Short abstract
The objective of our pilot clinical, prospective study was to determine the serum levels of mature brain-derived neurotrophic factor, in of women with endometriosis and controls and explore whether mature brain-derived neurotrophic factor is a potential biomarker for the disease. The patients were selected from the Endometriosis Marker Austria prospective cohort study conducted at the tertiary referral certified Endometriosis Center of the Medical University of Vienna. All women underwent laparoscopic surgery because there was a suspicion of endometriosis, or the women had pelvic pain, adnexal cysts, unexplained infertility, or uterine fibroids. Our main outcome parameter was total levels of mature brain-derived neurotrophic factor in serum, measured using ELISA. Our results show that serum levels of mature brain-derived neurotrophic factor are significantly higher in women with endometriosis compared to women without endometriosis. The mean serum protein levels are significantly higher in women with rAFS stage I and II endometriosis, whereas no difference was found in women with stage III and IV endometriosis and controls. Postoperative follow-up at 6–10 weeks revealed that surgical intervention leads to equilibration of the levels of secreted mature brain-derived neurotrophic factor between women with and without endometriosis. The difference between serum mature brain-derived neurotrophic factor levels of women with endometriosis compared to women without endometriosis is independent of menstrual cycle phase and overall self-reported pelvic pain. ROC-curve analysis showed that, the mature brain-derived neurotrophic factor is not a useful biomarker for endometriosis. In conclusion, although women with stage I and II endometriosis have increased levels of mature brain-derived neurotrophic factor in serum compared to controls, the difference is not predictive for the disease.
Impact statement
Endometriosis is a disease that can have a significant impact on the quality of life of affected women. The gold standard for diagnosis to this day remains visualization through laparoscopic surgery with histological verification. Current studies are attempting to find a biomarker with high sensitivity and specificity, which would bypass the surgery-associated risks and would significantly reduce costs. In an attempt to elucidate whether mature serum BDNF can serve as diagnostic marker for the disease, we compared the levels of the protein in women with endometriosis to endometriosis-free controls. While our results showed that serum concentrations of the mature protein were significantly higher in women with endometriosis, we did not find this marker to have the sensitivity or specificity needed in order to allow a reliable diagnosis.
Keywords: Mature brain-derived neurotrophic factor, endometriosis, noninvasive biomarker
Introduction
Endometriosis is a benign but troublesome gynecological condition that affects up to 10% of women during their reproductive years.1 It is characterized by the presence of endometrial tissue outside the uterine cavity.2,3 The disease is a major cause of chronic pelvic pain and infertility.4 The gold standard for the diagnosis relies on surgical assessment and visualization by laparoscopy. A surgical diagnosis is not ideal, not only because of the inherent surgical and anesthetic risks, as well as the financial costs to the patient and the healthcare system, but also because there is often a significant delay in diagnosis and adequate treatment.5 Therefore, one of the top priorities in the field of endometriosis research is currently the discovery and validation of new non-invasive diagnostic biomarkers.6
Brain-derived neurotrophic factor (BDNF) is synthesized as a 32 kD N-glycosylated and glycosulfated pro-form which is proteolytically cleaved to the 14 kD mature form (mBDNF).7 Pro-BDNF can be cleaved intracellularly; however, the majority is secreted uncleaved into the extracellular environment,8 where a portion of the pro-protein is also cleaved to the mBDNF form by the serine protease plasmin and matrix metalloproteinases.9 The two forms have distinct effects and the pro-BDNF may also oppose those of mBDNF.10 In general, in the neuronal system, pro-BDNF induces cellular apoptosis,9 while the mature protein promotes cell development and differentiation, survival and plasticity.11 Results from our group and the work of others have shown that BDNF is implicated in the processes of endometriosis-associated innervation and lesion formation.12,13 The BDNF protein and its high affinity receptor, TrkB, were found to be higher in the uterus of women with endometriosis versus disease-free controls.14,15 Moreover, we have recently shown that increased levels of BDNF expression and mBDNF secretion in endometriosis stroma are associated with epigenetic alterations that affect the levels of DNA methylation within the 3′ end of the