Short abstract
Mechanical ventilation is extensively used to treat patients with lung injury but may result in ventilator-induced lung injury (VILI). The present study investigated the protective effect of alpha 1-antitrypsin (AAT) on VILI. Adult male rats were subjected to sham, ventilation + saline, or ventilation + AAT treatment and lung injuries were evaluated. Peripheral blood and bronchoalveolar lavage fluid (BALF) were obtained to assess systemic and local inflammatory responses, respectively. Mechanical ventilation resulted in lung injury, as evidenced by histological abnormalities as well as elevations in PaO2/FiO2 ratio, the wet-to-dry weight ratio, and the BALF level of proteins. The intravenous administration of AAT significantly improved these parameters of lung function, suggesting a protective role of AAT in VILI. Mechanistically, ventilator-induced inflammation was effectively reduced by AAT, as evidenced by decreases in BALF neutrophil counts, BALF cytokines, and serum adhesion factors. In contrast, anti-inflammatory interleukin-10 in BALF was increased in response to AAT. AAT treatment also inhibited the expression of nuclear factor-κB, Bax, and cleaved caspase-3 while promoting Bcl-2 expression in ventilator-injured lung tissues. AAT treatment can ameliorate VILI by inhibiting inflammatory mediator production and apoptosis.
Impact statement
Mechanical ventilation has been commonly used to treat patients with lung injury but may result in ventilator-induced lung injury (VILI). Few effective treatment options are currently available to reduce VILI. Alpha 1-antitrypsin (AAT) is an inhibitor of serine protease with anti-inflammatory and antiapoptotic properties, suggesting a possible role in attenuating lung injury.
The present study demonstrates that AAT inhibits the development of VILI by modulating inflammation- and apoptosis-related protein expression. Therefore, AAT may be a novel therapeutic agent for acute respiratory distress syndrome patients undergoing mechanical ventilation.
Keywords: Alpha 1-antitrypsin, ventilator-induced lung injury, inflammation, apoptosis
Introduction
Mechanical ventilation is extensively used in patients with lung injury or lung dysfunction. Approximately 39% of patients in intensive care units have been ventilated.1 Although small tidal volume ventilation has been proven to be beneficial in patients with acute respiratory distress syndrome (ARDS),2 ventilator-induced lung injury (VILI) may occur in 24% of ARDS patients who are subjected to mechanical ventilation and contributes to 40–50% of deaths.3 Thus, a better understanding of the pathogenesis of VILI and developing more effective therapeutic strategies are clinically urgent for the treatment of lung dysfunction.
High tidal volume ventilation may cause alveolar overstretching and increased alveolocapillary permeability, leading to local inflammation and pulmonary edema, which play critical roles in the pathogenesis of VILI.4–8 AAT is an inhibitor of serine protease with anti-inflammation and antiapoptotic properties.9–11 Previous studies reported that AAT can ameliorate islet injury11,12 and endothelial/epithelial cell injury by inhibiting inflammation,13–15 apoptosis, and neutrophils elastase. Based on these findings, we hypothesize that AAT may have a beneficial effect on VILI.
The present study explored the role of AAT in VILI and the underlying mechanisms using a rat model preconditioned with high tidal ventilation. Our results demonstrated that AAT may attenuate inflammation and apoptosis in VILI by modulating inflammation- and apoptosis-related protein expression, thereby improving VILI.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee at The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China. All the procedures and treatments in this study were conducted following the guidance of the Institutional Animal Care and Use Committee at the same institution noted above.
VILI rat model
Twenty-four male rats (250–300 g, 10 weeks old) were purchased from Qingdao University and maintained under pathogen-free conditions at the animal experiment center at Qingdao University. The rats were randomly divided into three groups (n = 8/group): sham group (S), ventilation/saline group (V), and ventilation/AAT group (VA). All rats were anesthetized with 3% pentobarbital sodium (30 mg/kg; Sigma-Aldrich, Shanghai, China) intraperitoneally. The anesthesia of all rats was maintained with pentobarbital sodium (10 mg/kg) and vecuronium (0.1 mg/kg) in 1 h intervals. The caudal vein and artery of the rat tail were cannulated for blood sample withdrawal and intravenous injections. The rats in the S group only received anesthesia, while those in the V and VA groups also received tracheotomy and 4 h of mechanical ventilation with a tidal volume of 30 mL/kg,16,17 respiratory rate of 50/min, and inspiratory to expiratory ratio of 1:1. The saline or a single dose of AAT (2 mg/kg) was intravenously injected in the V and VA groups18 immediately following ventilation. The body temperature of the rats was monitored with a rectal temperature probe and maintained at approximately 37°C with a thermal blanket. The state of the rats (alive or dead) during experimentation was judged by heart rate and blood pressure. Moribund animals were defined as bradycardia with a heart rate < 40 beats/min or a mean blood pressure < 30 mmHg. At the end of the experiment, all rats were sacrificed with an overdose of anesthetics, and the lungs were collected for further analyses.
