Short abstract
Hepatic fibrosis was caused by a number of signaling pathways that damage liver integrity. We have previously shown that microRNA-29a (miR-29a) protects against liver fibrosis. Aberrant endoplasmic reticulum (ER) and autophagy function reportedly exaggerate hepatic disorders. The aim of this study was to characterize the biological influence of miR-29a on ER function in injured livers with bile duct ligation (BDL). We performed BDL on miR-29a transgenic mice (miR-29aTg) and wild-type mice to induce cholestatic liver injury. Rat T6 cells were transfected with miR-29a mimic and tunicamycin. Compared to the wild-type mice, the BDL deterioration of liver function in terms of total bilirubin, alanine transaminase, and aspartate transaminase activity in the miR-29aTg mice was significantly reduced. Affected livers in the miR-29aTg mice demonstrated a slight fibrotic matrix formation. miR-29a over-expression reduced the BDL disturbance of the expressions of inositol-requiring kinase 1alpha, double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase, spliced-X-box binding protein 1 (sXBP1), CCAAT/enhancer-binding protein homologous protein (CHOP), ULK, LC3BII, p62, and cleaved caspase-8, 9 and 3. In vitro, T6 cells exposed to tunicamycin by increasing abundances of CHOP, sXBP1, cleaved caspase-3, and LC3BII were diminished in the cell cultures transfected with the miR-29a mimic. On the other hand, we observed that miR-29a signaling protected liver tissues from BDL-mediated metabolic dysfunction and excessive fibrosis histopathology. This study provides new molecular insight into the miR-29a stabilization of ER integrity that slows the progression of cholestatic liver deterioration.
Impact statement
Long-term hepatic damage caused by hepatitis and cholestasis can accelerate fibrosis matrix over-production, which is a harmful process attributed to the dysregulation of a number of cellular and molecular events. The purpose of this study is to characterize the biological influence of miR-29a on endoplasmic reticulum (ER) function in bile duct ligation (BDL)-injured livers. To the best of our knowledge, this report is the first demonstration that miR-29a over-expression diminishes BDL provocation of ER stress (unfolded protein response, UPR) effector protein expression. This work also demonstrates that miR-29a decreased caspases protein expression in cholestatic livers, while an increase in miR-29a function reduced sXBP1 and CHOP expressions in T6 cells in mice. Analyses of this study highlight that controlling miR-29a signaling can serve as an innovative strategy in the future for microRNA regulation of ER homeostasis to combat cholestasis induction hepatic disorders.
Keywords: MicroRNA-29a, bile duct ligation, cholestasis, liver fibrosis, endoplasmic reticulum stress, autophagy
Introduction
Long-term hepatic damage from hepatitis and cholestasis can accelerate fibrosis matrix over-production, which is a harmful process attributed to the dysregulation of a number of cellular and molecular events.1 Activated hepatic stellate cells (HSC) have been observed to transdifferentiate into myofibroblastic cells, a phenomenon that contributes to extracellular matrix deposition in a damaged liver.2
Endoplasmic reticulum (ER) stress, also known as unfolded protein response (UPR), is a harmful reaction caused by the irregular folding of proteins inside of the ER.3 In accordance with stress type, UPR reportedly contributes to the survival and apoptosis of cells.3 Inositol-requiring kinase 1α (IRE1α), activating transcription factor 6 (ATF6) a and b, and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) are three distinct molecules located in the ER membrane compartment that have been found to initiate UPR in cells exposed to harmful stress.4 In hepatic cells, ER stress is observed to induce fibrogenic reactions in HSCs through the regulation of autophagic activities.5 Prolonged ER stress increases apoptotic programs, ultimately causing cell death,6 while inhibiting the IRE1α pathway maintains the autophagic process and inactive status that allows HSCs to exhibit low fibrogenic activities.5 In addition to hepatocellular apoptosis’s role in the progression of liver fibrosis,7 the three UPR proteins of interest have been found to regulate CCAAT/enhancer-binding protein homologous protein (CHOP), which is a pro-apoptotic transcription factor and a central mediator of ER stress that induces apoptotic cell activities.8,9 Nevertheless, the molecular mechanism underlying ER stress modulation of liver fibrosis remains ununderstood.
Numerous studies have shown that miR-29 deficiency has been found in patients with liver cirrhosis, experimental animals with bile duct ligation (BDL), and carbon tetrachloride (CCl4) deteriorated hepatic fibrosis. A drop in miR-29 signaling also activates HSCs.10 In a previous study, we demonstrated that mice with an over-expression of miR-29a had minor responses to cholestasis induction of hepatocellular damage and fibrosis.11 miR-29a signaling has been observed to control a number of biochemical and epigenetic pathways, which weaken the fibrotic reactions in livers. For example, miR-29a decreases profibrogenic factor TGF-β1 and histone deacetylase 4 signaling,11,12 as well as regulates DNA methyltransferase function to sustain the hypomethylation state of DNA, diminishes fibrotic matrix anabolism in HSCs, and thus reduces liver fibrosis.13 Since miR-29a signaling inhibits BDL-provoked excessive fibrogenesis, we hypothesized that UPR pathways in injured liver tissue may be related to these protective actions.