gene.13 In addition, because it is a secreted protein, BDNF shows disease-associated differences in the circulation. For example, decreased serum levels of mBDNF, but not its precursor, pro-BDNF, were found in patients with major depressive disorder.16 Abnormalities in serum levels of pro- and mBDNF were also reported in mood-stabilized patients with bipolar disorder.17 Interestingly, in this pathological condition, the levels of the pro-BDNF form were found to be lower in stabilized patients compared to controls and this change was not associated with changes in the levels of secretion of the mBDNF. These results suggest that the two forms of the protein might have a different input for certain pathological conditions, and, when measured separately, might have stronger disease predictive value than the combined soluble fraction. In contrast to these psychic illnesses, information about differences in the levels of secretion of the pro- and mBDNF forms in other diseases, including endometriosis, is limited. Endometriosis studies have evaluated only the entire soluble fraction of the protein, which comprises both the pro- and mature form of BDNF. It has been reported that the levels of soluble BDNF are increased in the plasma of women with endometriosis compared to women without endometriosis.18,19 A study by Wessels et al.19 further suggested that the soluble plasma BDNF could serve as a putative non-invasive biomarker for rAFS stage I and II endometriosis. However, it is still unclear, whether there are differences in the mode of action of pro- and mBDNF forms in endometriosis lesion formation and disease progression, and whether the two forms of the protein show a different pattern in their secretion in women with and without endometriosis.
Considering the putative role of mBDNF in endometriosis lesion formation13 and the potential differences in the prediction power of the two forms (soluble BDNF and mBDNF) with regard to disease identification, the objective of this pilot clinical study was to determine the serum mBDNF levels in women with and without endometriosis and evaluate whether mBDNF could be a potential biomarker for the diagnosis of endometriosis or disease-stage classification.
Materials and methods
Study population
The patients for this pilot clinical study were selected from the Endometriosis Marker Austria (EMMA) study, a prospective cohort study that has been conducted at the tertiary referral-certified Endometriosis Center of the Medical University of Vienna since 2010. All patients gave their written, informed consent prior to study inclusion. Patients eligible for participation in the EMMA study were premenopausal women between 18 and 50 years of age who were undergoing laparoscopic surgery because of suspected endometriosis, pelvic pain of unknown origin, adnexal cysts, infertility work-up or uterine fibroids. Patients who were pregnant at the time, or with a history of any malignant disease, acute inflammatory process or infection, and systemic autoimmune disorders were excluded from study participation. Study blood was collected from participating patients preoperatively in a fasting state, directly on the day of surgery, and at a follow-up visit scheduled 6 to 10 weeks after the surgical intervention. Furthermore, all patients were asked to fill out a detailed questionnaire to evaluate pain symptoms potentially associated with endometriosis (visual analogue scale VAS, 0 = no pain; 10 = excessive pain). To exclude the influence of comorbidities which potentially may have an effect on the levels of serum BDNF, a detailed clinical anamnesis was obtained for each study participant. The information was collected using a study-specific questionnaire designed by our certified Endometriosis Center and filled out by the patients prior to laparoscopic intervention. Women with chronic inflammatory, autoimmune and neuropsychiatric diseases, polycystic ovary and/or premature ovarian insufficiency syndrome and history of malignancies were excluded from the study. The presence or absence of endometriosis was confirmed visually and by laparoscopic biopsy and histopathologic analysis. The different stages of endometriosis disease were classified according to the revised American Fertility Society Score (rAFS).20 Patients who did not show any endometriotic lesions at laparoscopic evaluation were included as the control group. The menstrual cycle phase at the day of the surgery was evaluated by histologic analysis of endometrial biopsy obtained from every patient via diagnostic dilatation and curettage (D&C) supplemented by hormonal analysis.
From all women who participated in the EMMA study from July, 2013 through May 2016, 128 women had not taken hormones for at least three months prior to inclusion, had completed both the perioperative and follow-up visit, showed no signs of malignancy or inflammation in their final histopathologic results.
