Abstract
Stem cells can self-renew and differentiate over extended periods of time. Understanding how stem cells acquire their fates is a central question in stem cell biology. Early work in Drosophila germ line and neuroblast showed that fate choice is achieved by strict asymmetric divisions that can generate each time one stem and one differentiated cell. More recent work suggests that during homeostasis, some stem cells can divide symmetrically to generate two differentiated cells or two identical stem cells to compensate for stem cell loss that occurred by direct differentiation or apoptosis. The interplay of all these factors ensures constant tissue regeneration and the maintenance of stem cell pool size. This interplay can be modeled as a population-deterministic dynamics that, at least in some systems, may be described as stochastic behavior. Here, we overview recent progress made on the characterization of stem cell dynamics in regenerative tissues.
1. INTRODUCTION
Stem cells are defined as the cells that have the long-term ability both to self-renew and to differentiate, maintaining tissue homeostasis and repair injury. Until recently, a great deal of our current understanding of tissue stem cell biology was largely based on studies done in invertebrates, which suggest that tissue stem cells have several characteristics. They (1) possess the lifetime potential of self-renewal; (2) place at the top of lineage hierarchies and produce all differentiated cell types; (3) give rise through an asymmetric cell division to one stem cell and one daughter that undergoes differentiation; (4) reside within a specialized microenvironment that promotes “stemness” and prevents differentiation; (5) divide more infrequently (or “slowly”) than their immediate progenies, termed transit-amplifying (TA) cells; and (6) are rare and constant in number during adult homeostasis. These concepts have been repeatedly used over the past couple of decades to interpret results obtained from many studies on stem cell biology from invertebrates and vertebrates alike. Recent development of mouse genetics tools for in vivo lineage tracing, live imaging and mathematical modeling allowed in-depth studies into the behavior of tissue stem cells in mammals. These studies seem to indicate a model that does not fit with the orthodox, traditional view of stem cell fate decision.
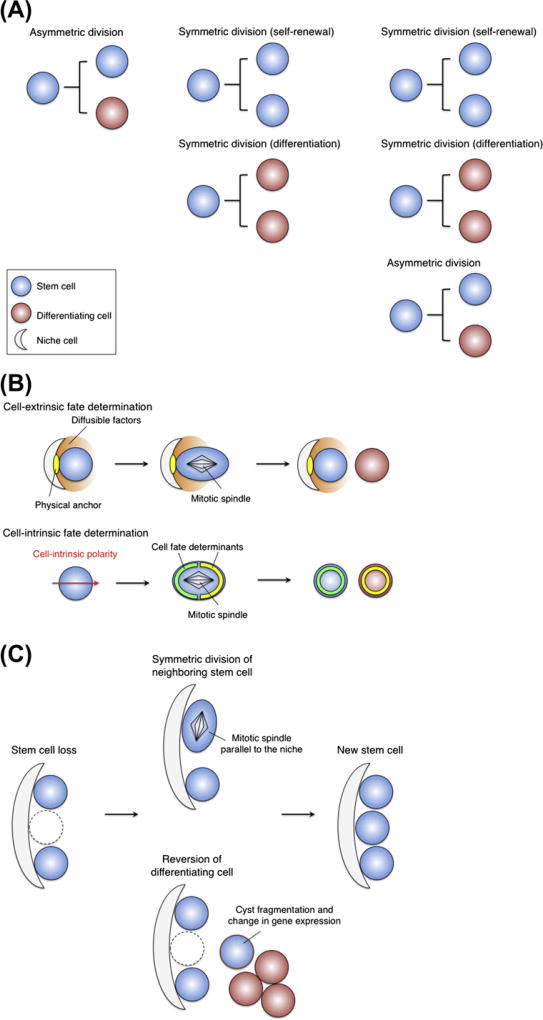
In principle, there are at least three possible divisional strategies that the stem cells would adopt to balance the number of stem cells and differentiated progeny produced in a tissue (Morrison and Kimble, 2006) (Fig. 1A). (1) Asymmetric cell division: each and every stem cell generates at each division one daughter stem cell and one daughter destined to differentiate. (2) Symmetric cell division: each stem cell can divide symmetrically to generate either two daughter stem cells or two differentiating daughters. (3) Combination of cell divisions: each stem cell can divide either symmetrically or asymmetrically. In the case of (2) or (3), if the probability of differentiation is matched by that of a self-duplicating stem cell division, in a somewhat stochastic manner or as a programmed ratio, homeostasis is achieved. This model is generally known as population asymmetry or population dynamics of stem cell behavior. In the first case, asymmetric cell division has been described in the Drosophila germ line or neuroblast. The second symmetric divisions have been observed in the developmental stem/progenitor cells or adult stem cells after tissue damage, in which a rapid expansion of stem cells or differentiated progenies is required (Morrison and Kimble, 2006). The Caenorhabditis elegans germ line may fit the second and third models although exact cellular mechanisms remain to be resolved. In most mammalian tissues, it has been unclear until recently whether homeostasis is maintained by asymmetric divisions or by a population strategy that uses symmetric (or both asymmetric/symmetric) divisions to balance stem cells and differentiated progeny.
Figure 1.
Stem cell behavior proposed in invertebrate model systems. (A) Three possible cell division strategies: invariant asymmetric division (left); invariant symmetric division (middle); combination of asymmetric and symmetric divisions (right). (B) Cell-extrinsic (upper) and -intrinsic (lower) regulation of asymmetric cell division. (C) Two possible stem cell behaviors to replenish a new stem cell: symmetric division (upper) and dedifferentiation (lower).
What mechanisms are used by stem cells to select two distinct cell fates (self-renewal and differentiation) during asymmetric cell division? It has been proposed that a stem cell (1) relies on external (cell-extrinsic) environmental factors; and/or (2) follows from internal (cell-autonomous or cell-intrinsic) regulations (Knoblich, 2008) (Fig. 1B). Drosophila germ line and neuroblast are well-studied examples of extrinsic and intrinsic modes of asymmetric cell division, respectively.
For extrinsic asymmetric divisions, the stem cell regulation is dependent on specific anatomical locations or cell type in a tissue known as a niche. Niches were first proposed as a theoretical concept that proposes stem cells can only survive and proliferate in specialized tissue locations (Schofield, 1978). Subsequently, the niche was defined in the Drosophila gonad at the anatomical and functional level (Kiger et al., 2001; Tulina and Matunis, 2001; Xie and Spradling, 2000). The niche employs physical supports to anchor stem cells in a particular place, as well as produces diffusible factors acting as short- and long-range signals to regulate stem cells (Scadden, 2006). Thus, only one of the two daughter cells maintains contact with the niche and stem cell identity after division. Candidate niches and regulatory molecules have also been identified in several mammalian tissues (Fuchs et al., 2004; Jones and Wagers, 2008; Morrison and Spradling, 2008).
For intrinsic asymmetric divisions, stem cells asymmetrically segregate cell-fate determinants at mitosis, leading one cell to follow a differentiation pathway and the other to keep stem cell identity (Knoblich, 2008; Roegiers and Jan, 2004). During this process, stem cells first set up an axis of asymmetry in interphase. As they enter mitosis, they use this axis to polarize the distribution of protein determinants and to orient the mitotic spindle (Morin and Bellaiche, 2011; Siller and Doe, 2009), so that determinants are inherited by only one of the two daughter cells (Bardin et al., 2004; Betschinger and Knoblich, 2004). Many key components of the genetic machinery that facilitate the intrinsic asymmetric division are also conserved in mammals (Knoblich, 2008).
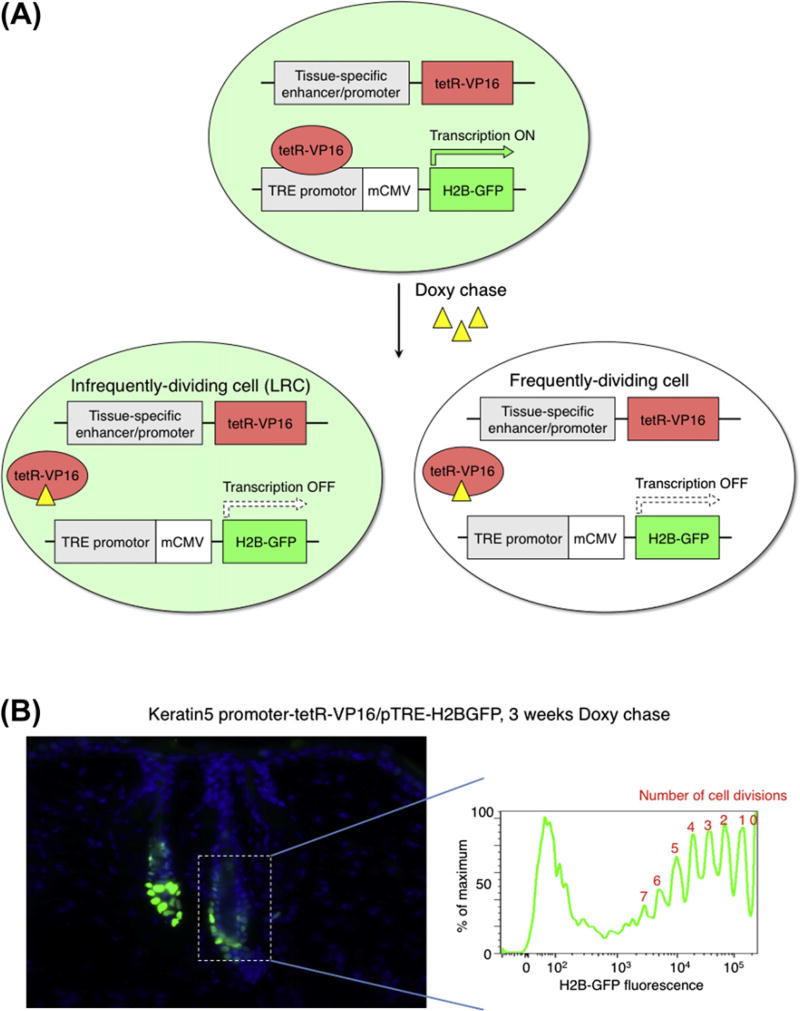
A stem/TA cell model predicts that stem cells divide more slowly (or more precisely less frequently) than their immediate daughter cells more differentiated to progenitors, which represent a short-lived population of cells called TA cells (Fuchs, 2009; Li and Clevers, 2010). TA cells enter terminal differentiation pathway after several rapid rounds of cell division. To identify slow-cycling cells within a tissue, the field has employed label-retaining assays, involving the incorporation of DNA analogs such as bromodeoxyuridine (BrdU) and tritiated thymidine during S phase of cell cycle. First proliferative cells can be labeled by administrating mice with the DNA analog. A subsequent long-term chase of the label allows the highly proliferative cells to dilute the label and to be shed out from the tissue by differentiation. The cells that had incorporated label, but that divided only a few times if at all, retain the label and are observed as label-retaining cells (LRCs). We have adapted the nucleotide pulse-chase concept to a tet-off gene regulation system using H2B-GFP, making now possible to label and isolate slow-cycling cells in vivo (Fuchs, 2009; Tumbar et al., 2004) (Fig. 2). The transgenic mice express histone H2B-GFP in a tissue-specific manner, and this expression can be turned off when tetracycline (doxycycline; doxy) is added to the diet. Upon administration of doxy, the dividing cells dilute out the label and differentiating cells are sloughed from the tissue, leaving only the slow-cycling cells detectable as H2B-GFP LRCs. Another way to explain the label retention of putative stem cells was suggested to be the socalled “immortal strand hypothesis” (Cairns, 1975; Lansdorp, 2007; Rando, 2007; Tajbakhsh, 2008). An old model proposed that stem cells asymmetrically segregate their DNA, keeping the old template DNA strand and transferring the newly synthesized strand to their daughter cells during mitosis. Selective chromosome segregation has been reported to occur in muscle (Conboy et al., 2007; Rocheteau et al., 2012; Shinin et al., 2006), nervous system (Karpowicz et al., 2005) and mammary gland (Smith, 2005) but not in blood (Kiel et al., 2007), skin (Sotiropoulou et al., 2008; Waghmare et al., 2008) and intestine (Schepers et al., 2011).
Figure 2.
H2B-GFP tet-off system to count cell division frequency in vivo. (A) Schematic representation of the strategy to detect slow-cycling cells with H2B-GFP. Doxy administration inhibits the binding of tetR-VP16 proteins to the TRE promoter, and thus turns off H2B-GFP transcription. Cells dilute H2B-GFP proteins after division, which enables quantification of the frequency of cell division during chase periods. (B) H2B-GFP pulse-chase in skin (Tumbar et al., 2004; Waghmare et al., 2008). The keratin5 promoter drives H2B-GFP expression in skin epithelial cells. After 3 weeks of doxy chase, bulge contains cells with different cell-division history shown in FACS profile on the right.