In this study, we used miR-29a transgenic mice (miR-29aTg) to confirm the association of liver fibrosis, UPR pathways in the ER compartment, and miR-29a signaling in livers with obstructive jaundice.
Materials and methods
Ethics statement
All animal use protocols have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Chang Gung Memorial Hospital (#201602301). We obtained male C57BL/6 mice (body weight 25–35 g) from BioLASCO Taiwan Co., Ltd. All animals were housed in an animal facility at 22°C, with a relative humidity of 55%, in a 12 h light/12 h dark cycle, with both food and sterile tap water ad libitum.
Construction and breeding of the miR-29a transgenic mouse colony
We bred transgenic mice that over-expressed miR-29a driven by a PGK promoter and housed them in a specific pathogen-free rodent barrier, as previously described.13 The genotype of the transgenic mice was probed with PCR and primers (forward: 5ʹ-GAGGATCCCCTCAAGGAT ACCAAGGGATGAAT-3ʹ and reverse 5ʹ-CTTCTAGAAGGAGTGTTTCTAGGTAT CCGTCA-3ʹ). We obtained wild-type mice from littermates that did not have said construct.
Animal model and experimental protocol
We used six to nine C57BL/6 male mice (BioLASCO, Yilan, Taiwan) weighing 25–35 g for all of our experiments. The mice were categorized into either the "BDL" group or the "sham" group based on whether it had received a ligation or a sham ligation of the common bile duct (CBD); we described said process in a previous study. In general, all surgical procedures are performed under ketamine (50 mg/kg) and xylazine (23 mg/kg) intramuscular injection (IM) anesthesia with clean surgical techniques. The surgical procedure is performed under sterile conditions. Midline laparotomy is performed to explore the hepatic hilum and identify the CBD. Under the dissecting microscope, the CBD is then isolated, doubly ligated, and transected between two ligatures.12 We euthanized all the mice one week after the operation. Liver tissues were dissected, snap-frozen, and processed to isolate total RNA and proteins. All specimens were stored at −80°C until biochemical analysis.
Culture of T6 cells and treatment with tunicaymycin and RNAi transfection
The rat T6 cells used in the present study were derived from immortalized mice HSCs transfected with the simian virus 40 (SV40) large T-antigens containing a Rous sarcoma virus promoter.14 The T6 cells were maintained in minimum essential media (MEM; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA) supplemented with 10% fetal bovine serum, glutamax-1, and penicillin/streptomycin (Gibco, MA) in a humidified incubator at 37°C with 5% CO2. The cells were plated at a density of 8 × 105 cells per 6-cm culture dish and were treated with a concentration of 0.6 μg/mL of tunicamycin to induce ER stress (Sigma, T7765, MO), which was dissolved in dimethyl sulfoxide (DMSO) and incubated for 6 h. Afterwards, the culture medium was discarded and washed by phosphate-buffered saline (PBS). Pursuant to the experimental condition, the cells were transfected with a miR-29a precursor (a miR-29a mimic, GE Healthcare Dharmacon, Inc., IN) or miR control (GE Healthcare Dharmacon, Inc.) for 24 h with Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen, CA) in accordance with the manufacturer's instructions.11 We performed each cell culture experiment four to six times.
Masson trichrome stain
Liver tissue samples were fixed in 10% formalin, embedded in paraffin, and then de-paraffinized and rehydrated. Then we cut 3 μm thick sections and mounted them on coating slides in order to carry out Masson's trichrome staining. We performed Masson's trichrome staining (Poly Scientific, Bay Shore, NY) according to the kit’s directions, except for the following: aniline blue-solution I, 90-min incubation. This prolonged incubation is standard procedure in our laboratory for trichrome liver stains. The staining intensity of sections was measured by independent color channel of an image J analysis.12 There were six to eight samples per group.
