotective effects of orientin on primary rat cortical neurons injured by OGD/RP, which can be used as an in vitro model of neonatal cerebral ischemia/reperfusion injury.
Materials and methods
Primary culture cortical neuron
The primary culture of cortical neurons was obtained from the brain cortex of embryonic day 17 (E17) and E18 Sprague-Dawley rat embryos. The dissociation and culture method was followed as described previously.24 The dissociated cerebrocortical cells were added to poly-l-lysine-coated culture plates and maintained in neurobasal medium (Gibco BRL, Rockville, MD, USA) with 2% B-27 (Gibco) supplement. After the cells has been seeded for 1–2 days, 1 µM cytosine arabinoside was added to the cultures to stop the cell proliferation of astroglia and microglia, and the purity of neuronal cultures was more than 90%.25 Twenty-four hours later, the media were totally changed, and then a 50% change of medium was performed every two days. All cells were cultured in an incubator with a humidified atmosphere of 5% CO2 at 37°C. Experiments were carried out 7–8 days after initial plating of cultures. All animal experimental procedures were in accordance with the NIH Guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
OGD/RP and drug treatment
The neurons were rinsed twice and cultured in glucose-free DMEM. Then, the culture media were introduced into a specialized, humidified chamber filled with 1% O2/94% N2/5% CO2 at 37°C for 6 h to produce OGD. Thereafter, the culture media were replaced with neurobasal medium supplemented with 2% B27 containing glucose under normoxic conditions for an additional 24 h at 37°C as OGD/RP. Orientin (C21H20O11, purity above 97%; Sigma, St. Louis, MO, USA) was diluted with distilled water at different concentrations. Then, neurons were pretreated with orientin (0, 10, 20, or 30 µM) or nimodipine (5 µg/mL; German Bayer Company) treatment for 24 h before OGD/RP exposure, and the levels of orientin were maintained during OGD/RP. The cells of the blank control group were incubated in normal DMEM medium under normoxic condition at 37°C in the absence of OGD/RP exposure and orientin pretreatment. The cells of the control group were treated with OGD/RP exposure in the absence of orientin pretreatment. The cells were harvested for subsequent experiments. When the JNK inhibitor SP600125, JNK activator anisomycin, ERK1/2 inhibitor PD98059, and ERK1/2 activator FGF-2 were introduced, the neurons were preincubated with 10 µM SP600125, 10 µM PD98059, 10 µg/mL anisomycin, or 1 nM FGF-2 for 30 min, or 30 µM orientin treatment for 24 h before OGD/RP exposure.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The neuronal cell viability was detected by the MTT assay or by determining the release of lactate dehydrogenase (LDH) into culture medium. Briefly, the cultured cells (5 × 103 cells/well) were grown in 96-well plates (Corning Inc., Corning, NY, USA). After exposure to OGD/RP, the cells were washed, and 20 µL modified tetrazolium salt MTT (5 mg/mL; Sigma, St. Louis, MO, USA) was added to each well, followed by incubation for 4 h at 37°C. The supernatant was then carefully removed and 100 µL DMSO was added to solubilize the formazan salt formed. Thereafter, the absorbance was determined at 490 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
LDH assay
The neuronal cell injury was also quantitatively assessed by detecting the LDH activity released from damaged or dead cells using the LDH activity assay kit (Nanjing Jiangcheng Bioengineering Institute, Nanjing, China). Briefly, the cells (5 × 103 cells/well) were grown in 96-well plates and cultured for 24 h. After OGD/RP treatment, 50 µL medium was removed and the amount of LDH leakage from the cells was measured according to the manufacturer’s protocol. The absorbance of the samples was measured spectrophotometrically at 490 nm, and the results were expressed as the percentage of LDH release relative to the control.
Measurement of intracellular ROS content
The ROS formation was measured using the fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology, Haimen, Jiangsu, China). Intracellular DCFH-DA was deacetylated by non-specific esterase, which was further oxidized to the fluorescent dichlorofluorescein (DCF) in response to ROS. The cells were rinsed twice with PBS, and then cultured with 10 µM DCFH-DA for 30 min in the dark at 37°C. The cells were then collected and suspended in PBS. The DCF fluorescence intensity was determined by a fluorospectrophotometer with excitation wavelength of 488 nm and emission wavelength of 525 nm.26
Intracellular catalase (CAT), Mn-superoxide dismutase (Mn-SOD), and glutathione peroxidase (GSH-PX) activity assay
After OGD/RP treatment, the cells were collected, resuspended in PBS, sonicated on ice, and then centrifugated. The supernatants were collected and assayed for CAT, Mn-SOD, and GSH-PX activities using the commercial kits (Nanjing Jiangcheng Bioengineering Institute). Activities of these antioxidases were normalized by protein concentration of each sample and presented as U/mg protein.
Measurement of [Ca2+]i
After OGD/RP treatment, the cells were collected, washed with PBS, centrifuged, and resuspended in PBS. The cell suspensions were then incubated with 5 µM Fluo-3 AM for 40 min in the dark at 37°C. Thereafter, the cells were centrifugated, washed with PBS, and then resuspended in PBS. Finally, the florescence was analyzed by flow cytometry at 488 nm and the relative florescence intensity of Fluo-3 was used to indicate the quantity of [Ca2+]i.27
Measurement of mitochondrial membrane potential (MMP)
The MMP was determined using a fluorescence method in primary cultures of rat cortical neurons under OGD/RP exposure.28 After OGD/RP treatment, we measured the MMP by the release of Rhodamine-123 from the mitochondria using RF-5000 spectroflourometer at an excitation wavelength of 488 nm and an emission wavelength of 535 nm. Additionally, 5 µM Rhodamine-123 was added for 15 min, and then the fluorescence intensity was monitored.