Institutional ethics committee approval
The study was approved by the institutional ethics committee of the Medical University of Vienna (EK 545/2010).
Sample preparation and mBDNF ELISA assay
The collected blood was centrifuged according to our standard protocol at 3000 r/min for 10 min at 4°C directly 30 min to 1 h after sampling. The sediment-free serum samples were frozen in aliquots at −80°C until further analysis. To avoid large inter-assay variation, the samples were analyzed in two batches, i.e. pre-surgical and post-surgical follow-up batch at the end of the study period. The analysis of each batch was accomplished within five consecutive days. For each analysis, we have used a single aliquot of previously frozen sample, thereby avoiding multiple rounds of freezing and refreezing procedures.
The concentrations of the mBDNF were quantified in duplicate using the Mature BDNF Rapid enzyme-linked immunosorbent assay (ELISA) kit from Biosensis (Australia, cat. number: BEK-2211–1P) and following the manufacturer’s protocol. Prior to each immunoassay, the serum samples were diluted 1:100 using the provided sample buffer. After incubation, the absorbance was measured at OD = 450 nm within 30 min using the FLUOstar Optima microplate reader (BMG Labtech Ltd) and the levels of serum mBDNF were calculated in pg/mL. The logistic delay in sample collection is typical for clinical studies and cannot be excluded. Therefore, to control for sample stability, we assessed and compared the levels of total serum mBDNF in the control serum samples stored for a relatively long (three to four years) with those stored for one to two years. As shown in Supplementary Figure 1(a), the duration of the sample storage at −80°C did not have an effect on mBDNF stability. Based on these findings, we concluded that storage time of up to four years at −80°C does not significantly influence the total serum levels of mBDNF.
Data analysis and statistics
The statistical tests were performed using either SPSS version 24 or GraphPadPrsm 7 software. Characteristics of the study population and differences between patients and the control group were accessed using a Kruskal–Wallis test (for variables reported as mean ± standard deviation). For multiple comparison tests, one-way ANOVA was applied. Differences with an adjusted P-value <0.05 were considered as significant. Receiver operating characteristics (ROC) analysis was used to examine the value of using mBDNF as a predictive marker for the diagnosis of endometriosis. To determine the sensitivity and specificity, we calculated the area under the ROC curve (AUC) at the 95% confidence interval. Correlation analysis was performed using GraphPadPrism7 software, with a Spearman rank correlation algorithm. The inter- and intra-assay variation coefficients (CVinter, CVintra) were computed using free online available platform (http://influentialpoints.com/Training/Coefficient_of_variation_Use_and_misuse.htm). The mean values of the concentrations of the positive control, supplied by the kit and measured in each individual plate, were used as reference for computing the inter-assay variation coefficient. The criteria for validity of our experimental data were set to %CVinter < 10% and %CVintra < 5%, respectively.
Results
Patient characteristics
Of the 128 women included in this study, 77 had endometriosis and 51 had no evidence of endometriosis and represented the control group (Table 1). A total of 19 women in the control group and five women in the case group had uterine fibroids, representing 24.7% and 6.5% of the individuals within the groups, respectively. The average BMI of women with endometriosis was significantly lower (P = 0.002; 23.1± 4.2 vs. 26.6 ± 6.4) compared to women without endometriosis (Table 1). However, the average age and the overall VAS score were similar between cases and controls. In contrast to the overall VAS score, menstruation-related pain intensity was significantly higher in women with endometriosis compared to women without endometriosis (P = 0.0002) (Table 1). Of the 77 women with endometriosis, 46.7% were classified as having minimal to mild (rAFS I, II) and 53.4% as having moderate to severe (rAFS III, IV) endometriosis (Table 2).
Table 1.