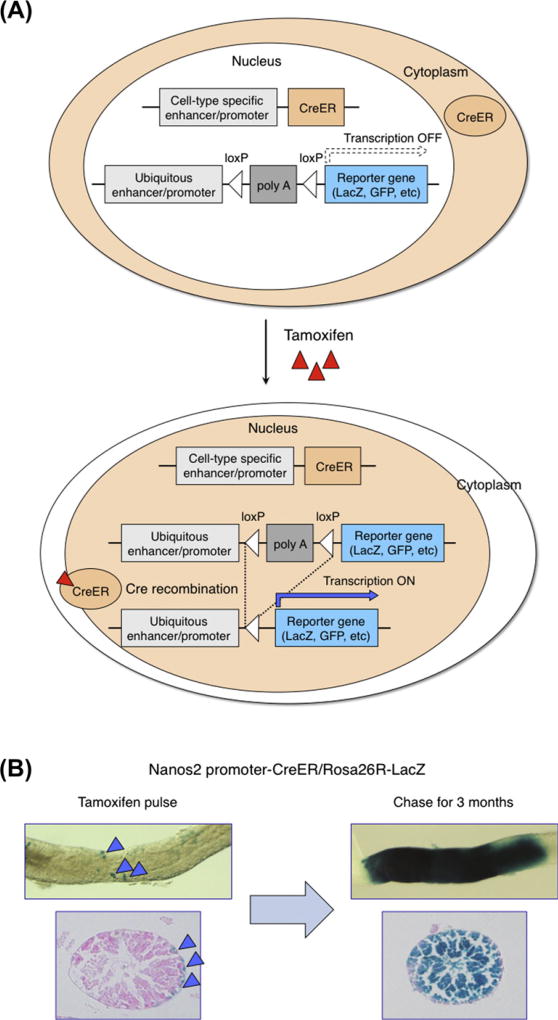
A major barrier in the field has been the relative complexity of mammalian tissues and the rarity of stem cells, which made it much more difficult to identify individual stem cells in their niche as compared to Drosophila or C. elegans models, in which stem and niche cells can be located quite precisely with single-cell resolution (Morrison and Spradling, 2008). As a surrogate for in vivo stem cell characterization, functional transplantation assays have been established and utilized in several mammalian tissues. However, it becomes increasingly clear that these assays perturb the normal tissue homeostasis and affect the normal cell behavior, providing a more accurate description of tissue injury rather than tissue homeostasis. Similarly, cell culture assays sometimes alter the patterning of cells in ways that modify their fates and even their developmental potentials. In the last decade, mouse genetics tools have been developed that allowed the visualization and tracking of specific cell populations in mammalian tissues. Particularly, an inducible lineage-tracing Cre–loxP system has been useful to describe the fates of stem cells in intact tissues (Saunders, 2011) (Fig. 3). In this system, expression of a fusion protein composed of Cre and a mutated estrogen ligand ER (or ERT, ERT2) is driven under the control of a cell-type-specific enhancer/promoter. Cre activation can be induced at any time through treatment with an estrogen ligand, such as tamoxifen or 4OH-tamoxifen, which drives the CreER fusion into the nucleus. The inducible CreER mouse is crossed with a reporter mouse that has a stop codon between loxP sites (“floxed”), allowing expression of the reporter gene following the Cre-induced recombination. Activation of the reporter gene is irreversible and inheritable, and allows tracing the lineage or fate of the recombined cells even after they lose the transcription of the Cre gene itself. Applying analytical methods from population dynamics and statistical physics to an inducible genetic fate mapping, it is now possible to describe the dynamics of entire stem cell population, even in complex mammalian tissues (Klein and Simons, 2011). Recent advances in deep-tissue imaging of single-cell live in their niche also provided insights into the behavior of individual stem cells, which is particularly important for rare and heterogeneous populations of mammalian stem cells (Schroeder, 2011).
Figure 3.
Genetic lineage-tracing experiments. (A) Schematic representation of the double transgenic DNA construct used for lineage-tracing experiments. Tamoxifen administration translocates CreER to the nucleus, where Cre-mediated recombination takes place. (B) Example of lineage-tracing experiments using Tamoxifen-inducible Cre driven by spermatogonia-specific Nanos2 enhancer/promoter (Sada et al., 2009). Two days after Tamoxifen injection, spermatogonia located on the basement membrane are labeled. Three months after labeling, a sufficiently long period for a repeated completion of spermatogenesis, seminiferous tubule of testis contained all stages of spermatogenic germ cells.
In this review, we integrate insights from invertebrate and vertebrate model systems to formulate cellular and molecular mechanisms of stem cell fate determination during homeostasis and regeneration.
2. STEM CELLS IN INVERTEBRATE MODEL SYSTEMS
2.1. Germline Stem Cells in Drosophila: Asymmetric Division Controlled by Niche
In the Drosophila testes, 6–12 germline stem cells lie at the anterior tip, surrounding a cluster of somatic cells called the hub (Fuller and Spradling, 2007; Gilboa and Lehmann, 2004). In females, the terminal filament, cap cells and inner germarial sheath cells constitute the stem cell niche, which closely abut 2–3 stem cells. In the gonad, germline stem cells share a niche with somatic stem cells, so-called “escort stem cell” in ovary, or “cyst progenitor cells” in testis. Drosophila germline stem cells are thought to exclusively undergo oriented asymmetric divisions: the cell within the niche remains as a stem cell, whereas the other daughter cell, which lies one-cell diameter away from the niche, begins to differentiate. Male and female stem cell daughters, known as gonialblasts or cystoblasts, respectively, undergo four rounds of TA mitotic divisions, with incomplete cytokinesis, to generate interconnected 16-cell germline cysts. Following mitosis, germ cells enter meiosis, and further differentiate to form mature sperm or egg. As differentiation is taking place, the more mature germ cells are displaced toward the posterior of the gonad. Thus, the cell fate of germline stem cells in Drosophila is geographically recapitulated in the polarized gonad, and is a consequence of the localization of stem cell relative to the niche that ultimately controls stem cell self-renewal and differentiation.
Studies of the Drosophila germ line have revealed several basic features of stem cell niches that are important for controlling stem cell behavior (Gilboa and Lehmann, 2004; Spradling et al., 2001; Yamashita et al., 2005). (1) Signals emanating from the niche regulate stem cell proliferation, survival and maintenance of undifferentiated state. For example, male germline stem cells are maintained by Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling initiated from the hub, which secretes the ligand unpaired, while the BMP homolog encoded by decapentaplegic (dpp) functions as a major signal for female germline stem cells. These signals maintain stem cell identity at least in part by repressing of genes that direct differentiation, such as Bam (bag of marbles) in germline stem cells. (2) Cell–cell adhesion mediated via E-cadherin or another adhesion molecule anchors stem cells to the niche and keeps them in the proximity of self-renewal signals. (3) The precise orientation of the stem cell mitotic spindle ensures the displacement of one of the daughter cells outside the stem cell niche. In female germline stem cells, the spindle is oriented via anchorage of one spindle pole to the spectrosome, a germ cell-specific subcellular organelle, which is always located at the apical side of stem cell. By contrast, in male, the spindle orientation is set up by centrosome positioning, where the mother centrosome is always anchored to the apical side of the stem cell by astral microtubules to adherens junctions formed between hub cells and stem cells (Yamashita, 2009).
In addition to stem cell–niche interaction, stem cells within a common niche also interact with each other via a “cell competition” mechanism. The cell competition was first discovered in the Drosophila wing imaginal discs, in which fast-growing cells induced by higher dMyc expression can outcompete and eliminate slow-growing neighbors by promoting their apoptosis (de la Cova et al., 2004; Moreno et al., 2002). In Drosophila gonad, stem cells seem to compete each other for niche occupancy, which leads to one stem cell forcing another out of the niche, ultimately resulting in one stem cell dominating the niche (Zhao and Xi, 2010). For example, competitive interaction could arise between ovarian germline stem cells as a consequence of differential expression of dMyc (Rhiner et al., 2009). Alternatively, the competitiveness of germline stem cells is determined by the physical strength of the niche–stem cell interaction, i.e. intensity of E-cadherin (Jin et al., 2008; Tian et al., 2012). In testes, an increased expression of integrin in somatic stem cells leads to their enhanced adhesion to the niche, enabling them to push out competitor germline stem cells (Issigonis et al., 2009; Sheng et al., 2009). The stem cell competition might play a role in the quality control of stem cells, excluding those with a low proliferative potential or a low capacity for interaction with niche cells. It is also possible that the cell competition might be a possible mechanism of how stem cell population size is maintained within niche.
Drosophila germ cells are thought to undergo strict asymmetric divisions. However, when stem cells are eliminated, a bona fide stem cell can arise by two distinct mechanisms (Fig. 1C). First, after one stem cell is lost, its neighboring stem cell divides parallel to the niche in a symmetric manner causing two daughter cells to occupy the environment (Xie and Spradling, 2000). Second, new stem cells can arise from the reversion or dedifferentiation of TA cells into fully functional stem cells (Brawley and Matunis, 2004; Kai and Spradling, 2004). This reversibility might be limited to cells that went down in differentiation to the 8-cell stage of germline cysts. During this process, interconnected germline cysts are breaking down into single cells and differentiation-related genes are downregulated in these cells. On the basis of these observations, either stem cells or their differentiating progeny may function as stem cells if they can respond to appropriate signals from the niche.
Symmetric division is not restricted to the circumstances of stem cell regeneration described above but also observed in the homeostatic condition. A recent live imaging study in the Drosophila testes showed that germline stem cells are generated in the niche by a previously undetected event “symmetric renewal,” where stem cell–daughter cell pairs both gain contacts to the hub (Sheng and Matunis, 2011). Furthermore, germline stem cells undergo direct differentiation by detaching from the hub. These symmetric renewal plus symmetric differentiation are observed at a frequency of approximately 20% during steady-state tissue maintenance although the majority of remaining germline stem cells employs asymmetric division. Therefore, Drosophila germline system provides a platform for determining regulators of stochastic fate choice of stem cells in vivo that may also be conserved in mammalian stem cell systems.
2.2. Drosophila Neural Precursor Cells: Cell-Intrinsic Regulation of Asymmetric Division
Neuroblasts are stem cell-like progenitors of Drosophila central nervous system (Knoblich, 2008; Reichert, 2011). Asymmetric division of a neuroblast yields a large, self-renewed neuroblast and a smaller intermediate progenitor called a ganglion mother cell. The ganglion mother cell undergoes one more division that gives rise to two postmitotic cells that become neurons or glial cells. Neuroblasts are specified within a monolayered epithelium called the ventral neuroectoderm and delaminate from the epithelium to undergo repeated rounds of asymmetric division along the apical–basal axis.
Key features and components associated with the neuroblast asymmetric division have been identified and characterized (Chia et al., 2008; Knoblich, 2008; Prehoda, 2009; Reichert, 2011; Siller and Doe, 2009): (1) the cell-fate determinants, which act as differentiation factors, are asymmetrically localized as cortical crescents during mitosis; (2) the mitotic spindle is oriented orthogonal to the cortical protein crescents to ensure their exclusive segregation to the ganglion mother cell; and (3) the mitotic spindle is itself asymmetrical, resulting in the production of a larger neuroblast daughter and a smaller ganglion mother cell.
Epithelial apical–basal polarity of neuroblasts is established by the asymmetric accumulation of Par-3, Par-6, and atypical PKC (aPKC) to the apical cortex (Goldstein and Macara, 2007; Suzuki and Ohno, 2006). The mitotic spindle orientation is regulated by the microtubule-binding protein Mud, which is recruited apically by Pins and Gαi (Siller and Doe, 2009). Gαi–Pins–Mud pathway works by recruiting the dynein–dynactin complex to the apical cortex, which exerts a pulling force to recruit and maintains one centrosome at the apical pole, thereby aligning the mitotic spindle along the apical/basal polarity axis. A factor called Inscuteable links the Par-3/6-aPKC and Gαi–Pins–Mud complexes by binding to both Par-3 and Pins.
Although most of these factors are preferentially inherited by the apical daughter cell, which remains a neuroblast, they do not appear to influence cell fate directly. Instead, they induce the asymmetric localization of cell-fate determinants such as Numb, Pros, and Brat to the opposite, basal side of the cell and their segregation into the basal ganglion mother cell (Bardin et al., 2004; Betschinger and Knoblich, 2004). The localizations of Numb, Pros and Brat are regulated by adaptor proteins, Pon and Miranda. In this cell, Numb represses Notch signaling by promoting endocytosis of the Notch receptor; Pros activates or inhibits transcription of cell cycle-related regulators and self-renewal/differentiation-related genes; Brat is involved in translational regulation and cell growth inhibition. Pros and Brat are thought to inhibit self-renewal and promote cell cycle exit and differentiation because mutant ganglion mother cells do not produce neurons, continue to proliferate like neuroblasts and give rise to tumors. Thus, defects in asymmetric cell division lead to the formation of a cell type that proliferates like a neuroblast but is immortal and no longer responds to the hormonal signals that inhibit proliferation.
2.3. Germline Stem Cells in C. elegans: Stem Cell Maintenance as a Population
Similar to the Drosophila, the C. elegans gonad is a polarized structure with immature germ cells at the distal end and mature gametes at the proximal end (Hubbard, 2007; Joshi et al., 2010; Kimble and Crittenden, 2007). In the distal-most part, termed the proliferative zone, a somatic distal tip cell provides the stem cell niche and maintains a population of ~250 mitotic germ cells. Those proximal are in transition zone, where cells exit mitosis and resume meiosis. As cells migrate further proximally, they progress through successive stages of meiosis.