Western blot analysis
We used approximately 20 mg of liver tissue in 500 μL protein lysis buffer (iNtRON, Seongnam-si), homogenized by MagNA Lyser system (Roche, Germany) at 6000 rpm for 20 s, and then centrifuged at 14,000×g. The protein (30 μg) obtained from the supernatant of each sample was mixed with a sample buffer and boiled for 10 min, followed by electrophoresis using 8–15% sodium dodecyl sulfate–polyacrylamide gels. The proteins in the gels were transferred to a polyvinylidene fluoride (PVDF) membrane, and blots were incubated with primary antibodies against collagen-4α1 (GeneTex, CA), IRE1α (Cell signaling, MA), p-PERK (Santa Cruz, CA), CHOP (Cell signaling), spliced-X-box binding protein 1 (sXBP1) (Cell signaling), caspase 8 (Cell signaling), caspase 9 (Cell signaling), caspase 3 (Cell signaling), p-ULK Ser317 (Cell signaling), SQSTM1/P62 (abcam, JHY), LC3B (Cell signaling), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (GeneTex) for protein control. Upon washing the blots with tris buffered saline with Tween 20 (TBST) and incubating them with horseradish peroxidase-coupled antirabbit immunoglobulin-G antibodies (dilution: 1:5000), horseradish peroxidase (HRP) antimouse immunoglobulin-G antibodies (dilution:1:10,000), and HRP antigoat immunoglobulin-G antibodies (dilution: 1:10,000) at room temperature for 1 h, we developed them with enhanced chemiluminescence detection (GE Healthcare Biosciences AB, Uppsala, Sweden), exposed them to film, and quantified the signals using densitometry.
Statistical analysis
All the values expressed in the figures and tables are mean ± standard error. We analyzed quantitative data using one-way analysis of variance when appropriate and adopted the least significant difference (LSD) test for post hoc testing. A two-sided P-value less than 0.05 was considered statistically significant.
Results
miR-29a over-expression reduced hepatocellular damage and fibrosis in cholestatic livers
In order to see ER stress signal change in early state of the cholestatic liver injury, we performed one-week BDL on miR-29a transgenic mice (miR-29aTg) and wild-type mice. Serum biochemical analyses demonstrated that BDL significantly increased total bilirubin, alanine transaminase (ALT), and aspartate transaminase (AST) levels in WT littermates (all P < 0.001, Table 1). However, the BDL escalation of total bilirubin, ALT, and AST levels was significantly weaker in the miR-29aTg mice (by 47.9%, 54.6%, and 60.0%, respectively) than in the wild-type littermates in the BDL group (P = 0.003, 0.002, and <0.001, respectively).
Table 1.
Comparison of the changes of liver functions in the four groups.
| Group | Number of mice, N | Bilirubin (mg/dL) | AST (IU/L) | ALT (IU/L) |
|---|---|---|---|---|
| Sham/ WT | 9 | <0.6 | 63.33 ± 6.17 | 22.00 ± 2.83 |
| BDL/ WT | 9 | 15.73 ± 1.30* | 890.69 ± 74.60* | 747.33 ± 80.40* |
| Sham/ miR-29a | 9 | <0.6 | 65.33 ± 5.61 | 16.00 ± 2.00 |
| BDL/ miR-29a | 9 | 7.53 ± 1.91*†‡ | 486.00 ± 62.79*†‡ | 448.67 ± 64.82*†‡ |
WT: wide type; BDL: bile duct ligation; miR-29a: transgenic miR29a; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Data expressed as mean ± standard error.
P < 0.05 versus sham/WT.
P < 0.05 versus BDL/WT.
P < 0.05 versus Sham/miR-29a.
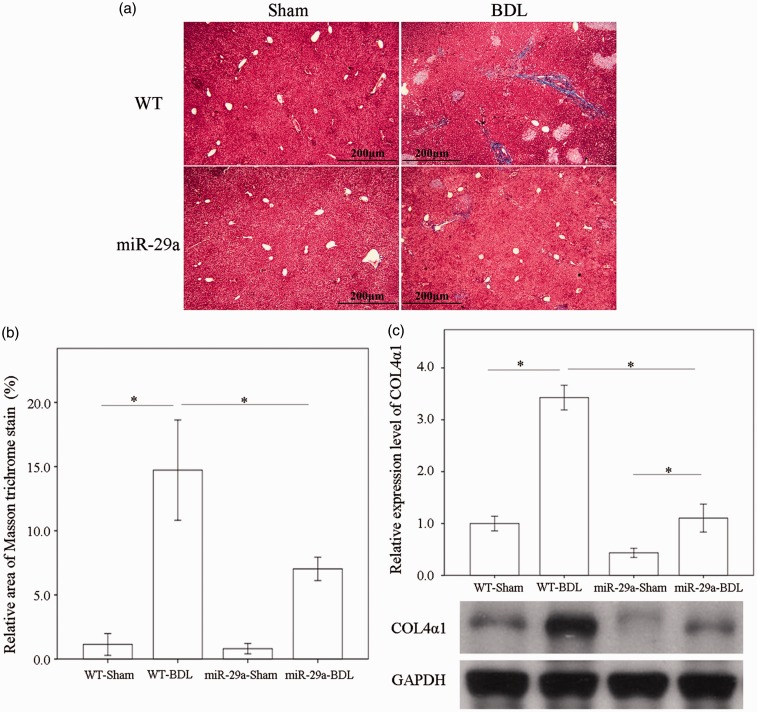
Masson trichrome stain analyses showed that abundant collagenous matrices accumulated blue staining around the portal regions in the BDL-affected liver specimens in the wild-type mice, which did not happen with those in the sham group. This histopathology was clearly alleviated in the BDL-affected miR-29Tg mice (Figure 1(a)). Similarly, BDL significantly increased collagen4α1 levels in the wild-type group, but the BDL elevation of collagen4α1 concentrations was significantly lessened in the miR-29aTg mice (P < 0.001; Figure 1(b)).