Baseline cohort characteristics.
| Characteristics | Control | Case | P | Missing values |
|---|---|---|---|---|
| Number of women | n=51 | n=77 | ||
| Age | 34.8 ± 6.9a | 33.7 ± 6.04a | NS | 0 |
| BMI | 26.6 ± 6.4a | 23.1 ± 4.2a | 0.002 | 0 |
| Overall pain score | 5.3 ± 2.9a | 6.2 ± 2.5a | NS | 0 |
| Pain score (menstruation) | 6.6 ± 0.4a | 7.8 ± 0.3a | 0.0002 | 9 |
| Dysmenorrhea, n (%) | ||||
| Yes | 49 (96.1) | 73 (94.8) | NS | 0 |
| No | 2 (3.9) | 4 (5.2) | ||
| Cycle phase, n (%) | ||||
| Proliferative | 23 (46) | 38 (50.7) | NS | 3 |
| Secretory | 27 (54) | 37 (49.3) |
Values are given in mean ± standard deviation.
Note: P-values were determined using Kruskal–Wallis test.
NS: not significant (P-value >0.05).
Table 2.
Endometriosis patient characteristics for disease stage and ectopic lesion location.
| Number of patients, n | 77 |
|---|---|
| rAFS stage of endometriosis, n (%) | |
| I, minimal | 21 (28) |
| II, mild | 14 (18.7) |
| III, moderate | 20 (26.7) |
| IV, severe | 20 (26.7) |
| Lesion type, n (%) | |
| Peritoneal | 22 (28.9) |
| Ovarian | 12 (15.8) |
| Deep infiltrating | 3 (3.9) |
| Combination of two lesion types | 32 (42) |
| Combination of three lesion types | 7 (9.2) |
mBDNF levels are increased in serum of woman with versus without endometriosis
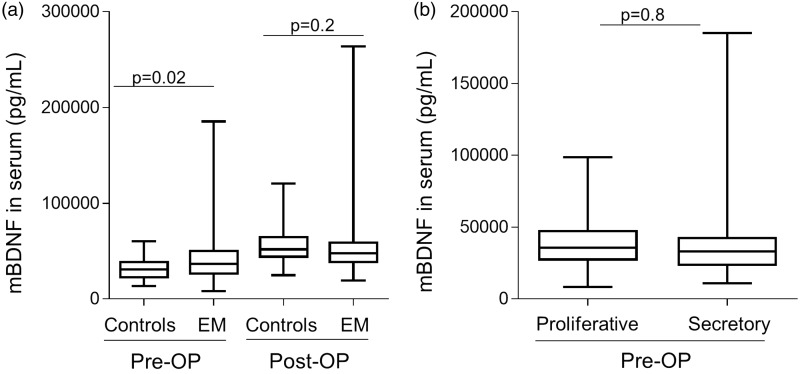
We first analyzed the levels of serum mBDNF regardless of stage of the disease or menstrual cycle phase in pre- and postoperative serum samples. Before surgery, the median serum concentration of the protein was greater in women with endometriosis compared to controls (1.3-fold, P = 0.0198, corresponding to mean mBDNF controls = 32,131 pg/mL and mean mBDNF endometriosis = 41,945 pg/mL) (Figure 1(a)). Interestingly, 6–10 weeks after surgery, the levels of secreted serum mBDNF in controls were higher compared to the respective preoperative group (1.8-fold, P < 0.0001, mean mBDNF postoperative controls = 56,741 pg/mL vs. 32,131 pg/mL in preoperative control samples). The same was found when comparing the levels of mBDNF in women with endometriosis before and after surgery (1.3-fold, P = 0.0038, mean mBDNF postoperative endometriosis =53,579 pg/mL vs. 41,945 pg/mL in preoperative cases). However, no significant difference was seen when postoperative serum mBDNF levels were compared between women with and without endometriosis (P = 0.183). The intra-assay variation within our pre- and post-operative serum samples was 4.3% (% CVintra-pre=4.3%) and 3.9% (CVintra-post=3, 9%), respectively, and the inter-assay variation coefficient was lower then 10% (%CVinter=9.5%), thereby fulfilling our criteria for assay validity. We further analyzed whether mBDNF levels correlated with the levels of overall self-reported pelvic pain and whether they were affected by menstrual cycle phase. As shown in Figure 1(b), the increased levels of mBDNF in women with endometriosis were independent of cycle phase. Furthermore, there was no significant correlation between the levels of either overall or menstruation-associated, self-reported pelvic pain and mBDNF (Supplemental Figure 1(b) and (c)).