Arterial blood gas (ABG) analysis
Arterial blood samples were withdrawn before and after ventilation, respectively. ABG analysis was performed with a Rapidlab 348 analyzer (Bayer, Leverkusen, Germany). The ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) was calculated.
The serum concentrations of adhesion factors
The peripheral blood samples were withdrawn before and after ventilation to determine the concentrations of intercellular adhesion molecule-1 (ICAM-1) and macrophage inflammatory protein-2 (MIP-2) using corresponding ELISA kits (Boster Bio-engineering, Wuhan, Hubei, China).
Bronchoalveolar lavage fluid (BALF) analysis
The right bronchus was blocked with an arterial clamp, and the BALF collection was performed in the left lung with five cycles of infusion/withdrawal with ice-cold saline containing EDTA-2Na (15 mL/kg), followed by centrifugation at 1000 g at 4°C for 15 min. The concentration of each cytokine was determined with corresponding ELISA kits (Boster Bio-engineering, Wuhan, Hubei, China). The Giemsa-stained neutrophils in the BALF were counted in randomly selected eight fields of each group by an independent pathologist under a Nikon microscope (Nikon, Tokyo, Japan).
Measurement of wet-to-dry weight (W/D) ratio
After ventilation, a portion of the right lung of each rat was excised and weighed. Following drying at 60°C for 48 h, it was reweighed. Lung tissue edema was assessed using W/D ratio calculation.
Histopathologic analysis
A piece of right lung tissue was fixed with 4% paraformaldehyde. Four-micrometer-thick lung tissue sections were prepared and stained with hematoxylin and eosin. The severity of the histopathologic injuries, including alveolar congestion, edema, neutrophil infiltration, hemorrhage, thickening of the alveolar wall, and hyaline membrane formation was assessed by independent pathologists in a blinded manner and scored from 0 to 4 (0, normal; 1, mild; 2, moderate; 3, severe; and 4, maximum). Images (×200 magnification) were captured under a Nikon eclipse 80i microscope (Nikon, Tokyo, Japan).
TUNEL staining
TUNEL staining was performed to evaluate lung tissue apoptosis using an apoptosis assay kit (Roche, Mannheim, Germany) following the manufacturer’s protocol. Briefly, after an incubation with proteinase K for 30 min and subsequent two rinses with phosphate-buffered saline, the sections were incubated with TUNEL reaction mixture for 1 h followed by DAPI staining (1 µg/mL) for 30 min in the dark. TUNEL-positive cells were visualized under a confocal microscope (absorption wavelength 490 nm). We randomly selected 10 sections in each lung sample of all the rats, and the TUNEL-positive cells were counted. The mean ratio of the number of positive cells to the total cells was used to quantify apoptosis.
Western blot assay
Proteins were extracted from lung tissues, and the concentration was determined using a Bradford assay. Proteins were separated by SDS-PAGE and transferred onto PVDF membranes. Following a blocking step with 5% dry milk, the membranes were probed with primary antibody against Bax, Bcl-2, cleaved caspase-3, or phosphorylated nuclear factor-κB (NF-κB) (Abcam, Cambridge, UK). After incubation with the horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, TX, USA), signals were visualized using an enhanced chemiluminescence detection kit (ComWin Biotech Co., Ltd, Beijing, China).
Statistical analysis
All experiments were performed at least three times, and the data are presented as the mean ± standard deviation. Data were analyzed with a two-way RM ANOVA test using SPSS 11.0 software (SPSS, Chicago, IL, USA). Non-normally distributed data were analyzed using the Mann–Whitney–Wilcoxon test. The data related to the protein and W/D ratio were analyzed with one-way ANOVA. P < 0.05 was considered significantly different.