Figure 1.
Over-expression of miR-29a in the murine model resulted in the downregulation of fibrosis in mice livers after BDL. (a) Masson trichrome stain showed moderate fibrosis (blue staining) in wild-type (WT) mice and mild fibrosis in miR-29aTg mice, which was limited to the immediate vicinity of the portal area (Masson trichrome stainX160). (b) The Masson trichrome stain intensity of sections was measured by independent color channel of an image J analysis. (c) The expression of collagen4α1 in tissues from the BDL group was significantly greater than in tissues from the sham-operated group in WT mice. Furthermore, miR-29a over-expression significantly downregulated collagen4α1 protein expression in miR-29aTg mice with cholestasis compared with their WT littermates. Data are expressed as the mean ± SE of six to eight samples per group. *Indicates a P < 0.05 between the groups. (A color version of this figure is available in the online journal.)
miR-29a over-expression prevented BDL provocation of ER stress and UPR effector protein expressions
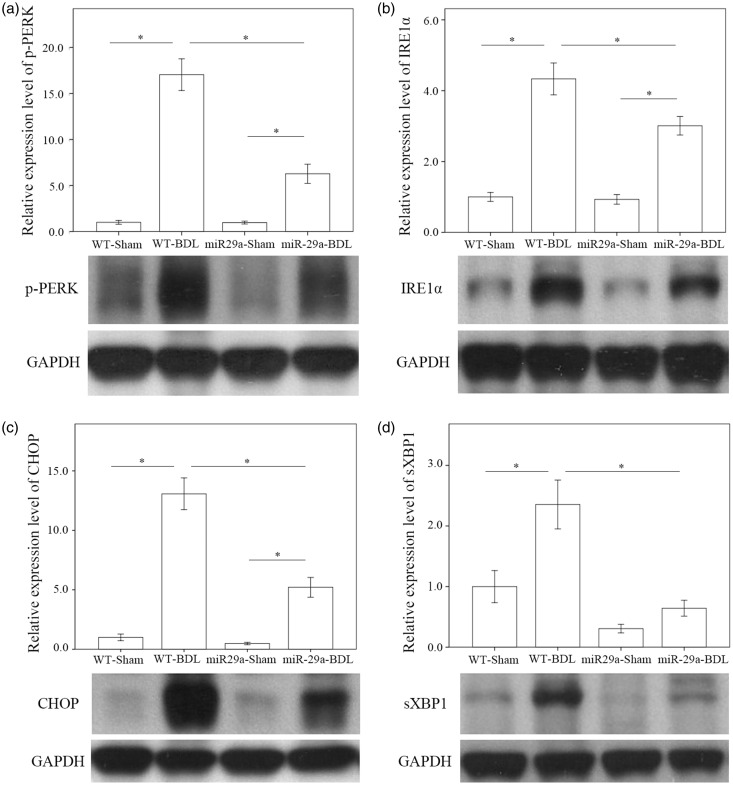
We tested whether miR-29a signaling could change ER stress in liver tissues injured by BDL. In the wild-type group, the abundances of p-PERK (P < 0.001) and IRE1α (P < 0.001) were significantly increased after BDL (Figure 2(a) and (b)). The BDL increase of p-PERK (P = 0.003) and IRE1α (P < 0.001) levels was clearly reduced in the miR-29aTg mice (Figure 2(a) and (b)), indicating that an active response to miR-29a signaling occurred in the ER compartment. We further investigated whether BDL or miR-29a signaling influenced sXBP1 or CHOP signaling, downstream effectors of ER stress that regulate HSC activation,15 and apoptotic reactions during liver injury.16 In the wild-type mice, the concentrations of CHOP (P < 0.001) and sXBP1 (P = 0.001) in the BDL group were significantly higher than those in the sham group (Figure 2(c) and (d)). Meanwhile, the miR-29aTg mice showed moderate responses to the BDL aggravation of CHOP (P < 0.001) and sXBP1 (P < 0.001) (Figure 2(c) and (d)).
Figure 2.