Figure 1.
mBDNF levels are increased in serum of women with versus without endometriosis and these changes are independent of menstrual cycle phase. (a) Box plots representing mBDNF levels in cases and controls before and 6 to 10 weeks after laparoscopic surgery, assessed with one way ANOVA adjusted for multiple testing using two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, are shown. (b) The effect of the cycle phase on mBDNF serum levels in patients and controls independent of disease status are plotted. Differences between the groups were calculated using non-parametrical T-Test.
Serum mBDNF levels are disease-stage specific and are not associated with specific lesion type
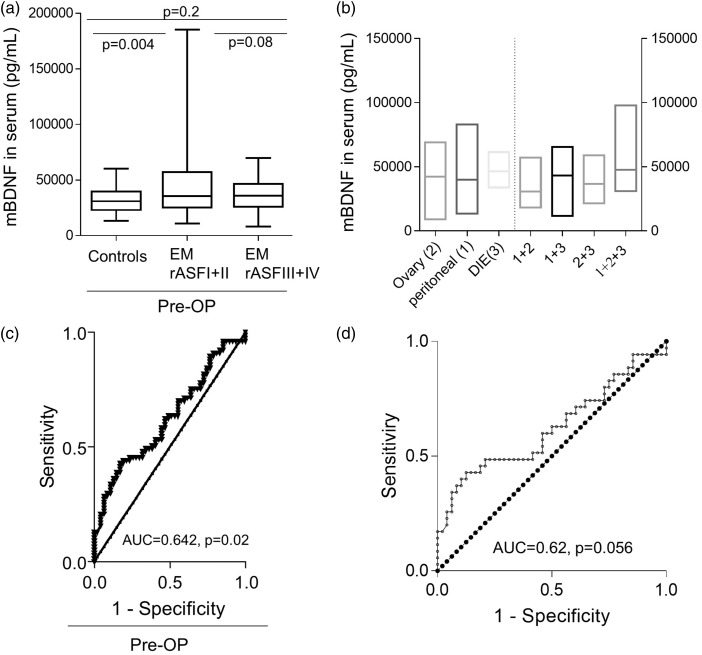
To evaluate the relationship between mBDNF and disease-stage, we looked at the levels of total serum protein in patients with rAFS I and II (n = 35), and rAFS III and IV (n = 40) and compared them to control (n = 51) serum levels. Women with stage I and II endometriosis had significantly higher mBDNF levels compared to endometriosis-free controls (1.43-fold, P = 0.004, mean mBDNF controls =32,379 pg/mL vs. 46,209 pg/mL for rAFS I and II endometriosis cases; Figure 2(a)). No significant difference in total serum mBDNF was found for women with stage I and II vs. stage III and IV (P = 0.08, mean mBDNFrAFS I,II = 46,209 pg/ml vs. mean mBDNFrAFS III,IV = 37,653 pg/mL), or between women with stage III and IV disease and controls (P = 0.2, mean mBDNFcontrols =32,379 pg/mL vs. mean mBDNFrAFS III,IV= 37,653 pg/mL).
Figure 2.
Serum mBDNF levels are disease-stage specific but they are not predictive for the disease. (a) Graphical representation of the influence of rAFS stages on total serum mBDNF levels is given. For each group, the comparison was performed using one-way ANOVA and Bartlett’s test to account for multiple group comparisons. The resulting P-values are indicated within the graphs. (b) Graphical representation of the influence of ectopic lesion location on total serum mBDNF is plotted. The mean values of the levels of the serum protein are indicated on each boxplot. Statistical multiple group analysis of the data (Kruskal–Wallis and Dunn multiple comparisons tests) showed that the differences in the levels of secreted protein between the groups are random (adjusted P-value, P > 0.05) and not associated with the type of the lesions. (c) Diagnostic predictive value of mBDNF for endometriosis expressed by ROC-curve analysis of the protein level in serum obtained in a cohort of 128 cases and controls. The AUC and P-values are indicated in each graph. (d) Prediction strength of mBDNF to discriminate between stages I and II endometriosis and endometriosis free controls – expressed by ROC-curve analysis of the protein level in serum obtained in a cohort of 35 women suffering from endometriosis stages I and II and 51 women making up the endometriosis free control group.