Results
AAT improves gas exchange and alveolocapillary permeability in VILI
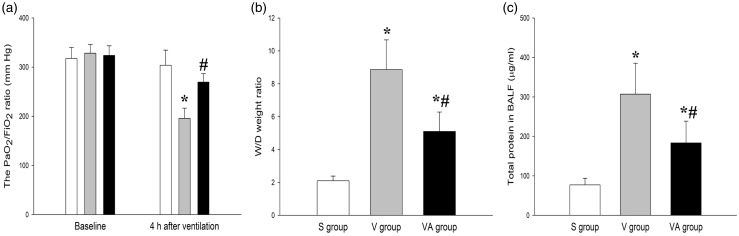
To determine whether AAT has a protective role in VILI, we measured the PaO2/FiO2ratio, W/D ratio, and the BALF level of proteins to assess gas exchange and alveolocapillary permeability of lung tissues, both of which are critical parameters to define acute lung injury (ALI). As shown in Figure 1, there were significant differences in PaO2/FiO2ratio, W/D ratio, and the BALF level of proteins between the three groups (P < 0.05), and the large-volume ventilation led to a marked decrease in PaO2/FiO2 ratio compared with the S group (P < 0.05). However, the AAT significantly restored the ventilation-induced reduction of PaO2/FiO2 ratio compared with the V group, less than 300 mmHg19 of which indicates ALI. Alveolocapillary permeability is assessed using the W/D ratio and the BALF level of proteins. As shown in Figure 1(b) and (c), after 4 h of ventilation, both the W/D ratio and BALF level of proteins were significantly increased in the V group but markedly decreased by AAT administration in the VA group compared with the S group. These data suggest that AAT may improve the function of ventilation-injured lungs.
Figure 1.
The effect of AAT on the PaO2/FiO2 ratio (a), W/D ratio (b), and protein concentration (c). *P < 0.05, versus S group; #P < 0.05, versus V group (n = 8). S: sham; V: ventilation + saline; VA: ventilation + AAT; W/D: wet-to-dry weight.
AAT ameliorates the histological damages in VILI
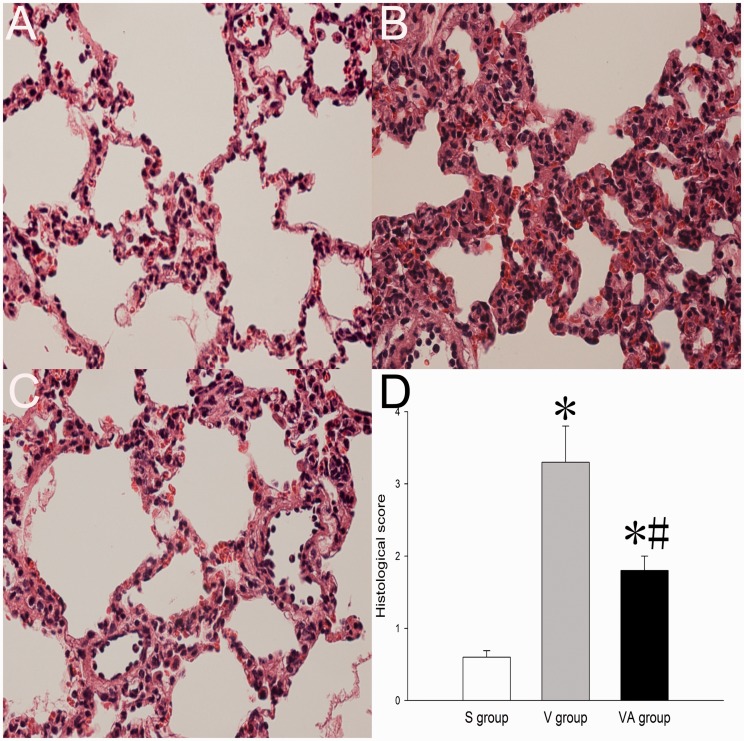
To further investigate whether AAT has a protective effect on lung morphology during VILI, histological assessment was performed on lung tissues. As shown in Figure 2, AAT treatment led to significant reductions in the degree of lung edema, alveolar wall thickening, local hemorrhage, and neutrophil infiltration in lung parenchyma compared with saline treatment, suggesting that AAT may improve the morphological abnormalities in VILI.
Figure 2.
Histological assessments of the lungs that received sham treatment (a), ventilation + saline treatment (b), and ventilation + AAT treatment (c). Magnification ×200. S: sham; V: ventilation; VA: ventilation + AAT. (A color version of this figure is available in the online journal.)