Comparison of the activation of endoplasmic reticulum stress: phspho-PERK (a), IRE1α (b), CHOP (c), and sXBP1 (d) in WT and miR-29Tg mice livers following BDL. Data from the six to eight samples per group are expressed as mean ± SE. *Indicates a P < 0.05 between the groups.
miR-29a alleviated autophagy in cholestatic livers
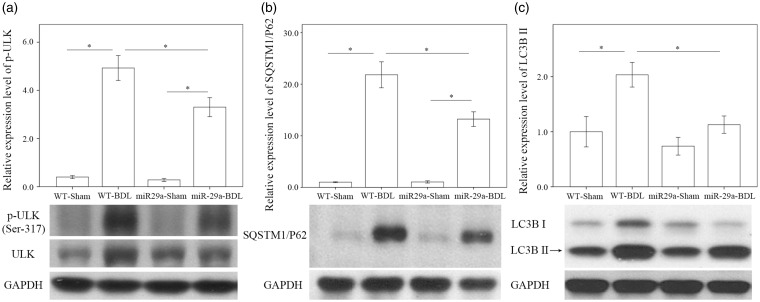
We investigated whether miR-29a signaling changed autophagy in liver tissues injured by BDL. In the wild-type mice, the abundances of p-ULK Ser317 (P < 0.001), SQSTM1/P62 (P < 0.001), and LC3B II (P = 0.002) were significantly higher after BDL (Figure 3). The BDL increase of p-ULK Ser317 (P = 0.001), SQSTM1/P62 (P = 0.001), and LC3B II (P = 0.006) levels was clearly reduced in the miR-29aTg mice (Figure 3), indicating that an active response to miR-29a signaling had occurred in the autophagy.
Figure 3.
Comparison of p-ULK Ser317 (a), SQSTM1/P62 (b), and LC3BII (c) expressions in WT and miR-29Tg mice livers after BDL. Data from the six to eight samples per group are expressed as mean ± SE. *Indicates a P < 0.05 between the groups.
miR-29a decreased caspases protein expression in cholestatic livers
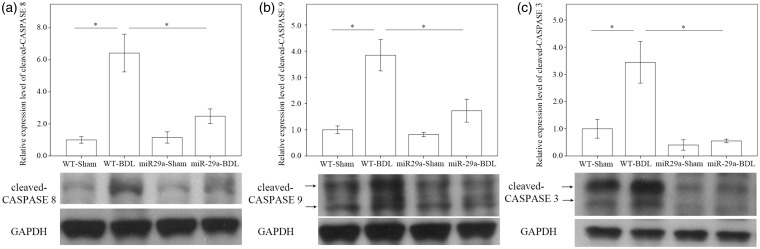
In addition to ER stress, we found considerable increases in the levels of cleaved caspase 8, 9, and 3 in the BDL-affected tissue in the wild-type mice. Of particular note, we observed that miR-29a over-expression significantly weakened the BDL-induced cleavage of caspase 8 (P < 0.001), caspase 9 (P = 0.005), and caspase 3 (P = 0.002) (Figure 4).
Figure 4.
Comparison of cleaved-caspase 8 (a), caspase 9 (b), and caspase 3 (c) expressions in WT and miR-29Tg mice livers after BDL. Data from the six to eight samples per group are expressed as mean ± SE. *Indicates a P < 0.05 between the groups.
Increased miR-29a function reduced sXBP1 and CHOP expressions in T6 cells
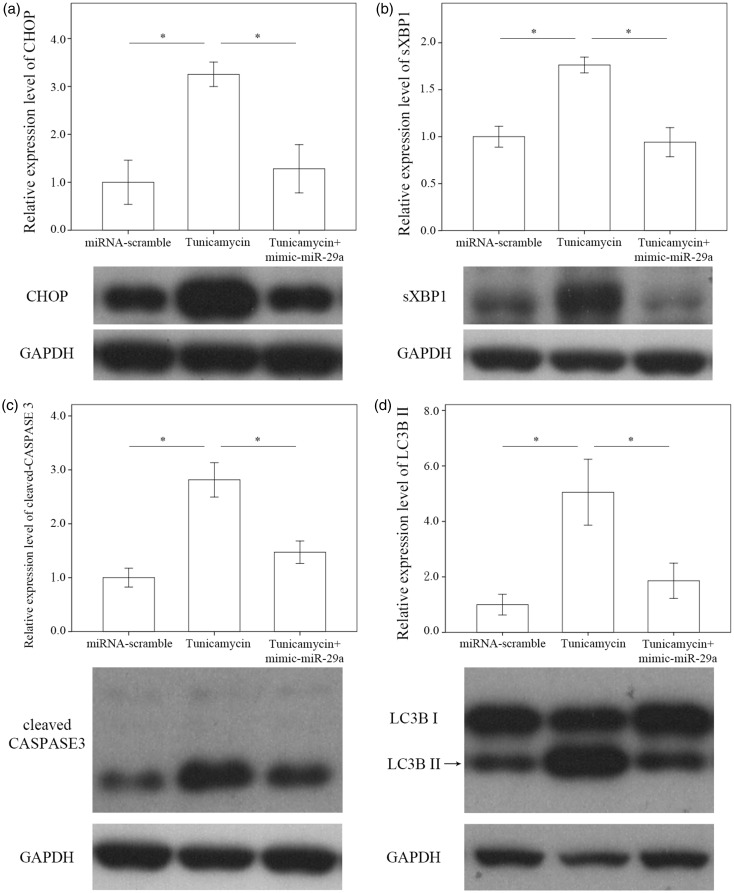
In this study, we explored whether miR-29a signaling regulated ER function in T6 HSCs. We exposed cell cultures to tunicamycin to induce ER stress as evidenced by considerable increases in the levels of CHOP (P = 0.004), sXBP1 (P = 0.001), cleaved caspase 3 (P < 0.001), and LC3BII (P = 0.004) in cell cultures. The elevations in CHOP (P = 0.009), sXBP1 (P = 0.001), cleaved caspase 3 (P = 0.001), and LC3BII (P = 0.014) levels were lowered significantly in the miR-29 mimic-transfected cell cultures (Figure 5).