Western blotting
Proteins were extracted from cells via the addition of cold RIPA lysis buffer. Western blotting was carried out according to the standard protocols. Total protein was quantified and separated by SDS-PAGE and blotted onto PVDF membranes (Millipore). The primary antibodies including cleaved caspase-3, phospho-ERK1/2, ERK1/2, phospho-JNK, and JNK antibodies (Cell Signaling Technology, Beverly, MA, USA); caspase-3, Bcl-2, and Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA); β-actin (Sigma, St Louis, MO, USA) were incubated at 4°C overnight. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The resultant protein bands were visualized by ECL reagents according to the manufacturer’s protocol. The optical density of each band was quantitatively analyzed through Gel-Pro Analyzer version 4.0 software (Media Cybernetics, Silver Spring, MD, USA).
Caspase-3 activity assay
The caspase-3 activity was measured using the commercialized kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. Ten microliters of protein were incubated with 10 µL caspase-3 substrate N-Acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) in a volume of 100 µL for 2 h at 37°C. The colorimetric release of p-nitroaniline from the Ac-DEVD-pNA substrate was recorded at 405 nm.
Cell apoptosis assay
Cell apoptosis rate was determined by flow cytometry analysis according to the instructions of Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis Detection Kit (R&D systems, Abingdon, UK). Cultured cells were washed twice in PBS, suspended with 500 µL binding buffer, and then treated with 5 µL Annexin V-FITC/PI for 12 min in the dark at room temperature. Thereafter, cell apoptosis rate was performed on a FACSAria flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The results were expressed as the means ± SD derived from three independent experiments. Statistical analysis was carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Multiple comparisons were carried out using the one-way ANOVA analysis. P < 0.05 was considered as statistically significant.
Results
Orientin protected cells against OGD/RP-induced cytotoxicity in primary culture of rat cortical neurons
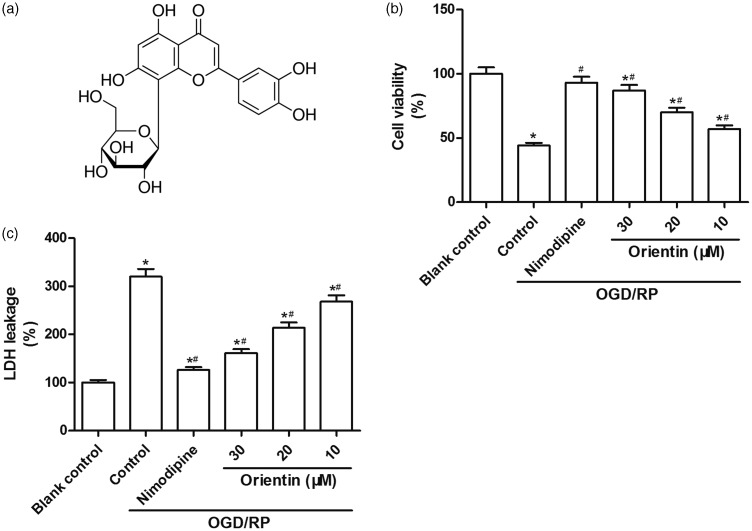
The chemical structure of orientin is shown in Figure 1(a). MTT assay and LDH assay were performed to measure the effects of orientin on OGD/RP-induced cytotoxicity in primary culture of rat cortical neurons. Compared to the blank control group, the cell viability was distinctly reduced under OGD/RP exposure and then dose dependently restored by orientin pretreatment at concentrations of 10, 20, and 30 µM (Figure 1(a)). In addition, the LDH leakage was elevated under OGD/RP treatment and then dose dependently weakened by orientin (10, 20, and 30 µM) incubation (Figure 1(b)). These findings showed that orientin pretreatment protected cells against OGD/RP-induced cytotoxicity in primary culture of rat cortical neurons.
Figure 1.
Effects of different concentrations of orientin on cytotoxicity in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a) The chemical structure of orientin was shown. (b) The cell viability was measured via the MTT assay. (c) The LDH leakage was evaluated via the commercialized LDH activity assay kit. *P < 0.05 versus Blank control, #P < 0.05 versus Control.LDH: lactate dehydrogenase; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OGD: oxygen-glucose deprivation.