Several lines of experimental evidence support that the distal tip cell is necessary and sufficient for maintaining germline stem cells and the mitotic zone of the gonad (Joshi et al., 2010). Upon laser ablation of the distal tip cell, mitotic germ cells stop mitotic divisions, enter meiosis, and differentiate. Furthermore, distal tip cell relocation leads to a positional change of the mitotic germ cells, and its duplication generates additional mitotic germ cells. At molecular level, the distal tip cell utilizes GLP-1 (homologous to Notch) signaling to control stem cell proliferation and maintenance. Loss of Notch signaling component results in a similar phenotype to that seen in the distal tip cell ablation, suggesting the necessity of direct cell contact with the niche in this system. Downstream of Notch signaling, many conserved RNA regulators act intrinsically within germ cells to control their decision between self-renewal and early differentiation.
The proliferative zone can be subdivided into at least three regions based on their distance from the distal tip cell and cell division kinetics (Crittenden et al., 2006; Hubbard, 2007; Jaramillo-Lambert et al., 2007; Maciejowski et al., 2006): (1) the distal-most zone comprising germ cells with lowest cell division kinetics in the 1–2 cell diameters from the distal tip cell; (2) the next-proximal zone containing cells with higher mitotic index in the 3–10 cell diameters from the distal tip cell; (3) a more proximal zone that includes cells completing their last mitotic cell cycle and in meiotic S phase. Unlike Drosophila, the division planes of mitotic germ cells are variable: the orientation of cell divisions is perpendicular or parallel with regard to the distal–proximal axis (Crittenden et al., 2006). More recently, Cinquin et al. (2010) showed that upon the removal of Notch signaling, proximal germ cell pool entered meiosis in a spatiotemporal wave from proximal to distal, subsequently distal pool (~7 cell diameters from the distal tip cell) entered meiosis in a synchronous manner. This result suggests that (1) the distal pool of cells exists in an essentially equivalent immature state within the niche; and (2) a proximal pool is in a gradual progression from the immature state to the early differentiation state while, at the same time, continuing to proliferate. Hence, we can consider the C. elegans proliferative zone to resemble the stem/TA-cell system, but the stem cells are maintained by the niche as a group of cells. They are not subjected to programmed-asymmetric divisions as observed for stem cells in the Drosophila germ line. It will be necessary to define the relationship between cell division history, location, and cell fate of individual germ cells in future.
In summary, asymmetric cell division has emerged as a central mechanism of fate determination in Drosophila germ line and neuroblast. In the former, the two daughter cells can be placed in different microenvironments, which might then specify different cell-fate choices through intercellular signaling. In the latter, asymmetric partitioning of cell-fate determinants in the mother stem cell can give rise to daughter cells that adopt different cell fates. In contrast, the population-based mechanism may work in the C. elegans germ line. The studies in invertebrate systems facilitate our understanding of the nature of mammalian regenerative tissues, which consist of greater number of stem cells, various cell types and more complicated structures and are more difficult to tease out.
3. CHARACTERISTICS OF MAMMALIAN STEM CELLS
3.1. Mouse Skin Stem Cells
Mammalian skin consists of epidermis and dermis, which are made in the majority of keratinocytes and fibroblast cells, respectively. A great deal is now known about the epidermal stem cells. Epidermis consists of layers of keratinocytes, which are organized into interfollicular epidermis and associated appendages, including hair follicles and sebaceous glands (Blanpain and Fuchs, 2006, 2009). The interfollicular epidermis, sebaceous gland and hair follicles have distinct stem cell population that sustain adult tissue turnover: cells in the innermost (basal) layer replenish the interfollicular epidermis and the sebaceous gland, whereas the hair follicle is cyclically regenerated by stem cells in the bulge region.
3.1.1. Interfollicular Epidermis: Hierarchal versus Stochastic Cell Fate Choice Models
The epidermis and its appendages develop from a multipotent embryonic progenitor of keratinocytes (Blanpain and Fuchs, 2006, 2009; Fuchs, 2007). During the early stages of embryonic skin development, most cell divisions are symmetric, which ensures the increase of the surface area and maintains the epithelium as a single layer. A shift from symmetric to predominantly asymmetric divisions occurs at embryonic day 14 coincident with differentiation and stratification. In symmetric cell divisions, mitotic spindles are oriented parallel to the underlying basement membrane, whereas asymmetric cell divisions have spindles perpendicular to it. During asymmetric division of developing skin, a conserved complex of proteins (including Par-3–Par-6–aPKC, mouse Inscuteable, Leu–Gly–Asn enriched protein [LGN], and NuMA) that play roles in Drosophila neuroblast localizes to the apical cell cortex (Lechler and Fuchs, 2005; Poulson and Lechler, 2010; Ray and Lechler, 2011; Williams et al., 2011). Impaired asymmetric cell divisions caused defects in stratification, differentiation and barrier formation. Thus, in developing epidermis, proper columnar stratification and tissue organization are driven at least in part by oriented, asymmetric cell divisions.
In adult interfollicular epidermis, proliferating cells are located in the basal cell layer (Ambler and Maatta, 2009; Blanpain and Fuchs, 2009; Fuchs and Horsley, 2008) (Fig. 4A). The skin epithelium is separated from the dermis by a basement membrane, which may act as niche by providing extracellular matrix proteins and proliferative stimuli to the basal cells. On commitment to terminal differentiation, basal cells exit the cell cycle and subsequently migrate into the suprabasal cell layers. The cells terminally differentiate while migrating outward toward the skin surface, and are eventually shed and replaced by inner layer cells moving outward.
Figure 4.
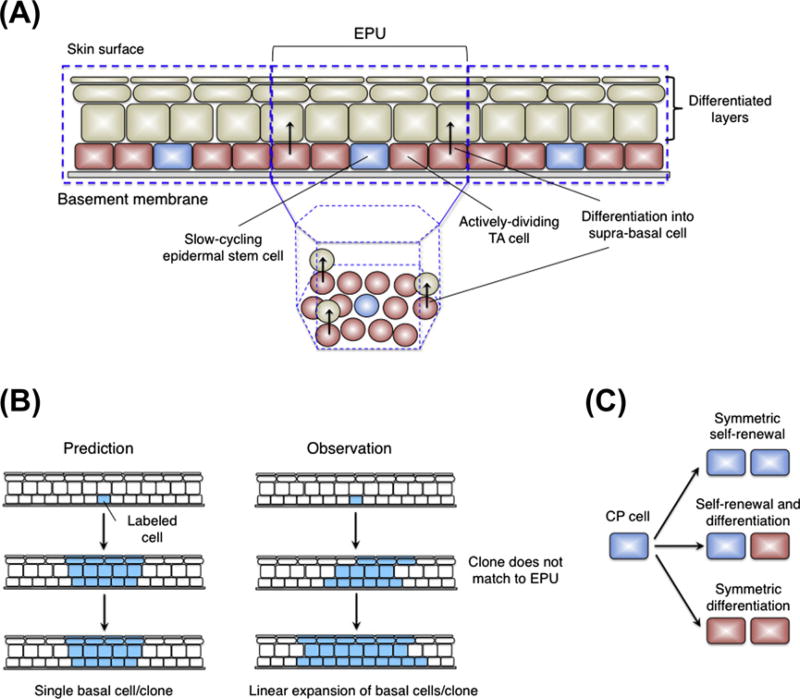
Interfollicular epidermis. (A) EPU model. A slow-cycling stem cell lies at the center of each unit and generates TA cells. Postmitotic basal cells leave the basal layer and migrate vertically to the suprabasal layer. (B) Distribution of epidermal clone. In EPU model, the size and shape of the labeled clones is constant (left). In actual observation, the clone size increases with time (right). (C) New model proposes three outcomes of the committed progenitor (CP) cell division.
Label-retaining studies show that interfollicular epidermis contains slowly cycling basal cells, which have been interpreted as stem cells that would produce a short-lived population of TA cells also localized to the basal layer, according to the stem/TA cell hypothesis (Bickenbach, 1981). The heterogeneity in proliferative potential is also observed in primary human keratinocytes, which contain three clonal types: stem cell-like holoclones; TA cell-like paraclones; and intermediate meroclones (Barrandon and Green, 1987). The relationship between stem cell activity, gene expression and cell cycle kinetics is further tested in human epidermis where α 6 integrin-bright/CD71-dim quiescent and α 6 integrin-bright/CD71-bright cycling cells showed distinct capacity of long-term tissue reconstitution (Schluter et al., 2011). These studies provide evidence for a hierarchical organization in the epithelial proliferative compartment and that the slow-cycling cells might represent a stem cell population of the epidermis.
On the basis of observation that there were slightly fewer mitoses in basal cells lying beneath the center of the columns than in those at the periphery, it was also proposed that epidermis is organized into columns of clonal units known as epidermal proliferative units (EPUs) (Janes et al., 2002; Jones et al., 2007; Jones and Simons, 2008; Kaur, 2006; Strachan and Ghadially, 2008) (Fig. 4A). In this model, the slow-cycling stem cell lies at the center of each EPU and generates an adjacent cluster of TA cells, which in turn maintain the overlying column of suprabasal cells. However, several reports bring this model into question. For example, retroviral and transgenic labeling studies demonstrated the existence of labeled cells that are organized in groups that are larger and more irregular in shape than predicted by the EPU boundaries (Kameda et al., 2003; Ro and Rannala, 2004, 2005). Thus, although interfollicular epidermis contains LRCs and stem/progenitor cells that support epidermal homeostasis, their clonal units do not always resemble classical EPUs.
A new model of epidermal homeostasis has been proposed based on quantitative lineage tracing in mouse tail (Clayton et al., 2007) and ear (Doupe et al., 2010) epidermis. These experiments tracked the fate of interfollicular cells over a one-year time course at single-cell resolution in intact tissues (Fig. 4B). Labeled clones were irregular in shape, across the predicted boundaries of EPU, consistent with previous labeling studies. The authors quantified the number of basal cells per persistent clones and found that the basal cells number was increased linearly with time in the long term. Strikingly, cell fate (generating two proliferating daughters, two postmitotic daughters, or one cell of each type) and the time between consecutive cell divisions were random (Fig. 4C). The results are not only incompatible with the EPU hypothesis but in addition, can be explained by a simpler model than needs not involve stem and TA cells. Instead, the new model proposes that all proliferating basal cells (termed committed progenitor (CP) cells) are identical in terms of their fate and cell cycle kinetics. The behavior of any individual CP cell is stochastic, but probabilities of the fates toward either self-renewal or differentiation are balanced as a population, so that tissue homeostasis is achieved. Although the new model is attractive, the following issues will need to be resolved. Several experimental evidences have shown that LRCs exist in the epidermal basal layer, but what is their biological function, if any? The previous lineage-tracing experiments examined the behavior of proliferation basal cells marked by non-cell-type-specific Ah-CreERT (Clayton et al., 2007; Doupe et al., 2010; Kemp et al., 2004), so could not discriminate among the actual characteristics of the pulse-labeled cells. Recently it has been shown by lineage tracing that a more quiescent or slow-cycling population of cells that can be genetically marked by the K14-CreER transgene is longer-lived and has a differential dynamics from a shorter-lived faster proliferating cell population that could be marked by the Involucrin-CreER (Mascre G et al, 2012). This clearly eliminated the possibility that a single population of progenitors maintain the adult epidermis. It has been proposed that these two populations are organized in a hierarchical manner, in which the slow-cycling cells generate the more frequently dividing cells based on a mathematical fit of the data. Given other more complicated models would likely fit these data, and given the caveat of being unable to rule out additional heterogeneity in the epidermal compartment the hierarchical model requires further experimental substantiation. Identification of specific genes to distinguish between active-versus slow-cycling populations in mice will enable us to directly address their possible differences and interplay between them.
3.1.2. Hair Follicle Stem Cells: Symmetric Fate Determination with Each Hair Cycle
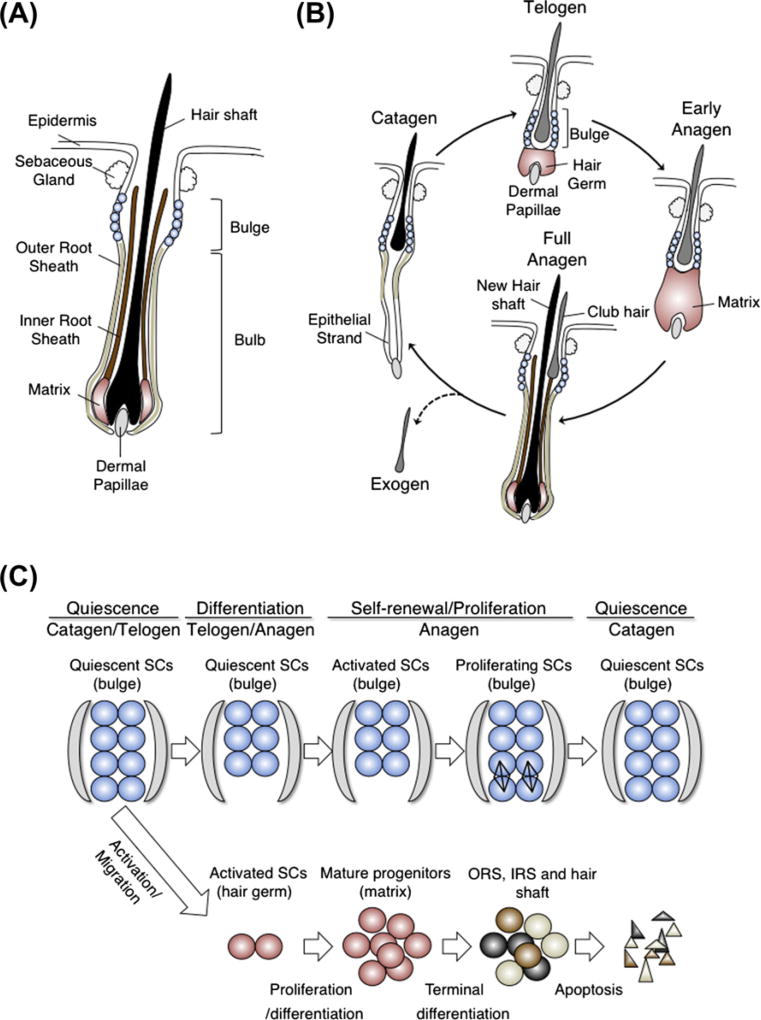
Hair follicle stem cells are clustered in bulge structures in the skin, making them easier to study than stem cells in other organs, where they are usually scattered randomly. Hair follicles have an upper permanent (bulge) region containing hair follicle stem cells and a lower temporary (bulb) region that periodically dies out and is regenerated again from the permanent region (Blanpain and Fuchs, 2006, 2009; Cotsarelis, 2006) (Fig. 5A). Concentric layers of cells surround the centrally located hair shaft, and the hair follicle stem cells are localized in the outermost layer, called the outer root sheath. At the bulb base, there is a pocket of progenitor cells known as matrix, which divides rapidly and generates terminally differentiated cells forming the inner root sheath and the hair shaft. The matrix encloses a mesenchymal pocket of cells called dermal papillae, a signaling center with fate instructive properties. Hair follicle employs a cyclic destruction and regeneration process known as the hair cycle, which consists of morphologically recognizable and synchronous phases (Muller-Rover et al., 2001) (Fig. 5B): anagen, for growth and proliferation with production of a new hair; catagen, for apoptosis-driven regression; and telogen, for rest.