AAT attenuates the inflammatory response in VILI
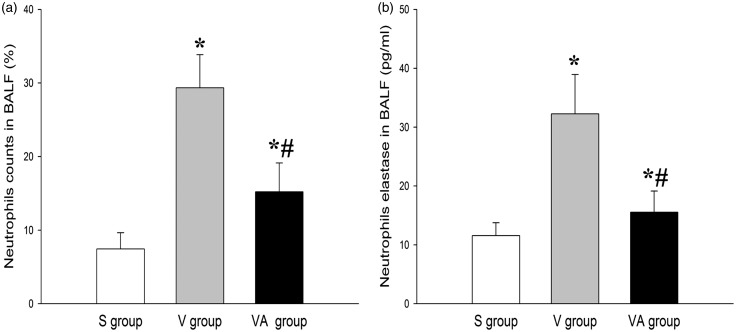
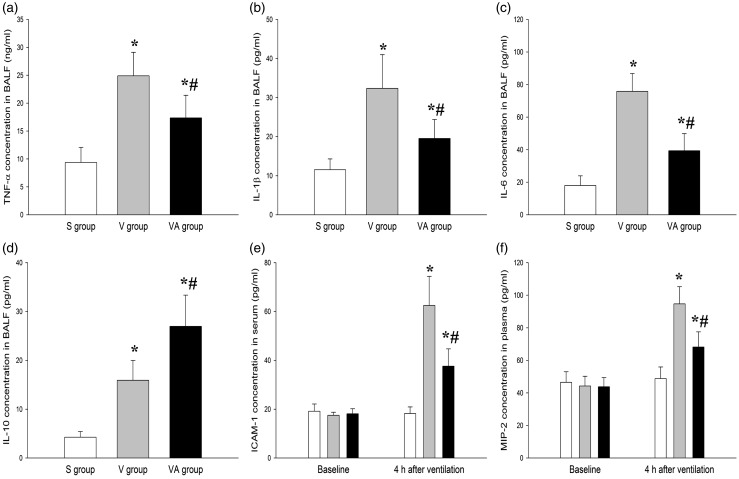
To determine whether AAT plays a role in the modulation of systemic and local inflammation in response to ventilation, we determined neutrophil counts in BALF and the levels of inflammatory mediators in BALF and serum.20–22 As shown in Figures 3 and 4, there were significant differences in inflammatory responses between the three groups (P < 0.05). Figure 3(a) indicated that the percentage of BALF neutrophils in the V group was significantly increased compared with that in the S group. However, the percentage of the neutrophils was markedly lowered in the VA group compared with the V group, suggesting the role of AAT in inhibiting lung inflammation. The BALF level of neutrophil elastase was consistently and significantly lower in the VA group than those in V group (Figure 3(b)). Similar results were also observed with regard to the BALF levels of pulmonary fibrosis-related proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β), and IL-6 (Figure 4(a) to (c)). In contrast, the BALF level of anti-inflammatory factor IL-10 was significantly elevated in the V and VA groups compared with the S group. However, the IL-10 in the VA group was significantly higher than that in the V group (Figure 4(d)). In addition, the serum levels of ICAM-1 and MIP-2 were lower in the VA group than in the V group (Figure 4(e) and (f)). Taken together, these data indicate that AAT may effectively protect lung tissues against VILI as an inflammatory modulator.
Figure 3.
The effect of AAT on neutrophil counts (percentage) and the BALF level of neutrophil elastase. *P < 0.05 versus S group; #P < 0.05 versus V group (n = 8). BALF: bronchoalveolar lavage fluid; S: sham; V: ventilation + saline; VA: ventilation + AAT.
Figure 4.
The effect of AAT on the BALF concentrations of TNF-α (a), IL-1β (b), IL-6 (c), and IL-10 (d); and serum levels of ICAM-1 (e) and MIP-2 (f). *P < 0.05 versus S group; #P < 0.05 versus V group (n = 8). BALF: bronchoalveolar lavage fluid; ICAM-1: intercellular adhesion molecule 1; IL-1β: interleukin 1 beta; IL-6: interleukin 6; IL-10: interleukin 10; MIP-2: macrophage inflammatory protein 2; S: sham; TNF-α: tumor necrosis factor alpha; V: ventilation + saline; VA: ventilation + AAT.