Furthermore, when we looked at mBDNF levels in women with endometriosis based on the type of the ectopic lesion location, we did not find significant differences in the serum protein between the analyzed groups (Figure 2(b)).
mBDNF is not a strong predictive marker for endometriosis
The ROC curve analysis, generated by using the entire study population of cases and controls (Figure 2(c)), showed that, although mBDNF levels differ between cases and controls, the protein is not a strong candidate for a noninvasive biomarker for the diagnosis of the disease. The area under the curve was 0.6242, P = 0.02 (95% CI = 0.526 to 0.7224, standard arrow =0.050). Furthermore, the test did not identify a cut-off level of the protein with both high sensitivity and specificity. We obtained similar results when we tested the prediction power of the mBDNF to distinguish between patients with rAFS stage I and II and endometriosis-free controls (Figure 2(d)).
Discussion
Altered and increased levels of soluble BDNF in patients with endometriosis have been previously reported by three independent groups.18,19,21 In the present study, we confirmed and extended this observation, showing that the mature form of the protein contributes to endometriosis-associated differences in BDNF in the circulation. We found that mBDNF is elevated in the serum of patients with endometriosis compared to women without endometriosis. Consistent with the findings of Wessels et al.19 in plasma, the changes in the serum mBDNF were also restricted to rAFS stage I and II disease and were not seen in patients with rAFS stage III and IV. BDNF is known to up-regulate antioxidants and block the development of oxidative stress.22 It has been suggested that reactive oxygen species of free radicals, the major factor that leads to oxidative stress, may promote the growth and adhesion of endometrial cells on the peritoneal surface and lead to endometriosis and infertility.23–25 Experimental evidence suggests that the level of oxidative stress positively correlates with the stage of the disease.26 Thus, the potential biological activities of BDNF in oxidative stress suggest that increased levels of the protein in the blood of patients with minimal and mild endometriosis might be associated with more restricted growth of the lesions at the site of implantation.
When looking at specific endometriosis lesion location and not taking into account the stage of the disease, Rocha et al.21 reported that women with ovarian endometrioma have higher preoperative plasma-soluble BDNF compared to women with other benign ovarian tumors. However, plasma levels of the protein were not indicative of the presence of peritoneal or deep infiltrating endometriosis. Moreover, these findings support the idea that the alterations in the circulating soluble and/or mBDNF are disease-stage- and/or lesion-type-specific, rather than a general endometriosis feature. In agreement with this hypothesis, the results of our receiver operating curve analysis showed a low prediction power of serum mBDNF to discriminate women with and without endometriosis. This is also in accordance with the report of Wessel et al.,19 showing that soluble plasma BDNF is a putative marker able to identify rAFS stage I and II disease with high sensitivity (91.7%) and good specificity (69.4%) at an arbitrary cutoff value of 1000 pg/mL. In contrast, we were not able to demonstrate that total serum mBDNF levels are useful for the diagnosis of minimal and mild endometriosis. The discrepancy between our results and the results of Wessel et al.19 points to a general need for additional and large scale validation studies to evaluate the putative use of both plasma and/or serum soluble and mBDNF as markers for endometriosis.