Figure 5.
Hair follicle stem cells. (A) The hair follicle structure. (B) The hair follicle cycle: stages of rest (telogen), growth (anagen), regression (catagen), and a less synchronous stage of hair shedding (exogen). (C) Models for symmetric fate decisions for hair follicle stem cells during hair cycle.
Nucleotide-tracing experiments and H2B-GFP pulse-chase studies showed that slow-cycling LRCs concentrated in the bulge (Cotsarelis et al., 1990; Fuchs, 2009; Tumbar et al., 2004). Our previous work quantified that the bulge cells divide on average 3 ×/hair cycle, and ~50–100 times in a life (Waghmare et al., 2008). In addition to their infrequent divisions, specific surface expression of CD34 and α6-integrin allows isolation of an enriched bulge cell population, which behaves as stem cells in vivo and in vitro (Cotsarelis, 2006; Tumbar, 2006). In addition, several promoters, such as Keratin15 (Morris et al., 2004), Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5) (Jaks et al., 2008) and Gli1 (Brownell et al., 2011), marked distinct population of bulge cells in lineage-tracing experiments and confirmed the long-term contribution of progeny to the entire hair lineages (Jaks et al., 2010). Hsu et al. (2011) recently demonstrate that hair follicle stem cell derivatives return to the bulge to serve as future stem cells, while more committed progeny home back to a distinct layer of the bulge to maintain stem cell quiescence. In summary, hair follicle stem cells reside in the bulge, which in turn generate TA namely the matrix cells. Having established a range of properties and fates for bulge cells, it will be interesting to address what are the critical differences between heterogeneous population in hair follicle stem cells correlated with gene expression and frequency of cell division. Hsu et al. (2011) proposed that the most infrequently dividing cells are a reserve population of cells only utilized in injury, but lineage-tracing experiments that specifically mark these cells is missing.
Lineage-tracing experiments by using hair follicle- or bulge-specific marks suggested that in response to wounding, hair follicle stem cells leave their stem cell niche and contribute to repopulation of the epidermis (Brownell et al., 2011; Claudinot et al., 2005; Ito et al., 2005; Jaks et al., 2010; Levy et al., 2007). During this process, bulge cells are transiently recruited out of the follicle and then proliferate to regenerate the epidermis. After contributing to wound healing, bulge-derived cells did not express follicle markers, but did express epidermal markers, indicating that they had converted into functional epidermal keratinocytes. Bulge cells are heterogeneous in terms of their capacity to become epidermal lineage cells: Keratin15+ middle/lower bulge do not persist in healed wounds, with no epidermal contribution beyond 50 days (Ito et al., 2005), while Gli1+ upper bulge established long-term progenitors that maintain the regenerated epidermis (Brownell et al., 2011). Intriguingly, it is a perineural microenvironment in the follicle that instills Gli1+ cells with the capacity to be similarly reprogrammed into epidermal stem cells (Brownell et al., 2011). Thus, stem cell replacement across neighboring tissues is another strategy to compensate for the stem cell loss upon injury.
The analysis of dynamics of clonal behavior reported in hair follicle also contradicted a strictly asymmetric cell fate decision model for immediate daughters of stem cells. Our laboratory conducted a single bulge cell genetic lineage tracing combined with a proliferation history analysis in vivo (Zhang et al., 2009). If a labeled bulge cell followed a simple model of asymmetric fate decisions, we would detect the labeled cells as dividing and generating one stem and one differentiating cell. In contrast, we found that soon after labeling at the telogen to anagen transition, the majority of labeled cells did not follow such simultaneous self-renewal and differentiation: the labeled cells did not divide, and instead, either remained as a single bulge cell or migrated into the differentiating hair germ where they began divisions (Zhang et al., 2009). At anagen stage, the labeled bulge cells that did not migrate previously divided 1–3 times in the niche without producing differentiating cells at that time. The newly generated bulge cells at this stage maintained stem cell-signature gene expression after division, indicating that their division is “self-renewing.” We further analyzed the placement of two daughter cells descending from one cell division with respect to the basement membrane (Zhang et al., 2010). The orientation of doublet cells generated by one division of a single-labeled cell reveals parallel orientation with respect to the basement membrane in the bulge (where the cells self-renew) during anagen. In contrast, in the hair germ and matrix (where the cells differentiate), cells divide at a perpendicular orientation to the basement membrane. On the basis of these results, we suggested that hair follicle stem cells undergo two processes of self-renewal and differentiation at distinct phases of hair cycle (Fig. 5C). During catagen to telogen transition, the stem cells remain in a nondividing, quiescent state. At telogen to anagen transition, some stem cells depart the bulge region; they do so without a self-renewing division and subsequently proliferate outside the niche, and begin to differentiate. In anagen, the remaining stem cells replenish their pool by division (self-renew) in the bulge. At the end of anagen, the newly generated stem cells stop dividing, probably because of overcrowding of the niche and wait for the activation of the next hair cycle. Together these results suggest that hair follicle stem cells divide symmetrically and their fate is spatially and temporally segregated in the tissue (Tumbar, 2012).
Owing to the synchrony of the first adult hair cycle and the hair cycle-dependent stem cell behavior, hair follicle stem cells represent an ideal model system for fate choice mechanisms during homeostasis. Next, we summarize cell-extrinsic and -intrinsic mechanisms of spatiotemporal stem cell regulation. In telogen, Wnt antagonists in bulge and inhibitory BmpTGF β ligands derived from dermis collectively keep hair follicle stem cells in a quiescent state (Plikus et al., 2011, 2008). In addition, the Keratin6+ bulge inner layer underneath the stem cells maintains bulge quiescence by providing Fgf18 and Bmp6 (Hsu et al., 2011). Loss of these inhibitors from the macroenvironment activates canonical Wnt pathway in bulge and dermal papillae, which is critical for anagen entry. Fgf or other signaling pathways are likely induced by Wnt-activated dermal papillae, which might stimulate hair follicle stem cells as short-range signaling cues to induce anagen initiation. Recent study defined an essential role for intradermal adipocytes in the regulation of cyclical hair follicle stem cell behavior (Festa et al., 2011). In parallel with the hair cycle, proliferation of adipocyte lineage cells is stimulated during catagen to increase immature adipocyte precursors during telogen and anagen initiation. Immature adipocytes express platelet-derived growth factor (PDGF) ligands, which drive hair follicle stem cell activation. Thus, in hair follicle, the microenvironment is changing during hair cycle and this might contribute to determining the timing of acquiring any one of the two possible fates—self-renewal or differentiation. Given that even in the same hair cycle stage, not all bulge cells undergo same fate, the probability of the fate choices is likely attributed to the location of bulge cells within a gradient of signaling molecules. More recently, Janich et al. (2011) revealed a cell-intrinsic mechanism to explain the heterogeneous signal-responsive states of hair follicle stem cells. They analyzed the activity of circadian molecular clock by using a core clock gene Per1 reporter mice. Telogen/early anagen bulge cells contained two populations with opposite phases of the clock, while full anagen bulge cells or epidermal basal cells did not. The clock genes directly bound the promoter of stem cell-signature genes, especially genes related to the responsiveness to the surrounding signals. These results indicate that internal oscillatory gene expressions may create subpopulations of bulge cells with different competency to activation and dormancy stimuli.
3.2. Murine Spermatogonial Stem Cells
3.2.1. Basic Properties of Spermatogonial Stem Cells in Mice
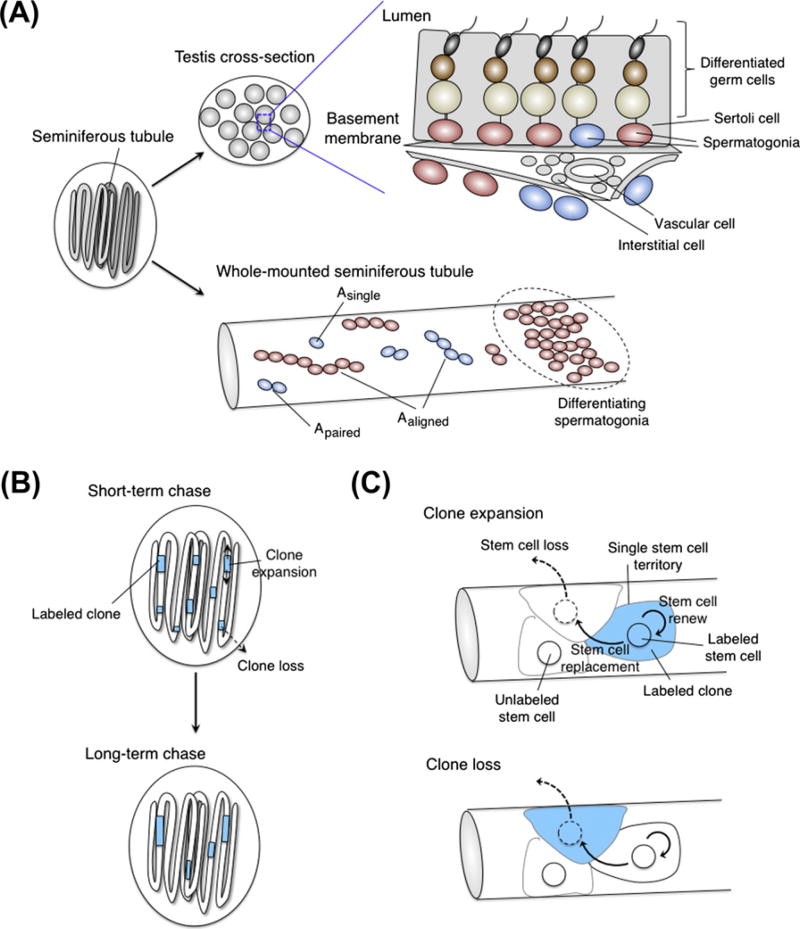
In the mouse testes, the entire developmental process from immature spermatogonia to mature spermatozoa occurs within seminiferous tubules of testes (de Rooij and Russell, 2000; Oatley and Brinster, 2008; Russell et al., 1990; Yoshida, 2010) (Fig. 6A). Within the tubules, spermatogonial stem cells are intermingled with nonstem spermatogonia as a monolayer on the basement membrane. The process of differentiation occurs periodically in accordance with the seminiferous epithelial cycle of 8.6 days: spermatogonia lose contact with the basement membrane, enter into meiosis and move toward luminal side until the matured spermatozoa are released into the lumen 35 days later (Russell et al., 1990). It has long been speculated that Sertoli cells form stem cell niches in the mouse testes as they are somatic cells that line up the inner part of the seminiferous tubules and physically interact with germ cells including spermatogonia. Sertoli cells secrete growth factors including glial cell-line derived neurotrophic factor (GDNF), which acts as one of the major niche signals for spermatogonial stem cells (Meng et al., 2000; Naughton et al., 2006). Recently, observations using time-lapse imaging and three-dimensional reconstitution of the seminiferous tubule suggest a different component of the niche in the blood vessels (Yoshida et al., 2007b). Specifically, the most primitive spermatogonia are localized in an area adjacent to the blood vessels and interstitium that surround the seminiferous tubules, and they migrate out of this region upon differentiation.
Figure 6.
Murine spermatogonial stem cells. (A) A testis is composed of long, coiled tubes called seminiferous tubules. Spermatogonia are located on the basement membrane of seminiferous tubules. As germ cells mature, they progressively locate toward the lumen of the tubules. Sertoli cells enclose germ cells within tubules, while vascular and surrounding interstitial cells are located outside of the tubules. Spermatogonia are interconnected by intercellular bridges and classified by the number of cell(s) in the same cluster. (B) Clonal analysis in the mouse testis. The number of labeled clones per testis decreases with time, while the average clone length increases. (C) Interpretation of clone expansion or loss. The clone expansion is caused by a loss of unlabeled stem cell and a subsequent replacement by labeled stem cell (upper), whereas the clone loss occurs by the opposite labeling pattern of stem cells (lower).