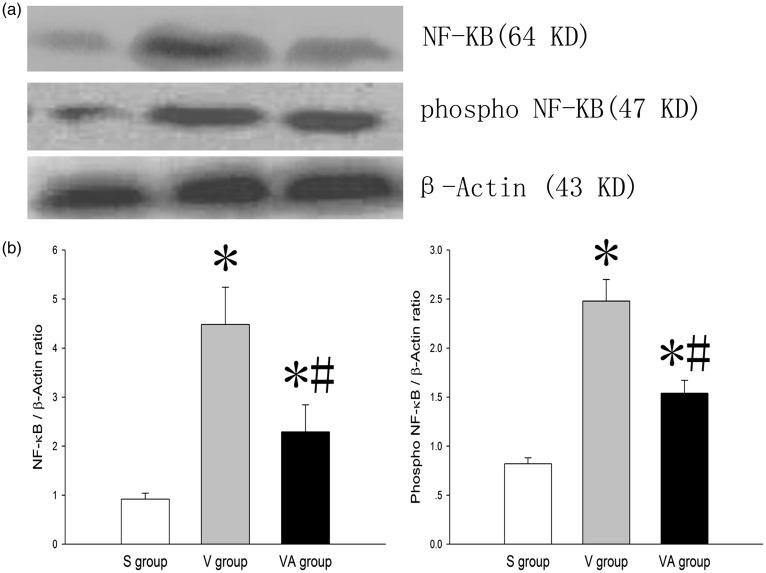
AAT inactivates NF-κB in VILI
Because transcription factor NF-κB is critically important in regulating inflammatory genes,23 we next sought to determine whether AAT affects the NF-κB activity in VILI. Figure 5 showed that the ventilation significantly up-regulated the expression of NF-κB in the V and VA groups compared with the S group (P < 0.05), and AAT significantly inhibited the VILI-induced activation of NF-κB, as evidenced by a decreased level of phosphorylated NF-κB in the VA group compared with the V group, suggesting that AAT attenuates inflammatory responses in VILI at least partially through the inactivation of NF-κB.
Figure 5.
The effect of AAT on the expression of phosphorylated NF-κB in lung tissues. * P < 0.05 versus S group; #P < 0.05 versus V group (n = 8). NF-κB: nuclear factor-κB; S: sham; V: ventilation + saline; VA: ventilation + AAT.
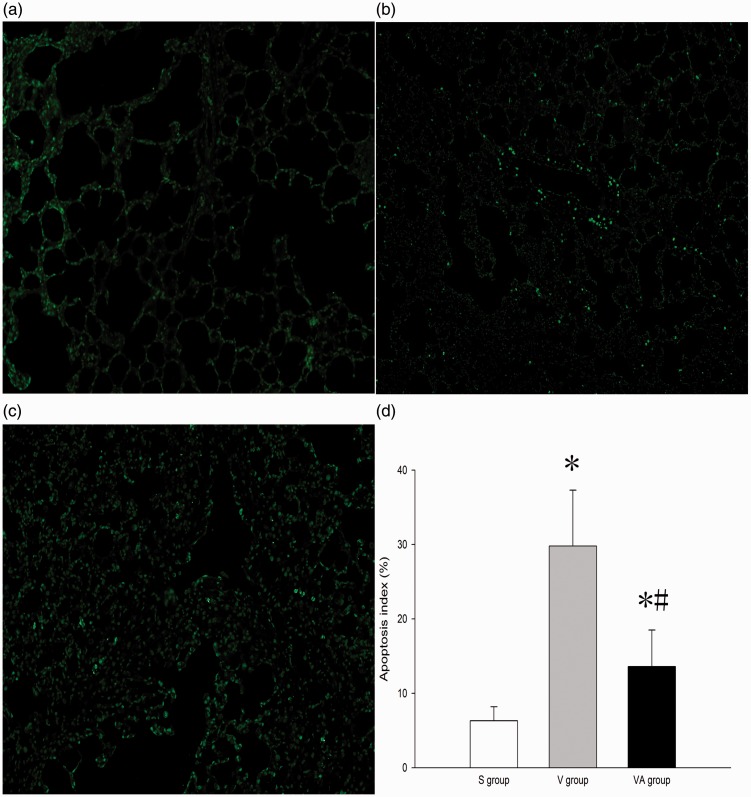
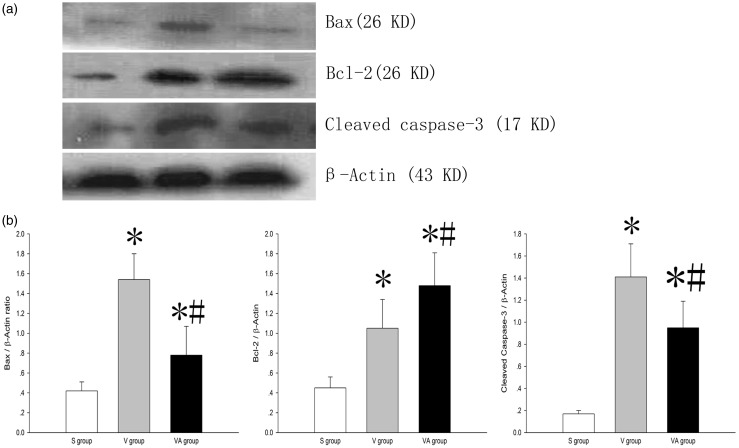
AAT reduces cell apoptosis in VILI
Apoptosis is an important contributor to VILI. To evaluate the role of AAT in cell apoptosis in lung tissues subjected to VILI, TUNEL staining and Western blot assays were performed. As shown in Figure 6(a) to (c), the number of TUNEL-positive cells (green) in the V and VA groups was notably increased compared with the S group (P < 0.05) and was dramatically decreased in the VA group compared with the V group (P < 0.05). The expression of all apoptosis-related protein in the V and VA groups was significantly altered compared with S group (P < 0.05). The expression of proapoptotic Bax and cleaved caspase-3 was consistently and appreciably down-regulated, whereas that of antiapoptotic Bcl-2 was markedly up-regulated in the VA group compared with the V group (P < 0.05) (Figure 7(a) and (b)), suggesting that AAT may reduce cell apoptosis in VILI under the regulation of the expression of apoptosis-related proteins. Collectively, these data indicate that AAT may suppress ventilator-induced cell apoptosis in lung tissues.
Figure 6.
TUNEL assay for cell apoptosis in the lung tissues that received sham treatment (a), ventilation + saline treatment (b), and ventilation + AAT treatment (c). Magnification ×100. (d) The apoptosis index (n = 8). *P < 0.05 versus S group; #P < 0.05 versus V group. S: sham; V: ventilation + saline; VA: ventilation + AAT. (A color version of this figure is available in the online journal.)
Figure 7.
The effect of AAT on the protein levels of Bax, Bcl-2, and cleaved caspase-3 in the lung tissues in VILI. (a) Western blot analysis of protein expression as indicated and (b) quantification of Western blot analysis in A. *P < 0.05, versus S group; #P < 0.05, versus V group (n = 8). S: sham; V: ventilation + saline; VA: ventilation + AAT.