Figure 5.
Comparison of CHOP (a) sXBP1 (b), caspase 3 (c), and LC3BII (d) on T6 cells which were derived from immortalized rat HSCs transfected with the simian virus 40 large T-antigens containing a Rous sarcoma virus promoter14 after treatment with Tunicamycin with or without a miR-29a mimic as well as miRNA scramble only for 24 h. Data are expressed as mean ± SE of the four to six samples per group. *Indicates a P < 0.05 between the groups.
Discussions
Extracellular fibrotic matrix accumulation is a prominent feature of the transdifferentiation of HSCs in the development of liver fibrosis.2 Furthermore, the HSC-T6 cells came from an immortalized hepatic rat stellate cell line and exhibited an activated phenotype, as seen in their fibroblast-like shape and rapid proliferation in culture.14 Said cells also express cytoskeletal proteins, including desmin, alpha smooth muscle actin, glial acidic fibrillary protein, and vimentin, all of which are typical of activated stellate cells. The miR-29 family produced inhibitory actions against excessive fibrogenesis in various tissues in both pathological and oncological contexts, so miR-29 signaling’s contribution to the organelle function during tissue fibrosis is worth investigating. The present study provides the first indication that miR-29a signaling may protect the hepatic cell microenvironment from ER stress, which mitigates autophagic activity, apoptotic activities, and fibrotic tissue formation, thus ultimately improving hepatic function. Analyses have shed a new light on the mechanisms underlying the miR-29a reduction of liver fibrosis.
Liver fibrosis and cirrhosis are caused by long-term liver injuries attributed to the infiltration of inflammatory cells, apoptosis of hepatocytes, and transactivation of HSCs.16 Accumulated hydrophobic bile acids in the liver have been found to over-produce unfolded proteins within the ER lumen.17 The UPR functions as an adaptive mechanism, which maintains cellular homeostasis in response to external stimuli.18 An over-abundance of UPR has been observed to produce adverse effects on the function and structural integrity of hepatic parenchymal cells when toxic agents are present. If ER stress remains excessive, ER stress-induced cell death signaling switched by the UPR molecule CHOP can deteriorate cell function and decrease survival.18 Persistent UPR has been reported to become a critical pathway involved in the activation of HSCs and collagen I secretion.19 HSCs in the presence of Brefeldin, an ER stress activator, have been observed to increase type I collagen and Smad 3 expressions in HSCs.20 Furthermore, we have discovered that miR-29a signaling produced inhibitory actions on TGF-β/Smad3-mediated liver fibrosis12 and renal fibrosis.15 These findings support our hypothesis in the current study that the miR-29a reduction of liver fibrosis is related to the maintenance of ER stress.
Although CHOP is rarely detectable in proliferating cells, it is highly expressed in cells that experience conditions that damage ER homeostasis. Furthermore, CHOP is involved in ER stress-mediated apoptosis.17 Regarding hepatic tissue metabolism, the CHOP-mediated proapoptotic pathways participate in cholestatic liver damage and fibrosis. Tauroursodeoxycholic acid, a hydrophilic bile acid, has been found to reduce CHOP-induced apoptotic cell death in a BDL animal model.21 A CHOP deficiency protects hepatocytes from liver damage and fibrosis caused by cholestasis, alcoholic hepatitis, and non-alcoholic steatohepatitis.8,22 Our study demonstrated that increased miR-29a expression resulted in decreased CHOP and cleavage caspase 3 signaling, which prevents liver fibrosis in cholestatic livers.
XBP1 is the key downstream fibrogenic cascade transcription factor of IRE1α.9 Recently, Kim et al. uncovered that XBP1 is a key fibrogenesis effector produced in HSCs in murine fibrosis models, as well as advanced human liver disease; meanwhile, ectopic over-expression of XBP1 produced collagen 1α expression in HSCs.23 Transport and Golgi organization 1 (TANGO1) participates in type I collagen biosynthesis; UPR signaling mediated by XBP1 up-regulates TANGO1 in response to TGF-β stress.19 Furthermore, XBP1-mediated UPR has been shown to contribute to fibrogenic HSC activation and is functionally related to autophagy.23 Our results indicate that an increased miR-29a expression resulted in the downregulation of IRE1a, PERK, CHOP, and XBP1 in cholestatic livers and HSCs, which ultimately protected against HSC activation and liver fibrosis.