Similar to Drosophila germ cells, mouse spermatogonia are connected by intercellular cytoplasmic bridges after mitosis and make chains of 2n cell cysts (de Rooij, 1998; de Rooij, 2001; de Rooij and Grootegoed, 1998; de Rooij and Russell, 2000; Russell et al., 1990) (Fig. 6A). The spermatogonial types Asingle (isolated single cells), Apaired (interconnected 2 cells), and Aaligned (interconnected 4, 8, 16 or 32 cells) are collectively described as undifferentiated spermatogonia. The undifferentiated spermatogonia differentiate into differentiating spermatogonia, which include A1, A2, A3, A4, Intermediate, and B spermatogonia. According to a classical “Asingle model”, Asingle spermatogonia represent stem cells: this type is recognized as the most primitive cells and exists as single cells without any intercellular connection with others, whereas Apaired and Aaligned spermatogonia are committed to differentiation (de Rooij and Russell, 2000; Huckins, 1971; Oakberg, 1971; Russell et al., 1990). Over the past few years, it has been discovered that the spermatogonial populations are characterized by heterogeneous gene expression in addition to distinct morphological classification (Nakagawa et al., 2010; Suzuki et al., 2009; Tokuda et al., 2007; Zheng et al., 2009). For example, promyelocytic leukemia zinc finger (Plzf) and E-cadherin are expressed in the entire undifferentiated spermatogonia; Nanos2 and GFR α 1 (glial cell line-derived neurotrophic factor family receptor alpha 1), a receptor of GDNF, are preferentially expressed in Asingle and Apaired; Nanos3 and Neurogenin3 (Ngn3) are expressed in a large subset ofAaligned spermatogonia: Kit marks the entire differentiating spermatogonia. All spermatogonial cells seem to be actively cycling since no LRCs are detected beyond 60 days (Grisanti et al., 2009). Studies using transplantation of isolated spermatogonia are based on cell-surface markers, which have demonstrated that spermatogonial stem cells are highly enriched in the undifferentiated spermatogonia containing Asingle to Aaligned (Oatley and Brinster, 2006, 2008). A conditional knockout of Nanos2 gene in adult testes resulted in the complete loss of spermatogenic germ cells (Sada et al., 2009), indicating that Nanos2+ cells might contain stem cell population. Lineage-tracing experiments directly showed that Nanos2+ cells could generate long-term spermatogenic clones in vivo (Sada et al., 2009). Interestingly, in Ngn3-lineage, labeled spermatogonia could also form long-lived clones with all types of spermatogenic germ cells (Nakagawa et al., 2007), indicating that both Nanos2+GFR α 1+ and Ngn3+Nanos3+ population may fit the criteria of stem cells. However, the average number of persistent clones in the Nanos2-lineage was about 10-fold higher than that of the Ngn3-lineage although the number of pulse-labeled spermatogonia was similar in both cases (Nakagawa et al., 2007; Sada et al., 2009). Furthermore, gene expression correlates with stem cell fate for short term after labeling, with Ngn3-expressing cells tend to differentiate, whereas Nanos2− or GFR α 1-expressing cells are more likely to self-renew (Nakagawa et al., 2007, 2010; Sada et al., 2009). Thus, undifferentiated spermatogonia contain two heterogeneous populations of stem cells with respect to their distinct cellular morphology, gene expressions and their short-term fate preference of self-renewal versus differentiation.
3.2.2. Dynamic Behavior of Spermatogonial Stem Cells during Regeneration
In murine spermatogonial stem cells, it is proposed that in addition to the spermatogonial population that actually acts as the stem cells (actual stem cells), a second set of undifferentiated spermatogonia also exists that possesses the potential to self-renew, but act as theTAcells in the normal situation (potential stem cells) (Nakagawa et al., 2007; Yoshida et al., 2007a). The potential stem cells seem to change their mode to self-renewal upon the loss of actual stem cells. Molecularly, Ngn3+ spermatogonia are capable of switching their own state to a GFR α 1+Nanos2+ state and of contributing to the self-renewing stem cells through cyst fragmentation and a change in gene expression as in the case of Drosophila (Nakagawa et al., 2007, 2010). Although the results are still under debate, this reversibility of fate was also observed in differentiating spermatogonia (Kit+/α6-integrin+ with side population phenotype), which have been generally considered to be irreversibly committed cells (Barroca et al., 2009; Yoshida, 2009). Importantly, the reversible behavior of Ngn3+ spermatogonia occurred not only in the damaged tissue but also in the normal homeostatic condition (Nakagawa et al., 2010), which might be critical for the stem cell maintenance as a population. It is unclear whether GFR α 1+Nanos2+ undergoes symmetric division or cyst fragmentation during regeneration and/or normal spermatogenesis.
3.2.3. Stochastic Fate Choice of Spermatogonial Stem Cells
As described above for the epidermis and the hair follicle, murine spermatogonia might also behave as a population rather than by a strict mode of asymmetric fate decisions. In murine testes, Klein et al. (2010) analyzed the property of long-lived clones by long-term clonal labeling of Ngn3+ spermatogonia (Fig. 6B). If the stem cells self-renew through invariant asymmetric division, the number and size of labeled clones should be constant. However, the number of long-lived clones decreases with time, whereas the size of the remaining clones expands continually. Intriguingly, labeling of Nanos2+ spermatogonia provides clone-fate dynamics consistent with that seen in Ngn3-based labeling experiments (Klein et al., 2010; Sada et al., 2009). Spermatogonial stem cells were continuously lost and subsequently replaced by their neighboring cells, on average within 2 weeks. Therefore, the concept of individual stem cells as immortal, long-lived, constantly generating both stem cells and committed daughters through asymmetric division does not fit the observed phenomenon. Rather, entire stem cell populations would actively turn over throughout life by stochastic fate choice (Fig. 6C).
One of the remaining questions is how the stem cells in the same or nearby niche quickly respond to the loss of stem cells and carry out subsequent repopulation of vacant niches. A possibility is that once a stem cell is lost, the neighboring stem cells will be triggered to divide symmetrically by receiving signal cues from the vacant niche. Alternatively, Klein et al. (2010) proposed that the stem cell loss and replacement can be activated by a stochastic and random migration of stem cells. Live imaging showed that Ngn3+ spermatogonia migrate out of the vasculature-proximal region across the seminiferous tubules, and into another vasculature-related region. This phenomenon may be due to the stem cells finding a vacant niche and switching their mode to self-renewal, probably by reverting to a GFR α 1+Nanos2+ state after migrating into an adjacent region that lacks stem cells. The authors claim that such rapid and ubiquitous migration of spermatogonia over the seminiferous tubule may provide the cellular basis for the stem cell replacement.
3.3. Intestinal Stem Cells
3.3.1. Frequently and Infrequently Dividing Cells in Intestine
The intestinal epithelium is one of the most rapidly self-renewing tissues in adult mammals (Barker et al., 2008). In the murine small intestine, stem cells and their short-lived TA cells reside in crypts (Fig. 7A). The stem cells produce a population of TA cells, which rapidly expand through multiple rounds of cell division as they migrate upward toward the crypt/villus border. TA cells exiting the crypts and entering the villi terminally differentiate into enterocytes, goblet cells, or enteroendocrine cells. These differentiated cells continue to move up the villus flanks to die upon reaching the villus tip. Paneth cells escape this course by migrating downward to crypt bottoms. Although epithelial crypts are in intimate contact with mesenchymal cells, recent in vitro data suggest that these cells might not be the specialized cellular niches (Sato et al., 2009). Rather, the stem cell progeny Paneth cells might be key for controlling stem cell fate decision (Sato et al., 2011) although recent study may contradict this idea (Kim et al., 2012).
Figure 7.
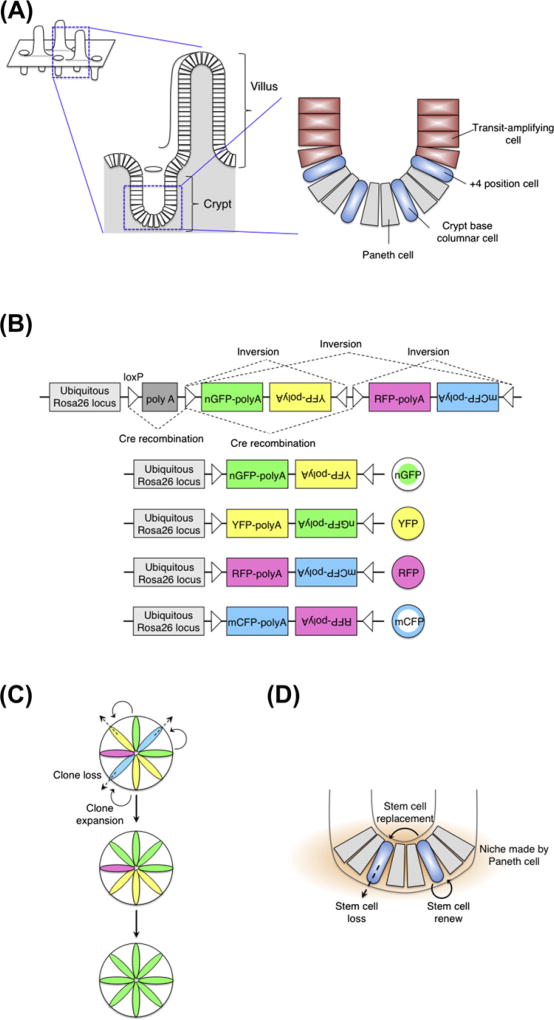
Intestinal stem cells. (A) The anatomy of the small intestinal epithelium. (B) Confetti reporter construct. Cre triggers both inversion and recombination in a random manner, which results in the four patterns of gene expression (nuclear GFP, cytoplasmic YFP and RFP, membrane-associated CFP). Cre-mediated inversion occurs at a sequence flanked by loxP sites in opposite orientation. In 50% of cells, inversion should lead to an antisense orientation and switch gene expression. (C) Multicolor lineage tracing shows a progressive monoclonality of the crypt. (D) Monoclonal conversion arises from turnover of an equipotent stem cell population. Paneth cells make a specialized microenvironment for intestinal stem cells.
By using three-dimensional images of whole-mount intestine, about half of the cells near the base of crypts have been shown to orient their spindles perpendicular to the apical surface of the epithelium, whereas TA cells oriented more parallel to it (Quyn et al., 2010). This orientation was proposed to correlate with the asymmetric segregation of chromosomes as predicted by the immortal strand hypothesis (Potten et al., 2002; Quyn et al., 2010), however, recent study opposed this interpretation as well (Schepers et al., 2011).
Since the late 1950s, a model has placed the intestinal stem cells at position +4 relative to the crypt bottom, with the first three positions being occupied by the terminally differentiated Paneth cells (Barker et al., 2008) (Fig. 7A). Potten et al. (1974) have reported the existence of LRCs residing specifically at this position. A study using in vivo lineage tracing has shown that cells expressing Bmi1 may predominantly mark the +4 position and are able to give rise to all four epithelial lineages (Sangiorgi and Capecchi, 2008). Moreover, selective killing of this population results in degeneration of the crypts underscoring their importance to intestinal homeostasis. Other markers, mouse telomerase reverse transcriptase (mTert) (Montgomery et al., 2010) and Hopx (Takeda et al., 2011), are also predominantly expressed at the +4 position, where they mark a slow-cycling, multipotent intestinal stem cell population. In addition, cryptbased columnar cells that are more rapidly dividing located at crypt bottoms among Paneth cells also seem to represent intestinal stem cells. The cell cycle time of these cells estimated at 1 day, implies that they go through 700–1000 divisions in the lifetime. The crypt-based columnar cells are marked by Lgr5 (Barker et al., 2007), Sox9 (Furuyama et al., 2011) and Prominin-1 (Zhu et al., 2009) expressions. Lineage tracing by using above markers demonstrated that these cells are or contain a long-lived multipotent stem cell population. In culture, single Lgr5+ cells can generate epithelial organoids with crypt–villus structures (Sato et al., 2009). The high rate of proliferation of Lgr5+ cells was a surprising characteristic, given most mammalian stem cell populations are thought to be slowly cycling. A most recent paper indicates a possibility that stem cell marker expression, including Lgr5, Bmi1 and mTert, might be overlapping each other, as shown by a highly sensitive in situ hybridization (Itzkovitz et al., 2012). Hence, we need to be careful to interpret the results obtained by using stem cell markers above.
It has recently been shown that +4 position cells marked by Bmi1+, mTert+, Hopx+ can give rise to Lgr5+ cells (Montgomery et al., 2010; Takeda et al., 2011; Tian et al., 2011). When Lgr5+ cells are selectively eliminated in vivo, progeny production by Bmi1+ cells increased and compensated for the loss of Lgr5+ cells, leaving the crypts relatively unaffected (Tian et al., 2011). Intriguingly, Lgr5+ cells could contribute to the +4 position cell population as well (Takeda et al., 2011), but they cannot rescue the loss of Bmi1+ cells. Hence, the intestine contains a relatively more quiescent stem cell population at the +4 position, which is indispensable for tissue homeostasis, and a cycling, more dispensable Lgr5+ stem cell population among the Paneth cells. These two stem cell populations can interconvert into each other and work together to maintain the intestine during homeostasis and regeneration.