Discussion
AAT, an endogenous glycoprotein protease inhibitor existing in human body fluids, including blood, neutralizes proteases derived from leukocytes in response to inflammatory stimuli and subsequently protects the body against the damage caused by immune cells.9 AAT deficiency increases the risk of chronic obstructive pulmonary disease and asthma.11,24,25 Although AAT has been successfully applied to treating patients with AAT deficiency,26,27 the role of AAT in VILI remains unknown. The present study demonstrated that AAT may provide morphological and functional protection against VILI by improving gas exchange, alveolocapillary permeability, lung edema, and neutrophil infiltration as well as inhibiting alveolar cell thickening, inflammatory responses, and cell apoptosis, suggesting that AAT is a potential therapeutic agent for VILI.
As a protease inhibitor, AAT can neutralize the activity of neutrophil elastase, a major source of endogenous proteases responsible for direct damage to lung epithelial cells and supportive tissues.28 Our study consistently showed that AAT treatment decreased the neutrophil counts and the neutrophil elastase level in BALF (Figure 2). Although the mechanism underlying these effects of AAT remains to be further investigated, AAT appears to improve gas exchange and alveolocapillary permeability likely by controlling the quality and quantity of neutrophil elastase, leading to an elevated PaO2/FiO2 ratio but decreased W/D ratio and BALF level of proteins in VILI (Figure 1). Because AAT can inhibit neutrophil elastase, we hypothesized that AAT may block protease-triggered inflammatory responses in VILI. Notably, compared with those in the V group, the BALF levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in the VA group were markedly reduced in response to AAT, whereas the level of anti-inflammatory IL-10 was significantly increased (Figure 3). Consistent with our findings, elevated BALF levels of TNF-α and IL-1β have been reported to play a pivotal role in the pathogenesis of VILI,29 which can be antagonized by IL-10.30 In addition, the anti-inflammatory effect of AAT is further confirmed by the AAT-induced inactivation of NF-κB (Figure 4), which in turn inhibits the transcription and translation of inflammatory genes, leading to decreased levels of circulating inflammatory cytokines. As a result, the AAT-suppressed inflammatory responses may improve the morphology of ventilator-injured lungs, as evidenced by attenuated lung hemorrhage and edema.