Autophagy enables the orderly degradation and recycling of cellular components, which is a mechanism for adapting to cellular stress, as well as a mechanism for programmed cell death.24 Growing evidence has indicated that autophagy is a vital regulatory pathway in liver fibrosis20 and has been correlated with directly contributing to HSC activation.23 Increased autophagy has been observed in mice with liver injury induced by CCl425 or BDL,26 while the inhibition of autophagic function reduces fibrogenesis and HSC activation.27 Meanwhile, p62 has been shown to be a negative regulator of liver inflammation and fibrosis.28 Our results demonstrated that increased miR-29a significantly inhibited autophagy and alleviated liver injury and fibrosis induced by BDL. Therefore, the data reported herein present the novel therapeutic potential of miR-29a by modulating ER stress and autophagy in the pathogenesis of liver fibrosis.
Conclusion
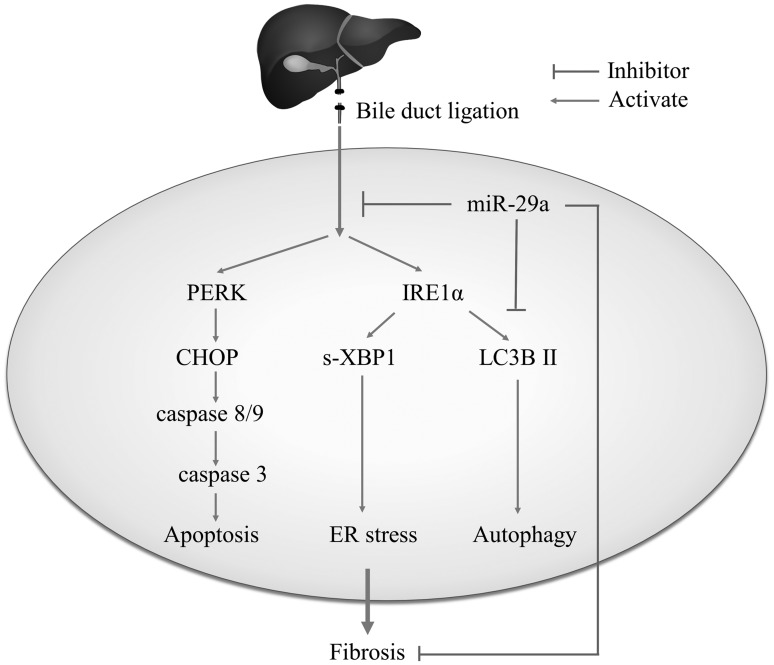
Inhibiting the profibrogenic effects of HSCs is vital for preventing the development of liver fibrosis.29 Analysis of the results presented here highlights the benefit that controlling miR-29a signaling can serve as an innovative strategy in the future for regulating microRNA of ER homeostasis to prevent cholestasis induction hepatic disorders (Figure 6).
Figure 6.
The proposed miR-29a signaling protection model in liver fibrosis by inhibiting endoplasmic reticulum (ER) stress. miR-29a is a crucial regulator of the profibrogenic phenotype of HSCs. Increased miR-29a function hinders sXBP1 and CHOP expressions, thus inhibiting the activation of HSCs.
Acknowledgments
The authors would like to thank Chia-Ling Wu and Yuan-Ting Chuang for assisting with this article.
Author contributions
All authors participated in the design of the study, interpretation of the study, analysis of the data, and review of the manuscript; YHH, YAY, FUH, MMT, YCL, and MHT conducted the experiments, and YHH, YAL, and FSW wrote the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of Conflicts of Interests
The authors hereby declare to have no financial interests or conflicts of interest to disclose in relation to this article.
Funding
This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 104-2314-B-182A-118-MY2 and 106-2314-B-182A-141 -MY3) and Chang Gung Memorial Hospital, Taiwan (CMRPG8B0993, 8E1171, 8E1172, 8F0281, and 8F1561). However, these organizations had no part in the study design, data collection and analysis, publication decisions, or preparation of the manuscript.