3.3.2. Monoclonal Conversion of Intestinal Stem Cells Explained by a Neutral Drift Model
After genetic marking of intestinal epithelial cells, crypts drift toward monoclonality with time, a phenomenon known as a monoclonal conversion (Winton and Ponder, 1990). This phenomenon rules out the idea that multiple stem cells maintain each crypt by a strict asymmetric division. Rather, this can be explained by a model, in which each crypt is supported by only a single long-lived stem cell and its shorter-lived progeny. An alternative possibility is that crypts may contain multiple stem cells that do not employ strict asymmetric division (Potten and Loeffler, 1990).
Two recent studies answered this classical question (Jones, 2010; Lopez-Garcia et al., 2010; Snippert et al., 2010). The authors genetically induced an intestinal clone by using either a single- (Lopez-Garcia et al., 2010) or a multicolor “confetti” reporter mouse (Livet et al., 2007; Snippert et al., 2010) (Fig. 7B) and analyzed their behavior in long term. Consistent with the monoclonal conversion observation, the intestinal clones expand and contract until, in some cases, either they take over the crypt or they are lost (Fig. 7C). This clonal behavior fits the model that intestinal stem cells form an equipotent population in which the loss of a stem cell is compensated by the multiplication of a neighbor. In time, there is a certain probability that the entire crypt might converge toward progeny deriving from a single stem cell by chance. Furthermore, Snippert et al. (2010) analyzed the behavior of clones derived from single Lgr5+ stem cells, using the Lgr5–EGFP–Ires–CreERT2 allele in conjunction with the confetti reporter. The stem cell replacement rate was comparable to the division rate, indicating that the loss of an Lgr5+ cell from crypt by differentiation is indeed replaced by the division of an adjacent Lgr5+ cell (Fig. 7D). Taken together, the monoclonal conversion arises from stochastic turnover of an equipotent intestinal stem cell population. Given that Paneth cells create an essential niche for Lgr5+ cells (Sato et al., 2009), Lgr5+ cells might compete for a limited surface of contact with Paneth cells. Unlike in the cases of Drosophila germ line or wing disc, competition is “neutral,” in which all stem cells are essentially equal. The probability of a stem cell to become a “winner” or a “loser” is determined by chance. After symmetrical division, stem cells compete each other to occupy one cell space within Paneth cell niche. Or if one stem cell loses by competition and departs from the niche, another stem cell divides to occupy the vacant niche space. In this way, a limited niche space created by Paneth cells might decide the short-term fate of equipotent stem cells and fix the total stem cell population size. Interestingly, Drosophila midgut stem cells also follow the neutral competition and utilize Delta/Notch-mediated lateral inhibition to achieve this process (de Navascues et al., 2012). It will be important to ask if this molecular mechanism is conserved in mammals.
3.4. Hematopoietic Stem Cells
3.4.1. Basic Properties of Hematopoietic Stem Cells and their Niches
The existence of hematopoietic stem cells within the bone marrow was demonstrated nearly 50 years ago by reconstitution of the hematopoietic system following irradiation. Hematopoietic stem cells are multipotent stem cells giving rise to differentiated blood cell types, including the cells of the T, B and myeloid lineages (Bryder et al., 2006; Orkin and Zon, 2008;Weissman et al., 2001). The cellular constituents forming the stemcell niche in the bone marrow have long been studied and implicated osteoblasts, endothelial and perivascular cells (Bianco et al., 2011). Osteoblasts influence stem cell numbers in the bone marrow. Hematopoietic stem cells also locate preferentially in perivascular regions. Real-time imaging traced the homing of purified hematopoietic stem cells after transplantation and showed that these cells preferentially colonize the endosteal zone, an inner bone surface, where both osteoblastic cells and vascular cells reside (Lo Celso et al., 2009; Xie et al., 2009). More recently, Nestin+ mesenchymal stem cells have been proposed to constitute an essential niche component of hematopoietic stem cells (Mendez-Ferrer et al., 2010). These cells show a close physical association with hematopoietic stem cells, very high expression levels of core stem cell maintenance genes and significant reductions in hematopoietic stem cells upon their deletion.
The hypothesis that hematopoietic stem cells cycle relatively slowly has also been shown by the BrdU label retention experiments. Mathematical modeling of H2B-GFP dilution in hematopoietic stem cells, identified with a stringent marker combination (Lineage markers SCA1+KIT+ [LSK] CD150+CD48−), revealed the heterogeneity in their proliferation rates: a larger population cycling faster and a smaller population cycling more slowly (Foudi et al., 2009; Raaijmakers and Scadden, 2008; Wilson et al., 2008). Hematopoietic stem cells with the highest H2B-GFP content were capable of long-term multilineage reconstitution but it was unlikely that they would participate in homeostasis. In contrast, the stem cells with low H2B-GFP may contribute more to the maintenance of hematopoietic system although their stem cell activity was low in long-term transplantations. Upon hematopoietic stress, the slow-cycling population can transit to an active state.
3.4.2. Symmetric and Asymmetric Division in Hematopoietic Stem/Progenitor Cells
Early work with single-cell transplantation assays clearly demonstrated the ability of hematopoietic stem cells to divide symmetrically in vivo. In lethally irradiated mice, single hematopoietic stem cells were capable of expansion and repopulation of the entire hematopoietic system, and continued to expand in serial transplants (Purton and Scadden, 2007; Weissman and Shizuru, 2008). However, even though it is clear that hematopoietic stem cells can expand symmetrically when found in challenging conditions induced by grafting and irradiation, it remains unclear what would their true behavior be in normal homeostasis. Evidence of asymmetric division in the murine hematopoietic system was showed by the fates of paired daughter cells generated from a single hematopoietic stem/progenitor cell (Ho, 2005; Ho and Wagner, 2007). The differentiation pattern of their progeny was analyzed by colony formation assays. This strategy revealed that about 20% of hematopoietic stem/progenitor cells divided asymmetrically, giving rise to progeny with different cell-cycle kinetics or different differentiation pathways. The disparate differentiation of paired hematopoietic progenitor cells was later confirmed by several live imaging studies. Wu et al. (2007) further developed a system to visualize the immature state of cultured hematopoietic precursors in mice. By using Notch-signaling GFP reporter in time-lapse imaging of single cells, the authors revealed three modes of cell division: (1) asymmetric divisions (one GFP+ immature cell and one GFP− committed daughter); (2) symmetric renewal (two GFP+ cells); or (3) symmetric commitment (two GFP− cells) occurred in hematopoietic precursors. These studies also showed that the cell-fate determinant Numb was asymmetrically segregated into the committed daughter. Interestingly, the cells cultured on prodifferentiation stroma primarily underwent asymmetric divisions, whereas those on prorenewal stroma primarily divided by symmetric renewal. These data indicate that control of divisional symmetry may be a key mechanism that can be altered to regulate the ultimate outcome of stem cell renewal and commitment.
While cell culture and transplantation assays are valuable tools to examine stem cell regulation, an important question of the future is how do hematopoietic stem cells truly behave in vivo, in the absence of any stress and injury induced by transplantation? Implementation of lineage-tracing mouse genetics tools awaits further development in the hematopoietic system, where the lack of defined structure in the bone marrow and the circulatory nature of differentiated cells in the peripheral blood introduce difficult technical challenges for statistical interpretation.
3.5. Murine Neural Stem/Progenitor Cells
3.5.1. Cellular Mechanisms of Symmetric and Asymmetric Divisions in Embryonic Neural Progenitors
During the development of the mammalian central nervous system, neural stem/progenitor cells generate neurons through a combination of asymmetric and symmetric divisions (Farkas and Huttner, 2008; Gotz and Huttner, 2005; Huttner and Kosodo, 2005; Willardsen and Link, 2011). All neurons of the mammalian central nervous system derive from a neuroepithelium. Before neurogenesis, the primary neural progenitors called neuroepithelial cells, which directly or indirectly generate all other neural progenitors and all neurons, expand via symmetric divisions. With the onset of neurogenesis, neuroepithelial cells divide asymmetrically to give rise to distinct types of secondary neural progenitor cells (radial glial cells, basal progenitors) and neurons. The secondary neural progenitors also undergo both symmetric and asymmetric divisions to renew their own population and to generate neurons. Unlike stem cells, neural progenitor cells eventually undergo symmetric differentiation into neurons, thus depleting the pool of proliferative cells. Concomitant with the production of neurons, the neuroepithelium changes into a multilayered structure, consisting of the apical-most ventricular zone, the adjacent subventricular zone and the basalmost cortical plate. Mammalian neural progenitors use many of common molecular players utilized in the invertebrate systems during asymmetric cell division (Doe, 2008; Knoblich, 2008; Siller and Doe, 2009; Zhong and Chia, 2008) in addition to the mechanisms introduced below, which were discovered in mammals.
During mitosis, the cell bodies of neuroepithelial cells and radial glial cells localize to the apical surface and their basal processes extend up to the basal lamina. In the rare event, when the cleavage plane is perpendicular to the apical–basal axis, the apical cell compartment will be inherited by one daughter cell and the basal compartment by the other, which would result in an asymmetric production of a progenitor cell and a differentiated neuron. Interestingly, the cleavage planes parallel to this axis (planar division) leads not only to symmetric division but also to asymmetric division (Konno et al., 2008; Kosodo et al., 2004). This is achieved because the apical plasma membrane of progenitors represents only a minute fraction of the entire plasma membrane. It allows the parallel cleavage planes to either bisect or bypass the apical plasma membrane. As a result, cells that inherit both apical and basal components will self-renew as progenitors (the “bisecting” case); cells containing only the apical plasma membrane remain as progenitors, whereas cells containing only the basal process become neurons (the “bypassing” case). This novel concept is important to understand how stem cell daughters can select different fates even if their cleavage plane is NOT oriented perpendicular to the niche structure (e.g. basement membrane).
Another important concept proposed in the mammalian neural system, known as the cell cycle length hypothesis, is that the cell cycle length is sufficient to direct daughter cell fates (Calegari and Huttner, 2003; Hodge et al., 2004; Pilaz et al., 2009). For example, if the cell cycle is short, the cell-fate determinants cannot reach the threshold required to induce differentiation. Even if one daughter cell has more determinants compared with the other daughter cell, both of them will adopt a symmetric, self-renewal fate. If the cell cycle is sufficiently long, then the cell-fate determinants can induce differentiation in one daughter cell that inherited more but not in the other daughter, hence the fates will be asymmetric. If the cell cycle is even longer, the cell-fate determinants have enough time to induce differentiation in both daughter cells, and both will adopt a symmetric differentiation. Consistent with the phenomenon that the G1 phase of cell cycle in neuroepithelial and radial glial cells became longer and longer during development, they switched their mode of cell division from symmetric self-renewal to asymmetric self-renewal/ differentiation and eventually to symmetric differentiation (Calegari et al., 2005; Takahashi et al., 1995).
Another interesting aspect of neural cell fate choice of neural progenitors is nuclear migration during cell cycle, called interkinetic nuclear migration (Baye and Link, 2008; Takahashi et al., 1993; Taverna and Huttner, 2010). The nuclei of neural progenitors migrate up and down during interphase and migrate back to the apical side and undergo mitosis. When progenitor cell nuclei migrate more basally, the neurogenic division in the next cell cycle is accelerated (Baye and Link, 2007; Del Bene et al., 2008; Murciano et al., 2002; Schenk et al., 2009). This relationship between nuclear position and neurogenesis was found to depend on apical–basal polarity within progenitor cells, so that nuclei that migrate to different apicobasal positions respond differentially to influence the subsequent cell division manner. Furthermore, computational analysis of neuroepithelial cell behavior in vitro revealed that the nuclear movement is one of the essential parameters to predict the mode of cell division (Cohen et al., 2010). Together, the cell fates of embryonic neural progenitors are regulated by at least three key cellular mechanisms: (1) asymmetric inheritance of apicobasal cell components; (2) cell cycle length; and (3) nuclear migration. These studies support valuable concepts for the cell biological basis of asymmetric division in the mammalian stem cell systems.
3.5.2. Symmetric and Asymmetric Fate Choice in Adult Neural Stem Cells
A rapid progress in studies of adult mammalian neural stem cells has been made since the first discovery of adult neurogenesis in 1965 (Altman and Das, 1965). In early studies, the characterization of adult neural stem cells was accomplished using tritiated thymidine and BrdU pulse-chase experiments, which detect both the proliferating and de novo generating neural cells (Dhaliwal and Lagace, 2011). In vitro culture of neural cells accelerated the study of neural stem cell cellular properties, including self-renewal and multipotency. The neural stem cells expand as “neurospheres,” free-floating, spherical clusters of cells in culture (Ahmed, 2009). By using neurosphere assay, we can distinguish neural stem cells from lineage-restricted progenitors by their ability of serial neurosphere formation, and their multilineage neural differentiation in vitro and in vivo after transplantation. For the past decade, genetic mouse models, including reporter and inducible Cre lines, have been established and widely used in adult neural tissues (Dhaliwal and Lagace, 2011).