What is intriguing are our findings that the levels of serum mBDNF increase in women with and without endometriosis at 6–10 weeks after surgery. At sites of vascular injury, BDNF can induce mobilization and recruitment of myeloid cells, and thereby contributes to vessel formation or stabilization.27 Nerve growth factor and BDNF also promote hematopoietic colony growth and participate in wound-healing and tissue repair, contributing to skin or airway remodeling in inflammatory conditions.28 Thus, we speculate that increased levels of mBDNF protein in the serum of women after laparoscopic surgery might be due to the involvement of the protein in the process of postoperative wound-healing, such as neovascularization. The postoperative follow-up also revealed that the surgical intervention leads to equilibration of the levels of secreted mBDNF between women with and without endometriosis. This result in serum samples of women with and without endometriosis, confirmed and extended the findings of Giannini et al.,18 who showed that the surgical removal of endometriotic lesions causes a counterbalance of the levels of soluble BDNF in the plasma of women with and without endometriosis.
In healthy women, plasma levels of BDNF strongly correlate with estradiol levels29 and fluctuate during the menstrual cycle.30 We did not find a correlation between menstrual cycle phase and mBDNF in serum, suggesting that mBDNF and soluble BDNF might be differentially regulated by hormonal fluctuations associated with menstrual cycle phase regulation. However, we did not monitor the fluctuation of mBDNF throughout the entire cycle, and therefore our findings should be interpreted cautiously. The strengths of the study, include the prospective case-control design, the confirmation of the disease by surgery and pathology, the homogeneity of the study population with respect to cycle phase group distribution, the equal time-frame of blood collection for cases and controls, and the inclusion of potential confounders, such as overall pain, age, BMI, and cycle phase, as well as the exclusion of putative comorbidities which potentially may have an effect on the levels of serum BDNF.
Overall, we show that the mBDNF is elevated in the serum of women with endometriosis compared to women without endometriosis. This alteration is associated only with rAFS stage I and II endometriosis, is independent of menstrual cycle phase, and is not associated with levels of either overall or menstruation-related, self-reported pelvic pain intensity VAS scores. Our results are in accordance with previously published data that analyzed plasma levels of BDNF18,19 and add to the evolving picture in which the changes in humoral BDNF pool are associated with minimal and mild endometriosis. Due to the limited number of samples, the results of our analysis have to be interpreted carefully and only in the context of the current experimental settings. Further validation studies in larger cohorts are needed in order to draw any conclusions regarding the clinical relevance of mBDNF as a diagnostic tool for endometriosis. In conclusion, although we show that women with rAFS stage I and II endometriosis have increased levels of mBDNF in serum compared to controls, this difference is not predictive for the disease.
Supplementary Material
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. I. Yotova, K. Ashjaei and K. Proestling conducted the experiments, I. Yotova and A. Perricos wrote the manuscript, and R. Wenzl, L. Kuessel, H. Husslein and R. Obwegeser contributed through final editing.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Reference
- 1.Laganà AS, Vitale SG, Trovato MA, Palmara VI, Rapisarda AMC, Granese R, Sturlese E, De Dominici R, Alecci S, Padula F, Chiofalo B, Grasso R, Cignini P, D'amico P, Triolo O. Full-thickness excision versus shaving by laparoscopy for intestinal deep infiltrating endometriosis: rationale and potential treatment options. Biomed Res Int 2016; 2016:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HJ, Park YM, Jee BC, Kim YB, Suh CS. Various anatomic locations of surgically proven endometriosis: a single-center experience. Obstet Gynecol Sci 2015; 58:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins S, Olive DL, Haney AF. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol 1986; 67:335–8 [PubMed] [Google Scholar]