On the other hand, in our study, AAT diminished the serum levels of two important adhesion molecules, ICAM-1 and MIP-2, which are released from injured epithelial and endothelial cells and are essential for neutrophil adherence and aggregation in VILI.31,32 ICAM-1- and MIP-2-recruited neutrophil infiltration may cause secretion of reactive oxygen species and elastase, leading to pulmonary injury. The blockade of ICAM-1 could effectively ameliorate neutrophil influx-induced lung injury.33,34
Considering cell apoptosis also plays a key role in VILI,35,36 we investigated whether AAT exerts effects on apoptosis in ventilator-injured lung tissues. The results demonstrated that AAT inhibited the expression of apoptotic cleaved capspase-3, which is consistent with previous findings13,37 (Figure 7). In addition to caspase-3, we also detected the protein levels of proapoptotic Bax and antiapoptotic Bcl-2.38 An elevated Bax/Bcl-2 ratio is considered to correspond with the onset of cell apoptosis. The results of our study showed that AAT suppressed Bax expression while enhancing Bcl-2 expression in VILI, confirming the role of AAT in regulating apoptosis during VILI.
Conclusions
In conclusion, our study demonstrated that AAT attenuates VILI, as indicated by improved pulmonary capillary permeability, decreased edema, inhibited inflammatory responses, and suppressed apoptosis, which is likely attributed to the inactivation of NF-κB by AAT. The administration of AAT may represent an interventional approach for ARDS patients who need mechanical ventilation in clinical practice. Importantly, AAT is derived from human plasma. The easy availability of AAT potentially reduces the treatment costs of lung disease and dysfunction.
Authors’ contributions
SW and HZ designed this study. JL, JH, and PL participated in the design of this study. XZ and FY carried out the molecular detection and immunoassays of this study. JL, JH, and PL performed the statistical work of this study. SW and HZ drafted this manuscript. XZ, FY, JL, JH, and PL conceived of the study, and helped to draft the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, Cide D, Goldwaser R, Soto L, Bugedo G, Rodrigo C, Pimentel J, Raimondi G, Tobin MJ. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000; 161:1450–8 [DOI] [PubMed] [Google Scholar]
- 2.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med 2000; 342:1360–1 [DOI] [PubMed] [Google Scholar]
- 3.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care 2009; 13:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso P. Ventilator-induced lung injury distribution: the key to understanding injury mechanisms. Am J Respir Crit Care Med 2007; 175:95–6 [DOI] [PubMed] [Google Scholar]
- 5.Kneyber MC, Zhang H, Slutsky AS. Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med 2014; 190:258–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MR, O’dea KP, Zhang D, Shearman AD, van Rooijen N, Takata M. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am J Respir Crit Care Med 2009; 179:914–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MR, Choudhury S, Goddard ME, O’dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 2003; 95:1385–93 [DOI] [PubMed] [Google Scholar]
- 8.Belperio JA, Keane MP, Lynch JP, 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 2006; 27:350–64 [DOI] [PubMed] [Google Scholar]
- 9.Churg A, Dai J, Zay K, Karsan A, Hendricks R, Yee C, Martin R, MacKenzie R, Xie C, Zhang L, Shapiro S, Wright JL. Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Invest 2001; 81:1119–31 [DOI] [PubMed] [Google Scholar]
- 10.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA 2005; 102:12153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, Zhang B, Song S, Hicklin D, Voelkel NF, Flotte T, Tuder RM. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006; 173:1222–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koulmanda M, Bhasin M, Fan Z, Hanidziar D, Goel N, Putheti P, Movahedi B, Libermann TA, Strom TB. Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci USA 2012; 109:15443–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol 2006; 169:1155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockett AD, Kimani S, Ddungu G, Wrenger S, Tuder RM, Janciauskiene SM, Petrache I. Alpha(1)-antitrypsin modulates lung endothelial cell inflammatory responses to TNF-alpha. Am J Respir Cell Mol Biol 2013; 49:143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garratt LW, Sutanto EN, Ling KM, Looi K, Iosifidis T, Martinovich KM, Shaw NC, Buckley AG, Kicic-Starcevich E, Lannigan FJ, Knight DA, Stick SM, Kicic A, Australian Respiratory EST, For CF. Alpha-1 antitrypsin mitigates the inhibition of airway epithelial cell repair by neutrophil elastase. Am J Respir Cell Mol Biol 2016; 54:341–9 [DOI] [PubMed] [Google Scholar]