Adult neurogenesis occurs mainly in two specific brain regions, the subgranular zone in the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles (Ma et al., 2009; Ming and Song, 2011; Wang et al., 2011; Zhao et al., 2008). In the subgranular zone, radial (type I cells) and nonradial (type II cells) glia-like cells give rise to intermediate progenitors, which in turn generate neuroblasts. Immature neurons migrate into the inner granular cell layer and differentiate into dentate granular cells in the hippocampus. Genetic fate-mapping studies have showed that the radial glia-like cells were largely quiescent, and acted as the primary neural stem cells in vivo. In contrast, the nonradial cells were active neural stem cells that also give rise to new neurons and glia in the adult subgranular zone. In the subventricular zone, the radial glia-like cells (type B cells) are considered to be a neural stem cell population, which includes subpopulation of slow-cycling type B1 and more proliferative-type B2 cells. The radial glia-like cells give rise to transient amplifying cells (type C cells), which in turn generate neuroblasts (type A cells). New immature neurons are generated and then migrate through the rostral migratory stream to the olfactory bulb to become interneurons. The neural stem cells in adult brain are closely associated with astrocytes, vascular cells, ependyma cells, and mature neurons, which serve as signal source and physical support for neural stem cells (Riquelme et al., 2008). Thus, two populations of neural stem cells likely coexist in adult brain; however, their lineage relationship, differences in their stem cell activity and the signal competency are to be further determined in the future.
Neural stem cells are another example that contradicts a strict asymmetric stem cell division model. In the subgranular zone, Bonaguidi et al. (2011) monitored fate choices of Nestin+ radial glia-like cells immediately after cell division. They found that Nestin+ cells undergo multiple modes of fate choices: asymmetric cell divisions that gave rise to (1) one Nestin+ cell and one intermediate neural progenitor (2) one Nestin+ cell and one astroglia (3) one Nestin+ cell and one or more Sox2+ nonradial precursor(s) (Lugert et al., 2010); or symmetric cell division generating two Nestin+ cells, which have not been previously reported in the adult brain. Thus, the total neural stem cell pool might be maintained as a population, as seen in other mammalian stem cells. An alternative model termed “disposable stem cell model” has been proposed by Encinas et al. (Encinas et al., 2010; Lugert and Taylor, 2011). They indicate that once radial glia-like cells are activated, these cells abandoned their capacity to act as stem cells and are lost via terminal differentiation into astrocytes. These two independent studies were not compatible (Kempermann, 2011) and further analysis will be required to distinguish between these two possibilities.
3.6. Muscle Stem Cells: A Model System of Stem Cells to Regenerate Tissues upon Injury
The adult skeletal muscle has long been considered an ideal model to study how stem cells act upon injury. The muscle stem cells called satellite cells are quiescent mononucleated cells, and lie on the surface of the myofibers between the plasma membrane and the overlying basal lamina (Buckingham and Montarras, 2008; Kuang et al., 2008; Relaix and Marcelle, 2009). Upon damage, satellite cells become activated, proliferate, and give rise to a population of myogenic precursor cells, called myoblasts. The myoblasts undergo multiple rounds of division before terminal differentiation and fusion to form multinucleated myofibers. A combination of signals from the myofiber, circulation system, and extracellular matrix govern the quiescence, activation, and proliferation of satellite cells.
There are two possible mechanisms of satellite-cell self-renewal/differentiation. In a first model, Pax7+ quiescent satellite cells are activated synchronously upon muscle damage and coexpress both Pax7 and MyoD (Halevy et al., 2004; Olguin and Olwin, 2004; Zammit et al., 2004). This transitory proliferating Pax7+MyoD+ population adopts divergent fates: most of these Pax7+MyoD+ cells then downregulate Pax7, upregulate Myogenin, and enter the differentiation program (Pax7−MyoD+Myogenin+), whereas a few return to the Pax7+MyoD− state to renew the quiescent satellite-cell pool. Thus, Pax7+ quiescent satellite cells are essentially homogenous and activated Pax7+MyoD+ satellite cells can either enter terminal differentiation or regain characteristics of quiescence.
In an alternative model, it is proposed that the satellite cells contain two heterogeneous populations. By Cre–loxP (Myf5-Cre/Rosa-YFP)-mediated lineage tracing, ~90% of Pax7+ satellite cells have experienced Myf5 expression (Pax7+YFP+), while ~10% of them have not (Pax7+YFP−) (Kuang et al., 2007). Pax7+YFP− satellite cells were more efficient in reconstituting the satellite cell compartment after grafting than Pax7+YFP+ satellite cells. By contrast, Pax7+YFP+ satellite cells are more likely committed progenitors as they rapidly give rise to differentiating Pax7+MyoD+ cells. Furthermore, Pax7+YFP− satellite cells can asymmetrically generate a self-renewing (Pax7+YFP−) and a committed (Pax7+YFP+) daughter cell in vivo.
The asymmetric inheritance of template DNA strands has also been observed in the muscle system, and was recently shown to occur specifically in Pax7-GFPhigh subpopulation, which indicated their stem cell identity (Conboy et al., 2007; Rocheteau et al., 2012; Shinin et al., 2006). Thus, these results suggest that the heterogeneous satellite-cell populations have hierarchical lineage relationships with distinct potential and cell fates. Future questions will be whether muscle stem cells employ strict asymmetric cell division as seen in some invertebrate systems or they also undergo symmetric division. Given that many mammalian tissues have two distinct stem cell-populations in the same tissue, does this also apply to the muscle system to ensure quick response to the tissue damage?
4. CANCER CELL DYNAMICS WITH THE STOCHASTIC CELL-STATE TRANSITION MODEL
Cancer stem-like cells are characterized by increased tumor-initiating ability and resistance to chemotherapeutic drugs (Reya et al., 2001; Vermeulen et al., 2008). It has been thought that cancer stem-like cells are at the top of lineage-hierarchy, giving rise to noncancer stem cells. However, a recent study by Gupta et al. (2011) revealed that human breast cancer cells showed an interconversion between cancer stem and noncancer stem states. They found that isolated subpopulation of breast cancer cells (nonstem luminal, nonstem basal, and stem-like cells) could self-renew or transit into other states in vitro. Cell populations eventually returned to a fixed equilibrium of cell-state proportions regardless of the starting state. These observations can be explained by a Markov model, in which cell transition stochastically occurred between states. They further confirmed this model by in vivo transplantation assay, which showed that the luminal and basal fractions are capable of efficiently seeding tumors and regenerating functional stem-like cells. The proportions of luminal, basal, stem-like cells in tumors were comparable irrespective of the sorted subpopulation used to seed the tumor. Collectively, these observations strongly suggest that cancer stem cells are dependent on the population dynamics rather than traditional, hierarchal model of stem cell behavior.
5. SELF-RENEWAL AND DIFFERENTIATION OF STEM CELLS IN CULTURE
5.1. Stem Cell Behavior in a Homogeneous Environment
Work in Drosophila germ line suggested that a stem cell could only self-renew within a niche environment that promotes self-renewal and prevents differentiation, whereas differentiation occurs only outside the niche. However, cultured stem cells are exposed to a homogeneous environment with growth factors that maintain the self-renewing ability of stem cells. Two reports showed that rat spermatogonial stem cells (Wu et al., 2009) and mouse intestinal stem cells (Sato et al., 2009) give rise to two distinct types of progeny: new stem cells and new differentiating cells, even in a uniform growth-promoting environment. These results indicate a capacity of cell-autonomous fate determination of mammalian stem cells. By quantitative experimental measurements and mathematical modeling, Wu et al. (2009) further suggested that self-renewal and differentiation might be stochastic events occurring with a consistent probability.
If mammalian stem cells are able to specify self-renewal and differentiation cell autonomously and independently of differential extrinsic stimuli, what is then the role of the niche? Although the above results argue against a deterministic role of extrinsic cues in stem cell fate specification, it is likely that growth factors derived from the niche may bias the probability of cell-fate choices entirely toward self-renewal. In addition, we cannot rule out a possibility that fate choice is dependent on heterogeneity associated with the feeder cells, the extracellular matrix or how crowded cells are within a certain microenvironment in the dish. In intestinal cell culture, stem cell daughter, Paneth cells might provide a specialized niche for stem cells: the intestinal organoid formation is greatly enhanced in the presence of Paneth cells, one of the main sources of a Wnt ligand (Sato et al., 2011); and only cells in crypts display hallmarks of nuclear β-catenin and the expression of Wnt-target genes (Sato et al., 2009).
5.2. Intrinsic Variation in Gene Expression
As we have discussed for the hair follicle stem cells, one possible mechanism for the stochastic fate choice is the intrinsic variation in gene expression in stem cells, which influence the competency to extrinsic signals. In hematopoietic system, the expression levels of stem cell surface marker, SCA1, is highly variable among individual cells within a clonal population of multipotent progenitor cells (Chang et al., 2008). SCA1 levels correlate with different cell-fate decisions, indicating that noise in gene expression can lead to a stochastic cell-fate response.
Fluctuating expression of genes also causes heterogeneity of differentiation competency in mouse embryonic stem (ES) cells. For example, Hes1 expression in ES cells oscillates with a periodicity of 3–5 h (Kobayashi et al., 2009). Hes1 is known as a biological clock in several mammalian cell types (Kobayashi and Kageyama, 2010). This oscillatory expression is regulated by an autonegative feedback loop: Hes1 protein represses its own transcription, but Hes1 mRNA and protein are unstable and are rapidly degraded, which allows the system to initiate the next round of expression. In ES cells, the Hes1 level affects the different cell-fate choices: when the Hes1 level was low, cells preferred to differentiate into a neuroectodermal fate; but when the Hes1 level was high, cells preferred to express early mesodermal marker genes. A similar phenomenon was also reported, when fluctuations in a key pluripotency gene, Nanog, regulated the probability of stem cell self-renewal and propagation in vitro (Kalmar et al., 2009). Nanog levels undergo slow, random fluctuations in ES cells, giving rise to heterogeneous cell populations. In the Nanog low state, cells are more likely to differentiate. Thus, the heterogeneity in mammalian stem/progenitor populations bias progenitor fates. It is considered that the intrinsic feature of stem cells may account, at least in part, for a variety of responses to signals, which come from nonspecialized microenvironment in mammalian tissues.
6. CONCLUDING REMARKS
In our present review, we describe the stem cell fates at the tissue level, the entire stem cell population level, and the individual stem cell level. In a classical model, tissue maintenance is sustained by an invariant, constant asymmetric fate choice of individual stem cells. The asymmetric division has been observed in invertebrate and some mammalian tissues; however, recent advances in imaging technology found rare, symmetric divisions of stem cells as well. Recent studies with genetic labeling and computer simulation further revealed that fates of individual stem cells are stochastic, but overall, the tissue homeostasis is sustained by the balanced acts of the entire stem cell population. Furthermore, stem cells undergo symmetric or asymmetric self-renewal, dedifferentiation or lineage conversion upon stem cell loss or tissue damage induced by chemical damage, irradiation, or gene mutation. Thus, stem cells are flexible and dynamic rather than permanently unchanged population.
Interfollicular epidermal stem cells, murine spermatogonial stem cells, and intestinal stem cells exhibit similar but distinct behaviors. Klein et al. classify the patterns of their stem cell behavior into two groups (Klein and Simons, 2011): cell-intrinsic regulation and cell-extrinsic regulation. The interfollicular epidermis applies to the former pattern, in which the regulation of stem cell fate is cell-autonomous and there is no correlation between the fates of other stem cells. Individual stem cells divide to produce two stem cells or one stem and one differentiating cell or two differentiating cells with a fixed probability. By contrast, in testes or intestine, the fate of stem cells influences the fate of other stem cells. If some stem cells are lost, neighboring stem cells compensate for the loss, probably by symmetric self-renewal division and migration to the vacant niche. What is the advantage of each strategy in different types of tissues? It will be interesting to see which strategy is used in other stem cell-dependent tissues or in their developmental stage, and how does it change in the damaged or aged tissues.
The slow-cycling nature of stem cells has long been thought as a mechanism of protecting stem cells from mutations caused by a repeated, life-long cell division. However, on the basis of the new models, the acquisition of multiple mutations in stem cells is less likely as some stem cells, such as those of the intestine, may turn over constantly. An interesting question is then what is the biological meaning of slow-cycling cells in the tissue? If all stem cells should be functionally “equivalent” to achieve population dynamics, then why do we need two or more heterogeneous populations of stem cells? A possibility is that frequently dividing stem cells might act as a major source for the normal tissue homeostasis, whereas infrequently dividing stem cells activate upon the loss of frequently dividing stem cells and function as a backup in injury or stress conditions. This idea is consistent with the increased stem cell activity of slow-cycling cells upon the tissue damage (e.g. hematopoietic, intestinal stem cells) and the fact that actively dividing cells (e.g. Lgr5+ intestinal stem cells) are highly sensitive to radiation. In the spermatogenic system, Ngn3+ cells increase their capacity to make stem cell-derived clones after chemical injury or transplantation damage (Nakagawa et al., 2007) although the evidence for differences in the cell division frequency between Ngn3+ versus Nanos2+GFR α 1+ cells is lacking so far. Hence, the existence of heterogeneous stem cell populations might account for the robustness against tissue damage.
Another interesting phenomenon is that stem cells undergo interconversion between two stem cell populations by migration/replacement (e.g. testes, intestine) or internal oscillation of gene expression (e.g. hair follicle) even in the homeostatic condition. Given that distinct stem cells (or cell state) might be controlled by different self-renewal/differentiation signals because of their location (Li and Clevers, 2010) or their different intrinsic competency (Janich et al., 2011), switch to the other stem cell state equals switch of their ultimate fate. If the lineage conversion and subsequent fate choice (to stay or to leave) happens randomly, irrespective of their previous lifetime or cell division history, there will be less chance for same stem cells to stay in the tissue in long-term. This might enable a rapid turnover of stem cells to protect them from oncogenic mutation or dysfunction. In fact, the average lifetime of individual stem cells can be a lot shorter (e.g. two weeks in spermatogonial stem cells) than we expected (Klein et al., 2010).