Orientin protected cells against OGD/RP-induced oxidative stress in primary culture of rat cortical neurons
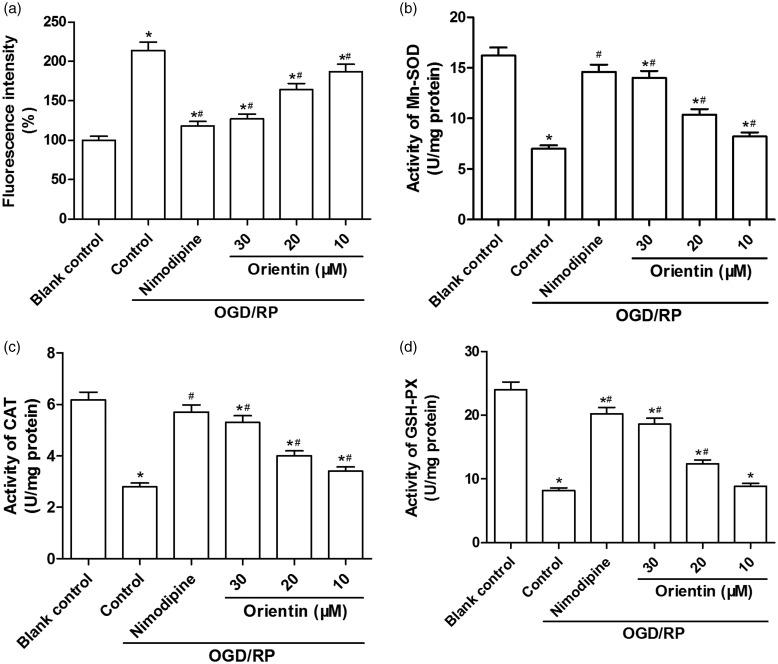
OGD/RP exposure markedly elevated intracellular ROS generation, whereas orientin (10, 20, and 30 µM) pretreatment resulted in a noticeable reduction of OGD/RP-induced ROS production in a dose-dependent manner (Figure 2(a)). An obvious depletion in the content of cellular Mn-SOD, CAT, and GSH-PX was found after OGD/RP exposure. Then, the effects of orientin on OGD/RP-induced oxidative stress were determined in primary culture of rat cortical neurons, and the data revealed that orientin (10, 20, and 30 µM) pretreatment dose dependently enhanced the activities of these intracellular antioxidases (Figure 2(b) to (d)). Therefore, these data suggested that orientin protected primary culture of rat cortical neurons against OGD/RP-induced oxidative stress.
Figure 2.
Effects of different concentrations of orientin on the oxidative stress in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a) The level of intracellular ROS was measured via DCF fluorescence intensity by a fluorospectrophotometer. The activities of the antioxidases; Mn-SOD (b), CAT (c), and GSH-PX (d) were examined via the commercial kits. *P < 0.05 versus Blank control, #P < 0.05 versus Control.CAT: catalase; DCF: dichlorofluorescein; GSH-PX: glutathione peroxidase; Mn-SOD: Mn-superoxide dismutase; OGD: oxygen-glucose deprivation; ROS: reactive oxygen species.
Orientin protected cells against OGD/RP-induced [Ca2+]i increase and MMP dissipation in primary culture of rat cortical neurons
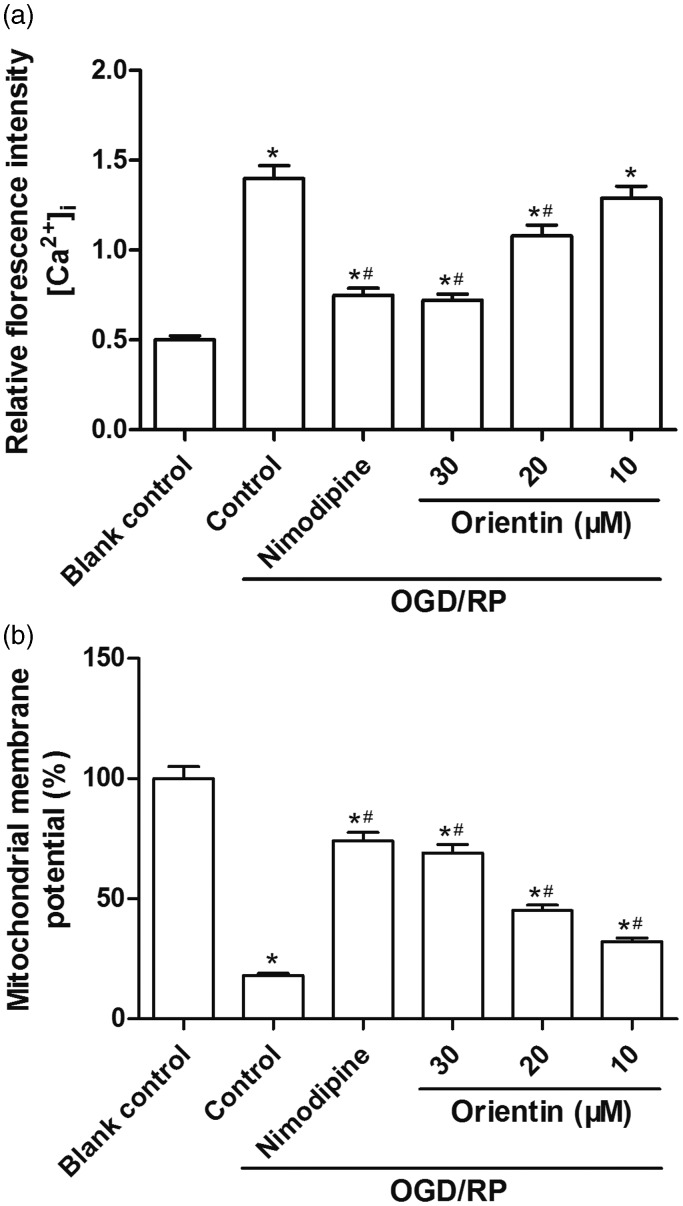
In this research, we also observed that the values of [Ca2+]i were distinctly enhanced under OGD/RP treatment in primary culture of rat cortical neurons. However, orientin (10, 20, and 30 µM) pretreatment dose dependently suppressed the elevated intracellular [Ca2+]i (Figure 3(a)). Additionally, OGD/RP exposure resulted in the dissipation of the MMP, but pretreatment with orientin (10, 20, and 30 µM) markedly stabilized the MMP in a dose-dependent manner (Figure 3(b)). Specially, the stabilized effect in 30 µM orientin-pretreated group was better than others.