- 16.Ju YN, Yu KJ, Wang GN. Budesonide ameliorates lung injury induced by large volume ventilation. BMC Pulm Med 2016; 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W, Ju YN. Budesonide attenuates ventilator-induced lung injury in a rat model of inflammatory acute respiratory distress syndrome. Arch Med Res 2016; 47:275–84 [DOI] [PubMed] [Google Scholar]
- 18.Jie Z, Cai Y, Yang W, Jin M, Zhu W, Zhu C. Protective effects of alpha 1-antitrypsin on acute lung injury in rabbits induced by endotoxin. Chin Med J 2003; 116:1678–82 [PubMed] [Google Scholar]
- 19.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. An official American thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011; 44:725–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med 2008; 177:1223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far?. Am J Physiol Lung Cell Mol Physiol 2002; 282:L892–6 [DOI] [PubMed] [Google Scholar]
- 22.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 2009; 182:8056–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med 2001; 163:711–6 [DOI] [PubMed] [Google Scholar]
- 24.Wencker M, Brantly ML. Cytotoxic concentrations of alpha-defensins in the lungs of individuals with alpha 1-antitrypsin deficiency and moderate to severe lung disease. Cytokine 2005; 32:1–6 [DOI] [PubMed] [Google Scholar]
- 25.Demeo DL, Sandhaus RA, Barker AF, Brantly ML, Eden E, McElvaney NG, Rennard S, Burchard E, Stocks JM, Stoller JK, Strange C, Turino GM, Campbell EJ, Silverman EK. Determinants of airflow obstruction in severe alpha-1-antitrypsin deficiency. Thorax 2007; 62:806–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrache I, Hajjar J, Campos M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics 2009; 3:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casas F, Blanco I, Martinez MT, Bustamante A, Miravitlles M, Cadenas S, Hernandez JM, Lazaro L, Rodriguez E, Rodriguez-Frias F, Torres M, Lara B. Indications for active case searches and intravenous alpha-1 antitrypsin treatment for patients with alpha-1 antitrypsin deficiency chronic pulmonary obstructive disease: an update. Arch Bronconeumol 2015; 51:185–92 [DOI] [PubMed] [Google Scholar]
- 28.Lomas DA, Parfrey H. Alpha1-antitrypsin deficiency. 4: molecular pathophysiology. Thorax 2004; 59:529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol 2005; 288:L599–607 [DOI] [PubMed] [Google Scholar]
- 30.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000; 117:1162–72 [DOI] [PubMed] [Google Scholar]
- 31.Miyao N, Suzuki Y, Takeshita K, Kudo H, Ishii M, Hiraoka R, Nishio K, Tamatani T, Sakamoto S, Suematsu M, Tsumura H, Ishizaka A, Yamaguchi K. Various adhesion molecules impair microvascular leukocyte kinetics in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2006; 290:L1059–68 [DOI] [PubMed] [Google Scholar]
- 32.Li LF, Liao SK, Lee CH, Huang CC, Quinn DA. Involvement of Akt and endothelial nitric oxide synthase in ventilation-induced neutrophil infiltration: a prospective, controlled animal experiment. Crit Care 2007; 11:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck-Schimmer B, Madjdpour C, Kneller S, Ziegler U, Pasch T, Wuthrich RP, Ward PA, Schimmer RC. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J 2002; 19:1142–50 [DOI] [PubMed] [Google Scholar]
- 34.Gao X, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, Malik AB. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol 2001; 167:2895–901 [DOI] [PubMed] [Google Scholar]
- 35.Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care 2007; 11:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CS, Kawamura T, Lee S, Tochigi N, Shigemura N, Buchholz BM, Kloke JD, Billiar TR, Toyoda Y, Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care 2010; 14:R234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockett AD, Van Demark M, Gu Y, Schweitzer KS, Sigua N, Kamocki K, Fijalkowska I, Garrison J, Fisher AJ, Serban K, Wise RA, Flotte TR, Mueller C, Presson RG, Jr, Petrache HI, Tuder RM, Petrache I. Effect of cigarette smoke exposure and structural modifications on the alpha-1 Antitrypsin interaction with caspases. Mol Med 2012; 18:-44554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cartron PF, Juin P, Oliver L, Meflah K, Vallette FM. Impact of proapoptotic proteins Bax and Bak in tumor progression and response to treatment. Expert Rev Anticancer Ther 2003; 3:563–70 [DOI] [PubMed] [Google Scholar]