What cellular and molecular mechanisms orchestrate seemingly random processes of the stem cell behavior? We discussed in this review four possibilities. (1) Periodical changing of the environment decides stem cell fate in a spatiotemporal manner (e.g. hair follicle stem cells). (2) Cell-intrinsic oscillation in gene expressions changes the competency to respond to environmental signals (e.g. hair follicle stem cells, cultured hematopoietic stem/ progenitor cells and ES cells). (3) A localized, limited niche space directs stem cells fate and decides the size of stem cell pool (e.g. intestinal stem cells). (4) Random and constant migration of stem cells contributes to find vacant niche after stem cell loss (e.g. murine spermatogonial stem cells). In addition to these mechanisms outlined above, the regulation of cell division (symmetric, asymmetric or combination), cell-cycle length, or other cellular events of stem cells (e.g. cell death, dedifferentiation, lineage conversion, etc.) might also affect the balanced production of differentiated daughters and the maintenance of the stem cell pool. We will reconsider and reassemble our knowledge of stem cell biology based on the novel idea of stem cell population dynamics. This would be an important basis for better understanding of stem cell features and manipulation of these cells for medical application.
Acknowledgments
This work is supported in part by NYSTEM Grant C024354 and NIH Grant R01AR053201 to T.T; and by a Long-Term Fellowship from Human Frontier Science Program to A.S. Thanks to Y. Saga for assistance with figures.
References
- Ahmed S. The culture of neural stem cells. J. Cell. Biochem. 2009;106:1–6. doi: 10.1002/jcb.21972. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambler CA, Maatta A. Epidermal stem cells: location, potential and contribution to cancer. J. Pathol. 2009;217:206–216. doi: 10.1002/path.2468. [DOI] [PubMed] [Google Scholar]
- Bardin AJ, et al. Asymmetric localization and function of cell-fate determinants: a fly’s view. Curr. Opin. Neurobiol. 2004;14:6–14. doi: 10.1016/j.conb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, et al. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroca V, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat. Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Bianco P, et al. Osteoprogenitors and the hematopoietic microenvironment. Best Pract. Res. Clin. Haematol. 2011;24:37–47. doi: 10.1016/j.beha.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. J. Dent. Res. 1981;60(Spec No C):1611–1620. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brownell I, et al. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryder D, et al. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Montarras D. Skeletal muscle stem cells. Curr. Opin. Genet. Dev. 2008;18:330–336. doi: 10.1016/j.gde.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Calegari F, et al. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J. Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Chang HH, et al. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W, et al. Drosophila neuroblast asymmetric divisions: cell cycle regulators, asymmetric protein localization, and tumorigenesis. J. Cell Biol. 2008;180:267–272. doi: 10.1083/jcb.200708159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinquin O, et al. Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc. Natl. Acad. Sci. USA. 2010;107:2048–2053. doi: 10.1073/pnas.0912704107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, et al. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Cohen AR, et al. Computational prediction of neural progenitor cell fates. Nat. Methods. 2010;7:213–218. doi: 10.1038/nmeth.1424. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, et al. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stemcells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J. Invest. Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, et al. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, et al. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol. Biol. Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, et al. Drosophila Myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de Navascues J, et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31:2473–2485. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG. Stem cells in the testis. Int. J. Exp. Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr. Opin. Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Del Bene F, et al. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J, Lagace DC. Visualization and genetic manipulation of adult neurogenesis using transgenic mice. Eur. J. Neurosci. 2011;33:1025–1036. doi: 10.1111/j.1460-9568.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Doupe DP, et al. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr. Opin. Cell Biol. 2008;20:707–715. doi: 10.1016/j.ceb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Horsley V. More than one way to skin. Genes Dev. 2008;22:976–985. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, et al. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. How different is Venus from Mars? The genetics of germline stem cells in Drosophila females and males. Development. 2004;131:4895–4905. doi: 10.1242/dev.01373. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grisanti L, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Halevy O, et al. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Ho AD. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp. Hematol. 2005;33:1–8. doi: 10.1016/j.exphem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ho AD, Wagner W. The beauty of asymmetry: asymmetric divisions and self-renewal in the haematopoietic system. Curr. Opin. Hematol. 2007;14:330–336. doi: 10.1097/MOH.0b013e3281900f12. [DOI] [PubMed] [Google Scholar]
- Hodge RD, et al. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J. Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, et al. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJ. Caenorhabditis elegans germ line: a model for stem cell biology. Dev. Dyn. 2007;236:3343–3357. doi: 10.1002/dvdy.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet. 1971;4:313–334. doi: 10.1111/j.1365-2184.1971.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Issigonis M, et al. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Itzkovitz S, et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 2012;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jaks V, et al. The hair follicle-a stem cell zoo. Exp. Cell Res. 2010;316:1422–1428. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Janes SM, et al. Epidermal stem cells. J. Pathol. 2002;197:479–491. doi: 10.1002/path.1156. [DOI] [PubMed] [Google Scholar]
- Janich P, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Lambert A, et al. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 2007;308:206–221. doi: 10.1016/j.ydbio.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Jin Z, et al. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Jones P, Simons BD. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat. Rev. Mol. Cell Biol. 2008;9:82–88. doi: 10.1038/nrm2292. [DOI] [PubMed] [Google Scholar]
- Jones PH. Stem cell fate in proliferating tissues: equal odds in a game of chance. Dev. Cell. 2010;19:489–490. doi: 10.1016/j.devcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Jones PH, et al. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell. 2007;1:371–381. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Joshi PM, et al. Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 2010;239:1539–1554. doi: 10.1002/dvdy.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kalmar T, et al. Regulated fluctuations in nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 2009;7:e1000149. doi: 10.1371/journal.pbio.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda T, et al. Analysis of the cellular heterogeneity in the basal layer of mouse ear epidermis: an approach from partial decomposition in vitro and retroviral cell marking in vivo. Exp. Cell Res. 2003;283:167–183. doi: 10.1016/s0014-4827(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, et al. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J. Cell Biol. 2005;170:721–732. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P. Interfollicular epidermal stem cells: identification, challenges, potential. J. Invest. Dermatol. 2006;126:1450–1458. doi: 10.1038/sj.jid.5700184. [DOI] [PubMed] [Google Scholar]
- Kemp R, et al. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 2004;32:e92. doi: 10.1093/nar/gnh090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The pessimist’s and optimist’s views of adult neurogenesis. Cell. 2011;145:1009–1011. doi: 10.1016/j.cell.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger AA, et al. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kim TH, et al. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc. Natl. Acad. Sci. USA. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Klein AM, et al. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7:214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kageyama R. Hes1 oscillation: making variable choices for stem cell differentiation. Cell Cycle. 2010;9:207–208. doi: 10.4161/cc.9.2.10478. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, et al. The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes Dev. 2009;23:1870–1875. doi: 10.1101/gad.1823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, et al. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp PM. Immortal strands? Give me a break. Cell. 2007;129:1244–1247. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, et al. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, et al. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Lugert S, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lugert S, Taylor V. Neural stem cells: disposable, end-state glia? Cell Stem Cell. 2011;8:464–465. doi: 10.1016/j.stem.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Ma DK, et al. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, et al. Quantitative analysis of germline mitosis in adult C. elegans. Dev. Biol. 2006;292:142–151. doi: 10.1016/j.ydbio.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Macre G, et al. Distinct contribution of stem an progenitor cells to the epidermal maintenance. Nature. 2012;489(7415):257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA. 2010;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, et al. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Morin X, Bellaiche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rover S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Murciano A, et al. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol. Cell. Neurosci. 2002;21:285–300. doi: 10.1006/mcne.2002.1174. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, et al. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, et al. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, et al. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat.Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz LJ, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc. Natl. Acad. Sci. USA. 2009;106:21924–21929. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, et al. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Potten CS, et al. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J. Cell Biol. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehoda KE. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect. Biol. 2009;1:a001388. doi: 10.1101/cshperspect.a001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Quyn AJ, et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Scadden DT. Divided within: heterogeneity within adult stem cell pools. Cell. 2008;135:1006–1008. doi: 10.1016/j.cell.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–1243. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Ray S, Lechler T. Regulation of asymmetric cell division in the epidermis. Cell Div. 2011;6:12. doi: 10.1186/1747-1028-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert H. Drosophila neural stem cells: cell cycle control of self-renewal, differentiation, and termination in brain development. Results Probl. Cell Differ. 2011;53:529–546. doi: 10.1007/978-3-642-19065-0_21. [DOI] [PubMed] [Google Scholar]
- Relaix F, Marcelle C. Muscle stem cells. Curr. Opin. Cell Biol. 2009;21:748–753. doi: 10.1016/j.ceb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rhiner C, et al. Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development. 2009;136:995–1006. doi: 10.1242/dev.033340. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, et al. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Rannala B. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep. 2004;5:914–920. doi: 10.1038/sj.embor.7400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Rannala B. Evidence from the stop-EGFP mouse supports a niche-sharing model of epidermal proliferative units. Exp. Dermatol. 2005;14:838–843. doi: 10.1111/j.1600-0625.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- Rocheteau P, et al. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN. Asymmetric cell division. Curr. Opin. Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Russell L, Ettlin R, Sinha Hikim A, Clegg E. Histological and Histopathological Evaluation of the Testis. Cache River Press; Clearwater, FL: 1990. [Google Scholar]
- Sada A, et al. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders TL. Inducible transgenic mouse models. Methods Mol. Biol. 2011;693:103–115. doi: 10.1007/978-1-60761-974-1_7. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schenk J, et al. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc. Natl. Acad. Sci. USA. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, et al. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30:1104–1109. doi: 10.1038/emboj.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter H, et al. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells. 2011;29:1256–1268. doi: 10.1002/stem.675. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schroeder T. Long-term single-cell imaging of mammalian stem cells. Nat. Methods. 2011;8:S30–S35. doi: 10.1038/nmeth.1577. [DOI] [PubMed] [Google Scholar]
- Sheng XR, et al. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinin V, et al. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, et al. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells. 2008;26:2964–2973. doi: 10.1634/stemcells.2008-0634. [DOI] [PubMed] [Google Scholar]
- Spradling A, et al. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Strachan LR, Ghadially R. Tiers of clonal organization in the epidermis: the epidermal proliferation unit revisited. Stem Cell Rev. 2008;4:149–157. doi: 10.1007/s12015-008-9020-6. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Suzuki H, et al. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S. Stem cell identity and template DNA strand segregation. Curr. Opin. Cell Biol. 2008;20:716–722. doi: 10.1016/j.ceb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Takahashi T, et al. Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J. Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, et al. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JP, et al. Mathematical model for two germline stem cells competing for niche occupancy. Bull. Math. Biol. 2012;74:1207–1225. doi: 10.1007/s11538-011-9713-x. [DOI] [PubMed] [Google Scholar]
- Tokuda M, et al. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol. Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Tumbar T. Epithelial skin stem cells. Methods Enzymol. 2006;419:73–99. doi: 10.1016/S0076-6879(06)19004-7. [DOI] [PubMed] [Google Scholar]
- Tumbar T. Ontogeny and homeostasis of adult epithelial skin stem cells. Stem Cell Rev. 2012;8:561–576. doi: 10.1007/s12015-012-9348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, et al. Cancer stem cells—old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- Waghmare SK, et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, et al. Concise review: quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells. 2011;29:907–912. doi: 10.1002/stem.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL, et al. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willardsen MI, Link BA. Cell biological regulation of division fate in vertebrate neuroepithelial cells. Dev. Dyn. 2011;240:1865–1879. doi: 10.1002/dvdy.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, et al. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Winton DJ, Ponder BA. Stem-cell organization in mouse small intestine. Proc. Biol. Sci. 1990;241:13–18. doi: 10.1098/rspb.1990.0059. [DOI] [PubMed] [Google Scholar]
- Wu M, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–554. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells. J. Cell Biol. 2009;187:513–524. doi: 10.1083/jcb.200907047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Yamashita YM. The centrosome and asymmetric cell division. Prion. 2009;3:84–88. doi: 10.4161/pri.3.2.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, et al. Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Casting back to stem cells. Nat. Cell Biol. 2009;11:118–120. doi: 10.1038/ncb0209-118. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Stem cells in mammalian spermatogenesis. Dev. Growth Differ. 2010;52:311–317. doi: 10.1111/j.1440-169X.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, et al. Stem cell heterogeneity: actual and potential stem cell compartments in the mouse spermatogenesis. Ann. N. Y. Acad. Sci. 2007a;1120:47–58. doi: 10.1196/annals.1411.003. [DOI] [PubMed] [Google Scholar]
- Yoshida S, et al. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007b;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zammit PS, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, et al. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, et al. Stem cell dynamics in mouse hair follicles: a story from cell division counting and single cell lineage tracing. Cell Cycle. 2010;9:1504–1510. doi: 10.4161/cc.9.8.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao R, Xi R. Stem cell competition for niche occupancy: emerging themes and mechanisms. Stem Cell Rev. 2010;6:345–350. doi: 10.1007/s12015-010-9128-3. [DOI] [PubMed] [Google Scholar]
- Zheng K, et al. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Chia W. Neurogenesis and asymmetric cell division. Curr. Opin. Neurobiol. 2008;18:4–11. doi: 10.1016/j.conb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Zhu L, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]