Figure 3.
Effects of different concentrations of orientin on [Ca2+]i and MMP alterations in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a) The [Ca2+]i was detected using flow cytometry analysis. The relative fluorescent intensity of Fluo-3 was used to indicate the [Ca2+]i quantity. (b) The MMP was monitored using a fluorescence method. *P < 0.05 versus Blank control, #P < 0.05 versus Control.MMP: mitochondrial membrane potential; OGD: oxygen-glucose deprivation.
Orientin protected cells against OGD/RP-induced cell apoptosis in primary culture of rat cortical neurons
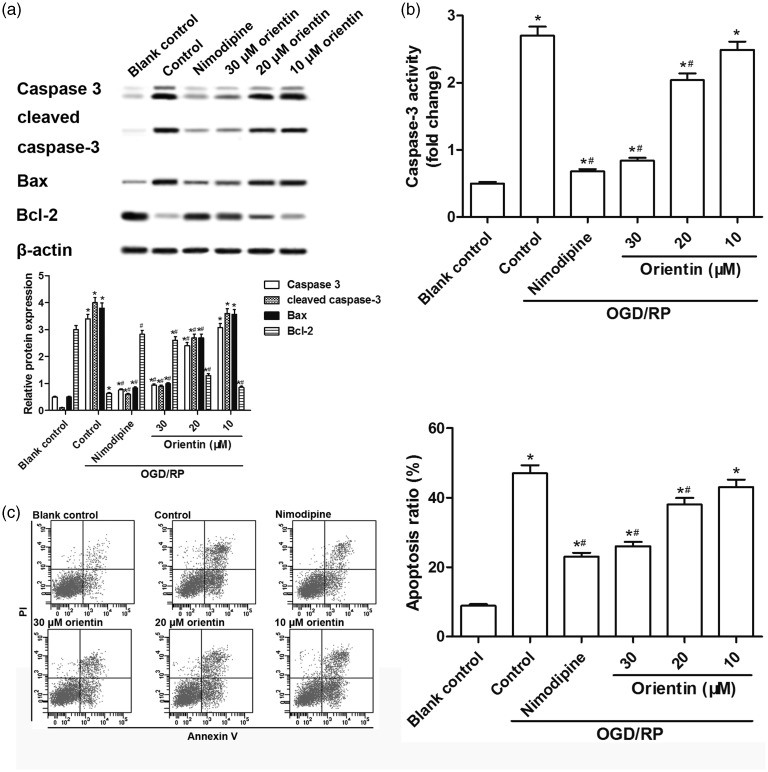
Western blot analysis showed that OGD/RP treatment induced a distinct decrease of Bcl-2 protein, and a marked elevation of Bax, caspase-3, and cleaved caspase-3 proteins, which were dose dependently reversed by orientin (10, 20, and 30 µM) incubation (Figure 4(a)). In addition, both the caspase-3 activity and the apoptosis rate were increased under OGD/RP treatment, but was then dose dependently down-regulated by orientin (10, 20, and 30 µM) incubation (Figure 4(b) and (c)). These results indicated that orientin pretreatment protected cells against OGD/RP-induced cell apoptosis in primary culture of rat cortical neurons.
Figure 4.
Effects of different concentrations of orientin on cell apoptosis in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a) The levels of caspase-3, cleaved caspase-3, Bcl-2, Bax, and β-actin proteins were measured by western blotting and the representative blots were presented. (b) The caspase-3 activity was measured via the commercial kit. (c) The cell apoptosis rate was detected by flow cytometry analysis. *P < 0.05 versus Blank control, #P < 0.05 versus Control.