- 4.Burghaus S, Klingsiek P, Fasching P, Engel A, Häberle L, Strissel P, Schmidt M, Jonas K, Strehl JD, Hartmann A, Lermann J, Boosz A, Thiel FC, Müller A, Beckmann MW, Renner SP. Risk factors for endometriosis in a German case-control study. Geburtsh Frauenheilk 2011; 71:1073–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, Prentice A, Saridogan E, Soriano D, Nelen W. European society of human reproduction and embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014; 29:400–12 [DOI] [PubMed] [Google Scholar]
- 6.Fassbender A, Dorien O, D, Moor B, Waelkens E, Meuleman C, Tomassetti C, Peeraer K, D'hooghe T. Biomarkers of endometriosis. Endometr Pathog Treat 2014; 99:321–39 [Google Scholar]
- 7.Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy R. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 2001; 276:12660–6 [DOI] [PubMed] [Google Scholar]
- 8.Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris S, Sossin W, Murphy R. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci 1999; 19:2069–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science 2001; 294:1945–8 [DOI] [PubMed] [Google Scholar]
- 10.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci 2005; 6:603–14 [DOI] [PubMed] [Google Scholar]
- 11.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphismaffects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112:257–69 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Yamada Y, Morioka S, Niiro E, Shigemitsu A, Ito F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch Gynecol Obstet 2014; 289:13–21 [DOI] [PubMed] [Google Scholar]
- 13.Yotova I, Hsu E, Do C, Gaba A, Sczabolcs M, Dekan S, Kenner L, Wenzl R, Tycko B. Epigenetic alterations affecting transcription factors and signaling pathways in stromal cells of endometriosis. PLoS One 2017; 12:e0170859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Ren H, Liu T, Yong M, Zhong H. Expression and significance of ERβ and TrkB in endometriosis. Clin Exp Obstet Gynecol 2016; 43:75–81 [PubMed] [Google Scholar]
- 15.Anger DL, Zhang B, Boutross-Tadross O, Foster WG. Tyrosine receptor kinase B (TrkB) protein expression in the human endometrium. Endocrine 2007; 31:167–73 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One 2012; 7:e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Södersten K, Pålsson E, Ishima T, Funa K, Landén M, Hashimoto K, Agren H. Abnormality in serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in mood-stabilized patients with bipolar disorder: a study of two independent cohorts. J Affect Disord 2014; 160:1–9 [DOI] [PubMed] [Google Scholar]
- 18.Giannini A, Bucci F, Luisi S, Cela V, Pluchino N, Merlini S, Casarosa E, Russo M, Cubeddu A, Daino D, Artini P, Genazzani A. Brain-derived neurotrophic factor in plasma of women with endometriosis. J Endometr Pelvic Pain Disord 2010; 2:144–50 [Google Scholar]
- 19.Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 2016; 105:119–28.e1–5 [DOI] [PubMed] [Google Scholar]
- 20.American Society for Reproductive AS for R. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997; 67:817–21 [DOI] [PubMed] [Google Scholar]
- 21.Rocha AL, Vieira EL, Ferreira MC, Maia LM, Teixeira AL, Reis FM. Plasma brain-derived neurotrophic factor in women with pelvic pain: a potential biomarker for endometriosis? Biomark Med 2017; 11:313–7 [DOI] [PubMed] [Google Scholar]
- 22.Boutahar N, Reynaud E, Lassabliere F, Borg J. Brain-derived neurotrophic factor inhibits cell cycle reentry but not endoplasmic reticulum stress in cultured neurons following oxidative or excitotoxic stress. J Neurosci Res 2010; 88:2263–71 [DOI] [PubMed] [Google Scholar]
- 23.Szczepańska M, Koźlik J, Skrzypczak J, Mikołajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril 2003; 79:1288–93 [DOI] [PubMed] [Google Scholar]
- 24.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod 2005; 20:2014–20 [DOI] [PubMed] [Google Scholar]
- 25.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol 2009; 25:75–81 [DOI] [PubMed] [Google Scholar]
- 26.Carvalho LFP, Abrão MS, Biscotti C, Sharma R, Nutter B, Falcone T. Oxidative cell injury as a predictor of endometriosis progression. Reprod Sci 2013; 20:688–98 [DOI] [PubMed] [Google Scholar]
- 27.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest 2005; 115:653–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micera A, Vigneti E, Pickholtz D, Reich R, Pappo O, Bonini S, Maquart FX, Aloe L, Levi-Schaffer F. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc Natl Acad Sci U S A 2001; 98:6162–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Casarosa E, Luisi M, Cela V, Genazzani AR. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod 2009; 24:2303. [DOI] [PubMed] [Google Scholar]
- 30.Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod 2007; 22:995–1002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.