References
- 1.Tiao MM, Lin TK, Chen JB, Liou CW, Wang PW, Huang CC, Chou YM, Huang YH, Chuang JH. Dexamethasone decreases cholestatic liver injury via inhibition of intrinsic pathway with simultaneous enhancement of mitochondrial biogenesis. Steroids 2011; 76:660–6 [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008; 134:1655–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833:3460–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivas A, Vidal RL, Hetz C. Targeting the unfolded protein response for disease intervention. Expert Opin Ther Targets 2015; 19:1203–18 [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, Devi LA, Friedman SL. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol 2013; 59:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J 2016; 283:2640–52 [DOI] [PubMed] [Google Scholar]
- 7.Higuchi H, Bronk SF, Taniai M, Canbay A, Gores GJ. Cholestasis increases tumor necrosis factor-related apoptotis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity. J Pharmacol Exp Ther 2002; 303:461–7 [DOI] [PubMed] [Google Scholar]
- 8.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol 2008; 294:G498–505 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang Y, Wang H, Huang C, Huang Y, Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res 2015; 64:1–7 [DOI] [PubMed] [Google Scholar]
- 10.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011; 53:209–18 [DOI] [PubMed] [Google Scholar]
- 11.Huang YH, Tiao MM, Huang LT, Chuang JH, Kuo KC, Yang YL, Wang FS. Activation of Mir-29a in activated hepatic stellate cells modulates its profibrogenic phenotype through inhibition of histone deacetylases 4. PloS One 2015; 10:e0136453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li SC, Wang FS, Yang YL, Tiao MM, Chuang JH, Huang YH. Microarray study of pathway analysis expression profile associated with microRNA-29a with regard to murine cholestatic liver injuries. Int J Mol Sci 2016; 17:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YL, Wang FS, Li SC, Tiao MM, Huang YH. MicroRNA-29a alleviates bile duct ligation exacerbation of hepatic fibrosis in mice through epigenetic control of methyltransferases. Int J Mol Sci 2017; 18:192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou MH, Huang YH, Lin TM, Du YY, Tsai PC, Hsieh CS, Chuang JH. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. Biochem J 2012; 447:25–34 [DOI] [PubMed] [Google Scholar]
- 15.Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS, Chiang WC, Reiser J, Wang FS. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol 2014; 25:1698–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiao MM, Wang FS, Huang LT, Chuang JH, Kuo HC, Yang YL, Huang YH. MicroRNA-29a protects against acute liver injury in a mouse model of obstructive jaundice via inhibition of the extrinsic apoptosis pathway. Apoptosis 2014; 19:30–41 [DOI] [PubMed] [Google Scholar]
- 17.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334:1081–6 [DOI] [PubMed] [Google Scholar]
- 18.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011; 54:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiers JL, Kostallari E, Mushref M, deAssuncao TM, Li H, Jalan-Sakrikar N, Huebert RC, Cao S, Malhi H, Shah VH. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 2017; 65:983–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi DQ, Yang XF, Liao DF, Wu Q, Fu N, Hu Y, Cao T. Effect of autophagy over liver diseases. Chin Med Sci J 2016; 31:65–8 [DOI] [PubMed] [Google Scholar]
- 21.Paridaens A, Raevens S, Devisscher L, Bogaerts E, Verhelst X, Hoorens A, Van Vlierberghe H, van Grunsven LA, Geerts A, Colle I. Modulation of the unfolded protein response by tauroursodeoxycholic acid counteracts apoptotic cell death and fibrosis in a mouse model for secondary biliary liver fibrosis. Int J Mol Sci 2017;18:214. [DOI] [PMC free article] [PubMed]
- 22.Masouminia M, Samadzadeh S, Mendoza AS, French BA, Tillman B, French SW. Upregulation of autophagy components in alcoholic hepatitis and nonalcoholic steatohepatitis. Exp Mol Pathol 2016; 101:81–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim RS, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, Robinson CL, Bhattacharya D, Chou HI, Zhang DY, Fuchs BC, Lee Y, Hoshida Y, Friedman SL. The XBP1 arm of the unfolded protein response induces fibrogenic activity in hepatic stellate cells through autophagy. Sci Rep 2016; 6:39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8:741–52 [DOI] [PubMed] [Google Scholar]
- 25.He W, Wang B, Yang J, Zhuang Y, Wang L, Huang X, Chen J. Chloroquine improved carbon tetrachloride-induced liver fibrosis through its inhibition of the activation of hepatic stellate cells: role of autophagy. Biol Pharm Bull 2014; 37:1505–9 [DOI] [PubMed] [Google Scholar]
- 26.Mao Y, Zhang S, Yu F, Li H, Guo C, Fan X. Ghrelin attenuates liver fibrosis through regulation of TGF-beta1 expression and autophagy. Int J Mol Sci 2015; 16:21911–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoen LF, Guimaraes EL, Grunsven LA. Autophagy: a new player in hepatic stellate cell activation. Autophagy 2012; 8:126–8 [DOI] [PubMed] [Google Scholar]
- 28.Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, Roberts LR, Kisseleva T, Karin M, Diaz-Meco MT, Moscat J. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell 2016; 30:595–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schon HT, Bartneck M, Borkham-Kamphorst E, Nattermann J, Lammers T, Tacke F, Weiskirchen R. Pharmacological intervention in hepatic stellate cell activation and hepatic fibrosis. Front Pharmacol 2016; 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]