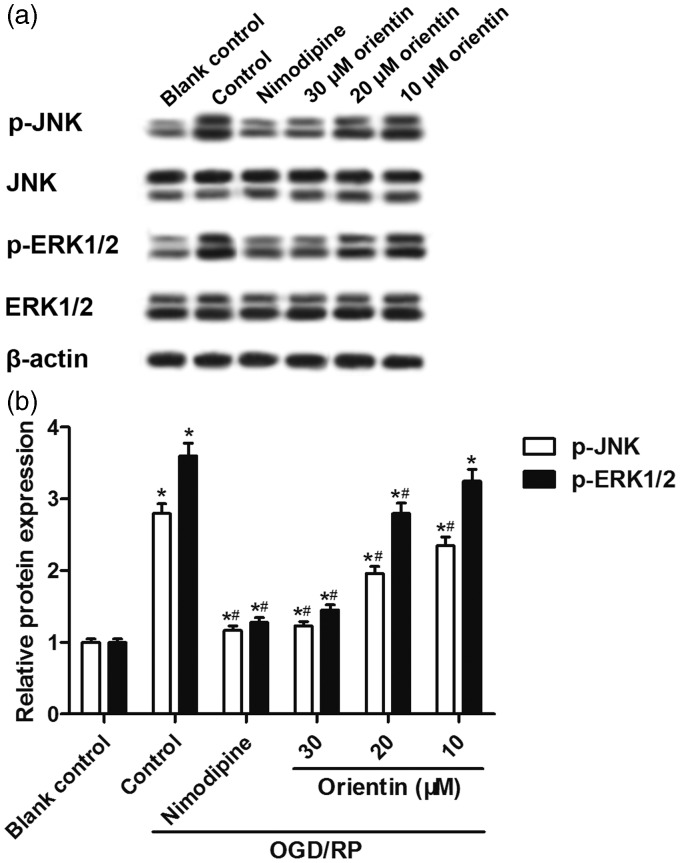
Orientin reduced OGD/RP-induced JNK and ERK1/2 activation in primary culture of rat cortical neurons
Furthermore, we explored whether the JNK and ERK1/2 signaling pathways were involved in the neuroprotective effects of orientin against OGD/RP-induced cell injury in primary rat cortical neurons. Western blot analysis showed that the levels of p-JNK and p-ERK1/2 proteins were up-regulated caused by OGD/RP exposure, but were then dose dependently down-regulated by orientin pretreatment at concentrations of 10, 20, and 30 µM (Figure 5(a) and (b)), implying that orientin reduced OGD/RP-induced JNK and ERK1/2 activation in primary culture of rat cortical neurons.
Figure 5.
Effects of different concentrations of orientin on the activation of JNK and ERK1/2 signaling pathways in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a, b) The levels of p-JNK, p-ERK1/2, JNK, ERK1/2, and β-actin proteins were detected by western blotting and representative blots were presented. *P < 0.05 versus Blank control, #P < 0.05 versus Control.ERK1/2:extracellular signal-regulated protein kinases 1/2; JNK: c-Jun N-terminal kinase; OGD: oxygen-glucose deprivation; p-ERK1/2: phosphorylated extracellular signal-regulated protein kinases 1/2; p-JNK: phosphorylated c-Jun N-terminal kinase.
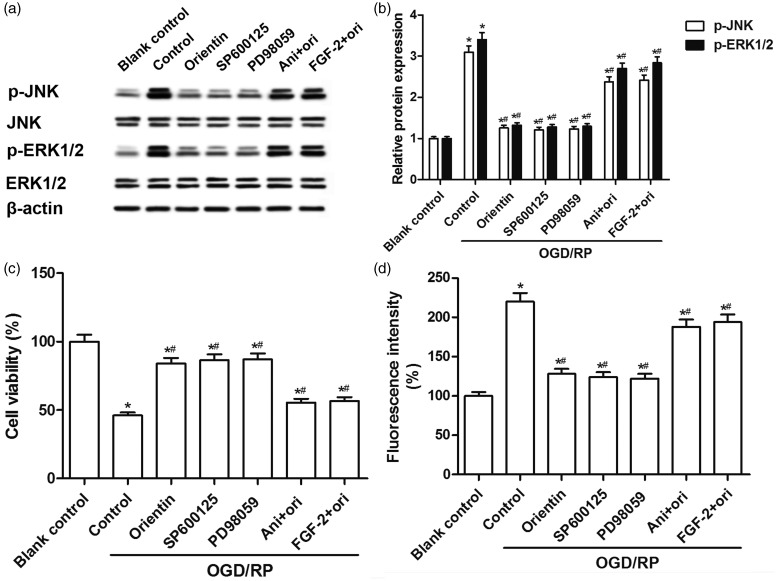
Orientin attenuated OGD/RP-induced cell injury via JNK and ERK1/2 signaling pathways in primary rat cortical neurons
Notably, we observed that JNK inhibitor SP600125 and ERK1/2 inhibitor PD98059 suppressed OGD/RP-induced phosphorylation of JNK and ERK1/2 proteins, whereas JNK activator anisomycin and ERK1/2 activator FGF-2 blocked orientin-inhibited phosphorylation of JNK and ERK1/2 proteins caused by OGD/RP in primary rat cortical neurons (Figure 6(a) and (b)). SP600125 and PD98059 dramatically attenuated OGD/RP-induced cell viability loss and ROS generation, and further, orientin failed to protect cortical neurons with the interference of JNK activator anisomycin or ERK1/2 activator FGF-2 (Figure 6(c) and (d)). Thus, it may be concluded that orientin attenuated OGD/RP-induced cell injury via JNK and ERK1/2 signaling pathways in primary rat cortical neurons.
Figure 6.
Effects of orientin, JNK, and ERK1/2 signaling pathways on cell injury in primary rat cortical neurons under OGD exposure for 6 h followed by reoxygenation for 24 h. (a, b) The neurons were preincubated with 10 µM SP600125, 10 µM PD98059, 10 µg/mL anisomycin, or 1 nM FGF-2 for 30 min, or 30 µM orientin treatment for 24 h before OGD/RP exposure. The levels of p-JNK, p-ERK1/2, JNK, ERK1/2, and β-actin proteins were detected by western blotting and representative blots were presented. (c) The cell viability was determined via the MTT assay. (d) The levels of intracellular ROS were measured via DCF fluorescence intensity by a fluorospectrophotometer. *P < 0.05 versus Blank control, #P < 0.05 versus Control.DCF: dichlorofluorescein; ERK1/2: extracellular signal-regulated protein kinases 1/2; FGF-2: fibroblast growth factor-2; JNK: c-Jun N-terminal kinase; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OGD/RP: oxygen-glucose deprivation/reperfusion; p-ERK1/2: phosphorylated extracellular signal-regulated protein kinases 1/2; p-JNK: phosphorylated c-Jun N-terminal kinase; ROS: reactive oxygen species.
Discussion
Neonatal hypoxic–ischemic brain injury is also an important cause of long-term disabilities and neurological disorders.29 Orientin has been shown to have antioxidant properties, and it is possible that orientin may contribute to the recovery function after ischemia/reperfusion brain injury. In this study, we used OGD/RP as an in vitro model that mimics the in vivo neonatal ischemia/reperfusion brain injury, and then investigated whether orientin could reduce cell injury following OGD/RP treatment in primary culture of rat cortical neurons.
After transient deprivation of oxygen and glucose, the reperfusion disrupts the permeability of cell membrane, and a series of devastating cascades are induced, for instance, cell viability loss, ROS generation, reduced antioxidases activities, mitochondrial dysfunction, expression of pro-apoptotic factors, excessive excitatory amino acid release as well as inflammation, and ultimately results in neuronal cell death.23,30 Hence, multiple interventions have been introduced to attenuate cell injury induced by OGD/RP, for instance, maintaining intracellular Ca2+ levels. Ca2+ overloading can cause elevated cell vulnerability and oxidative stress in the process of cell apoptosis, and subsequently trigger the activation of downstream signaling pathways.31 Excessive Ca2+ flow eventually causes acute or delayed neuronal death.32 Indeed, we found that OGD/RP induced cytotoxicity, cell apoptosis, oxidative stress, [Ca2+]i increase, and MMP dissipation as well as the activation of JNK and ERK1/2 pathways in primary culture of rat cortical neurons.
Orientin, a C-glycosyl flavonoid, has been traditionally used in the treatment of rheumatoid arthritis, coronary heart disease, and hernia. It has been reported to have various pharmacological properties, such as antianxiety, antiapoptosis, antiglycation, antithrombus, antioxidant, and antimicrobial, anti-inflammation, and radioprotection.18,33,34 It has also been demonstrated that orientin protects against hypoxia reoxygenation injury accompanied by increased cell viability and reduced cell apoptosis in neonatal rat cardiomyocytes.19 Orientin treatment significantly reduces the increased percentage of apoptotic cells and Caspases-3 activity induced by hydrogen peroxide in SH-SY5Y cells.14 Our results indicated that orientin protected cells against OGD/RP-induced cytotoxicity and cell apoptosis in primary culture of rat cortical neurons.
Increasing evidence has suggested that natural antioxidants possess potential roles in blunting lipid peroxidation in neurons challenged with oxidative stress stimuli. Previous study suggests that the possible neuroprotective role of orientin might be attributed to the alleviation of mitochondrial apoptotic pathway caused by oxidative stress.14 Orientin provides protection against radiation-induced lipid peroxidation in mouse liver and shows a significantly greater free radical-inhibiting activity in vitro, implying that orientin may exert radiation protection via the mechanism of free radical scavenging.35 In addition, orientin, as one of C-glycosyl-flavones, may act as inhibitors of non-enzymic lipid peroxidation.36 Specifically, orientin reduces cognitive deficits and levels of oxidative stress, indicated by decreased ROS production, mitochondrial dysfunction, and mitochondrial apoptotic pathway in the mouse model of Alzheimer’s disease caused by Aβ1–42.37 Orientin treatment markedly increases the production of glutathione and the activities of SOD and CAT, and further, attenuates cognitive impairments in the brain of noise-exposed mice.13 Orientin produces antidepressant-like effects by elevating central oxidative stress levels in chronic unpredictable mild stress mice.38 In the current study, the results demonstrated that orientin protected cells against OGD/RP-induced oxidative stress in primary culture of rat cortical neurons. Importantly, orientin exerts protective effects against mitochondrial dysfunction and apoptosis induced by ischemia/reperfusion injury in H9c2 cells, mainly via the inhibition of ROS generation, MMP repolarization, reduction of mitochondrial cytochrome C release, increase of Bcl-2 level, and decrease of Bax level.39 Here, our data also revealed that orientin could protect cells against OGD/RP-induced [Ca2+]i increase and MMP dissipation in primary culture of rat cortical neurons.
To further explore the underlying signaling pathways related to the neuroprotective effects of orientin on cell injury caused by OGD/RP in primary rat cortical neurons, we examined the activation of JNK and ERK1/2 proteins via the introduction of signaling pathway inhibitors and activators. Orientin is one of the main phenolics of Dendropanax morbifera, which has been reported to possess antiamnesic effect on cognitive dysfunction through JNK signaling pathway in diabetic mice caused by high-fat diet.40 It has also been considered that orientin suppresses the production of inflammatory factor and ERK 1/2 activation induced by LPS.41 Orientin inhibits LPS-mediated levels of secretory group IIA phospholipase A2 by reducing the activation of cytosolic phospholipase A2 and ERK1/2 in human umbilical vein endothelial cells.42 Furthermore, PD98059 protects brain from cells death by inhibiting ROS/ERK activation in a cardiac arrest rat model.43 It is noteworthy that ROS may act as upstream signaling of JNK activation, and SP600125 significantly decreases JNK-activated apoptosis in human cancer cells.44 In the present study, our data confirmed that orientin attenuated OGD/RP-induced cell injury via JNK and ERK1/2 signaling pathways in primary rat cortical neurons.
Taken together, our results showed that orientin exerts significant neuroprotective effects on cell injury caused by OGD/RP via the JNK and ERK1/2 signaling pathways in primary culture of rat cortical neurons. This research highlighted the potential therapeutic application of orientin via the suppression of oxidative stress. Further investigations are still required to evaluate the potential use of orientin as protective agent in vivo in cerebral ischemia/reperfusion injury.
Authors’ contributions
TT conceived and designed the experiments. JZ conducted the experiments. GZ analyzed the data. WZ and SG contributed reagents/materials/analysis tools. TT and LL wrote the manuscript. TT and JZ contributed equally to this paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol 2011; 69:743–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res 2001; 49:735–41 [DOI] [PubMed] [Google Scholar]
- 3.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001; 21:2–14 [DOI] [PubMed] [Google Scholar]
- 4.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res 1999; 424:83–95 [DOI] [PubMed] [Google Scholar]
- 5.Platenik J, Stopka P, Vejrazka M, Stipek S. Quinolinic acid-iron(ii) complexes: slow autoxidation, but enhanced hydroxyl radical production in the Fenton reaction. Free Radic Res 2001; 34:445–59 [DOI] [PubMed] [Google Scholar]
- 6.Pratico D. Alzheimer’s disease and oxygen radicals: new insights. Biochem Pharmacol 2002; 63:563–7 [DOI] [PubMed] [Google Scholar]
- 7.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol 2000; 62:649–71 [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Zhang B, Zhang Y. Protective effects of Acanthopanax polysaccharides on cerebral ischemia-reperfusion injury and its mechanisms. Int J Biol Macromol 2015; 72:946–50 [DOI] [PubMed] [Google Scholar]
- 9.Ashafaq M, Khan MM, Shadab Raza S, Ahmad A, Khuwaja G, Javed H, Khan A, Islam F, Siddiqui MS, Safhi MM. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr Res 2012; 32:133–43 [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013; 2013:162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simirgiotis MJ, Schmeda-Hirschmann G, Borquez J, Kennelly EJ. The Passiflora tripartita (Banana Passion) fruit: a source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Molecules 2013; 18:1672–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazier-Hicks M, Evans KM, Gershater MC, Puschmann H, Steel PG, Edwards R. The C-glycosylation of flavonoids in cereals. J Biol Chem 2009; 284:17926–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Yu Y, Feng Y, Zou F, Zhang X, Huang J, Zhang Y, Zheng X, Huang XF, Zhu Y, Liu Y. Protective effect of the orientin on noise-induced cognitive impairments in mice. Behav Brain Res 2016; 296:290–300 [DOI] [PubMed] [Google Scholar]
- 14.Law BN, Ling AP, Koh RY, Chye SM, Wong YP. Neuroprotective effects of orientin on hydrogen peroxide induced apoptosis in SHSY5Y cells. Mol Med Rep 2014; 9:947–54 [DOI] [PubMed] [Google Scholar]
- 15.Mendonca ED, da Silva J, Dos Santos MS, Carvalho P, Papke DK, Ortmann CF, Picada JN, Reginatto FH, de Barros F, Ferraz A. Genotoxic, mutagenic and antigenotoxic effects of Cecropia pachystachya Trecul aqueous extract using in vivo and in vitro assays. J Ethnopharmacol 2016; 193:214–20 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, An F, Wang S, An Z. Orientin attenuates cerebral ischemia/reperfusion injury in rat model through the AQP-4 and TLR4/NF-kappaB/TNF-alpha signaling pathway. J Stroke Cerebrovasc Dis 2017;26:2199-214 [DOI] [PubMed]
- 17.Li D, Wang Q, Yuan ZF, Zhang L, Xu L, Cui Y, Duan K. Pharmacokinetics and tissue distribution study of orientin in rat by liquid chromatography. J Pharm Biomed Anal 2008; 47:429–34 [DOI] [PubMed] [Google Scholar]
- 18.An F, Yang G, Tian J, Wang S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in d-galactose-aged mice. Neural Regen Res 2012; 7:2565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Wu Y, Huang X. Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy. Eur J Pharmacol 2016; 776:90–8 [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Gan P, Hao L, Tao L, Jia J, Gao B, Liu JY, Zheng LT, Zhen X. Antiinflammatory effects of orientin-2″-O-galactopyranoside on lipopolysaccharide-stimulated microglia. Biol Pharm Bull 2014; 37:1282–94 [DOI] [PubMed] [Google Scholar]
- 21.Wadworth AN, McTavish D. Nimodipine. A review of its pharmacological properties, and therapeutic efficacy in cerebral disorders. Drugs Aging 1992; 2:262–86 [DOI] [PubMed] [Google Scholar]
- 22.Wang CP, Zhang LZ, Li GC, Shi YW, Li JL, Zhang XC, Wang ZW, Ding F, Liang XM. Mulberroside A protects against ischemic impairment in primary culture of rat cortical neurons after oxygen-glucose deprivation followed by reperfusion. J Neurosci Res 2014; 92:944–54 [DOI] [PubMed] [Google Scholar]
- 23.Wang CP, Li JL, Zhang LZ, Zhang XC, Yu S, Liang XM, Ding F, Wang ZW. Isoquercetin protects cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via suppression of TLR4-NF-small ka, CyrillicB signal pathway. Neurochem Int 2013; 63:741–9 [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Zhang Q, Yu S, Yang Y, Ding F. The protective effects of chitooligosaccharides against glucose deprivation-induced cell apoptosis in cultured cortical neurons through activation of PI3K/Akt and MEK/ERK1/2 pathways. Brain Res 2011; 1375:49–58 [DOI] [PubMed] [Google Scholar]
- 25.Negishi T, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Primary culture of cortical neurons, type-1 astrocytes, and microglial cells from cynomolgus monkey (Macaca fascicularis) fetuses. J Neurosci Methods 2003; 131:133–40 [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Xia Q, Luo RJ, Lin ZQ, Xue P. In vitro study of the antagonistic effect of low-dose liquiritigenin on gemcitabine-induced capillary leak syndrome in pancreatic adenocarcinoma via inhibiting ROS- mediated signalling pathways. Asian Pac J Cancer Prev 2015; 16:4369–76 [DOI] [PubMed] [Google Scholar]
- 27.Mo ZT, Fang YQ, He YP, Zhang S. beta-Asarone protects PC12 cells against OGD/R-induced injury via attenuating Beclin-1-dependent autophagy. Acta Pharmacol Sin 2012; 33:737–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikari A, Nakajima K, Kawano K, Suketa Y. Polyvalent cation-sensing mechanism increased Na(+)-independent Mg(2+) transport in renal epithelial cells. Biochem Biophys Res Commun 2001; 287:671–4 [DOI] [PubMed] [Google Scholar]
- 29.Perrone S, Stazzoni G, Tataranno ML, Buonocore G. New pharmacologic and therapeutic approaches for hypoxic-ischemic encephalopathy in the newborn. J Matern Fetal Neonatal Med 2012; 25:83–8 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ma T, Zhou L, Li M, Sun XJ, Wang YG, Gu S. Penehyclidine hydrochloride protects against oxygen and glucose deprivation injury by modulating amino acid neurotransmitters release. Neurol Res 2013; 35:1022–8 [DOI] [PubMed] [Google Scholar]
- 31.Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 2014; 21:123–37 [DOI] [PubMed] [Google Scholar]
- 32.D’Orsi B, Kilbride SM, Chen G, Perez Alvarez S, Bonner HP, Pfeiffer S, Plesnila N, Engel T, Henshall DC, Dussmann H, Prehn JH. Bax regulates neuronal Ca2+ homeostasis. J Neurosci 2015; 35:1706–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ku SK, Kwak S, Bae JS. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation 2014; 37:2164–73 [DOI] [PubMed] [Google Scholar]
- 34.Bouchouka E, Djilani A, Bekkouche A. Antibacterial and antioxidant activities of three endemic plants from Algerian Sahara. Acta Sci Pol Technol Aliment 2012; 11:61–5 [PubMed] [Google Scholar]
- 35.Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK. Radiation protection by the ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res 2000; 154:455–60 [DOI] [PubMed] [Google Scholar]
- 36.Mora A, Paya M, Rios JL, Alcaraz MJ. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem Pharmacol 1990; 40:793–7 [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Wang S, Chen X, Yang H, Li X, Xu Y, Zhu X. Orientin alleviates cognitive deficits and oxidative stress in Abeta1-42-induced mouse model of Alzheimer’s disease. Life Sci 2015; 121:104–9 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Lan N, Ren J, Wu Y, Wang ST, Huang XF, Yu Y. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol Nutr Food Res 2015; 59:1130–42 [DOI] [PubMed] [Google Scholar]
- 39.Lu N, Sun Y, Zheng X. Orientin-induced cardioprotection against reperfusion is associated with attenuation of mitochondrial permeability transition. Planta Med 2011; 77:984–91 [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Park SK, Guo TJ, Kang JY, Ha JS, Lee Du S, Lee U, Heo HJ. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav Brain Res 2016; 312:39–54 [DOI] [PubMed] [Google Scholar]
- 41.Lee W, Ku SK, Bae JS. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vascul Pharmacol 2014; 62:3–14 [DOI] [PubMed] [Google Scholar]
- 42.Bae JS. Inhibitory effect of orientin on secretory group IIA phospholipase A2. Inflammation 2015; 38:1631–8 [DOI] [PubMed] [Google Scholar]
- 43.Nguyen Thi PA, Chen MH, Li N, Zhuo XJ, Xie L. PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev 2016; 2016:3723762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LH, Li HH, Li M, Wang S, Jiang XR, Li Y, Ping GF, Cao Q, Liu X, Fang WH, Chen GL, Yang JY, Wu CF. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Prolif 2015; 48:718–28 [DOI] [PMC free article] [PubMed] [Google Scholar]