Abstract
Cryptosporidiosis, giardiasis, and microsporidiosis are important waterborne diseases. In the standard for wastewater treatment plant (WWTP) effluents in China and other countries, the fecal coliform count is the only microbial indicator, raising concerns about the potential for pathogen transmission through WWTP effluent reuse. In this study, we collected 50 effluent samples (30 L/sample) from three municipal WWTPs in Shanghai, China, and analyzed for Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi by microscopy and/or polymerase chain reaction (PCR). Moreover, propidium monoazide (PMA)-PCR was used to assess the viability of oocysts/cysts. The microscopy and PCR-positive rates for Cryptosporidium spp. were 62% and 40%, respectively. The occurrence rates of G. duodenalis were 96% by microscopy and 92–100% by PCR analysis of three genetic loci. Furthermore, E. bieneusi was detected in 70% (35/50) of samples by PCR. Altogether, 10 Cryptosporidium species or genotypes, two G. duodenalis genotypes, and 11 E. bieneusi genotypes were found, most of which were human-pathogenic. The chlorine dioxide disinfection employed in WWTP1 and WWTP3 failed to inactivate the residual pathogens; 93% of the samples from WWTP1 and 83% from WWTP3 did not meet the national standard on fecal coliform levels. Thus, urban WWTP effluents often contain residual waterborne human pathogens.
Keywords: Cryptosporidium, Enterocytozoon bieneusi, genotype, Giardia, wastewater, WWTP effluent
INTRODUCTION
Cryptosporidiosis and giardiasis are two major waterborne diseases worldwide. Cryptosporidiosis, caused by Cryptosporidium spp., is responsible for self-limited diarrhea in immunocompetent persons and chronic diarrhea, cholangitis, and other severe complications in immunocompromised persons (Chalmers & Davies 2010). Giardiasis, caused by Giardia duodenalis, mainly occurs in children and causes diarrhea (Cacciò & Sprong 2011). Hundreds of waterborne outbreaks of cryptosporidiosis and giardiasis have been reported, mostly in industrialized nations (Baldursson & Karanis 2011). As a result, Cryptosporidium spp. and G. duodenalis are two of the most important pathogens in drinking water standards of many developed nations including China. Microsporidiosis is also a significant cause of diarrhea in children and AIDS patients (Didier & Weiss 2011). Although its epidemiology is less clear, spores of microsporidia have been found in drinking water sources (Dowd et al. 1998; Fournier et al. 2000).
Among more than 70 Cryptosporidium species/genotypes identified, C. parvum and C. hominis are responsible for most human infections (Ryan et al. 2014). Similarly, among the at least eight genotypes of G. duodenalis (assemblages A–H), only assemblages A and B are major human pathogens (Feng & Xiao 2011). The most common species that causes human microsporidiosis is Enterocytozoon bieneusi (Didier & Weiss 2011), which consists of more than 200 genotypes in at least eight groups, with humans mainly infected by genotypes in Group 1 (Karim et al. 2014).
United States Environmental Protection Agency Method 1623 is the standard method for the detection and quantitation of Cryptosporidium oocysts and Giardia cysts in water samples (Weintraub 2006). Currently, there are no validated methods for the detection of E. bieneusi spores in water. Like other microcopy-based methods, Method 1623 cannot diagnose Cryptosporidium spp. and Giardia spp. at the species or genotype level, and thus cannot differentiate human pathogens from animal pathogens. In contrast, molecular biological techniques such as polymerase chain reaction (PCR) and sequence analysis not only allow sensitive detection of multiple pathogens including E. bieneusi but also may facilitate the assessment of sources and human-infective potential of pathogens in water (Kothavade 2012). In addition, the viability of Cryptosporidium oocysts and Giardia cysts can be assessed through the treatment of samples with propidium monoazide (PMA) prior to PCR analysis, as the incorporation of PMA into DNA in damaged oocysts or cysts prevents PCR amplification of genetic targets (Brescia et al. 2009; Alonso et al. 2014).
Effluents of wastewater treatment plants (WWTPs) have been increasingly used for irrigation, recreational impoundments, and wetland reconstruction (Hachich et al. 2013). However, the existence of residual waterborne pathogens is a potential problem in wastewater reuse. For example, the use of treated effluents for crop cultivation and recreation is regarded as a potential risk to human health (Carr etal. 2004). Moreover, the current discharge standard of pollutants from municipal WWTPs in China uses the fecal coliform count as a microbiological indictor and does not have any specifications for pathogens such as Cryptosporidium spp. and G. duodenalis. Currently, the permitted concentration of fecal coliforms in China is 100 MPN/100 mL for Level 1-A water discharge (reclaimed use for landscape irrigation and small river and lake recharge), and 1,000 MPN/100 mL for Level 1-B (fishery, mariculture, swimming, etc.) and Level 2 water discharge (recreational water without direct body contact, agricultural irrigation, industrial usage, coastal tourist areas, etc.) (SEPA & AQSIQ 2002).
In this study, we examined the occurrence and human-infective potential of Cryptosporidium spp., G. duodenalis and E. bieneusi in effluents from three municipal WWTPs in Shanghai by microscopy, PCR, and PMA-PCR. Data from the study showed a common occurrence of human-pathogenic Cryptosporidium, G. duodenalis, and E. bieneusi genotypes in WWTP effluents, and no consistent correlation between fecal coliform counts and the occurrence of waterborne pathogens.
MATERIALS AND METHODS
Study sites
The effluents from three municipal WWTPs in Shanghai were sampled in this study. WWTP1 lies in the suburbs, utilizing the suspended carrier inverted anaerobic-anoxic-oxic (AAO) process after the removal of large particles by screening and vortex-type grit chambers. The treated wastewater goes through chlorine dioxide (ClO2) disinfection (the designed dosage was 5 ppm) before discharge. The effluent flows into a canal that is bordered by farmlands. WWTP2 is in the city; the wastewater first traverses through screens to remove large particles, and then flows into an aerated grit chamber and through a primary clarifier. It employs the inverted AAO process as the biological treatment and ultraviolet (UV) (TrojanUV3000Plus system, London, Ontario) for disinfection. The high output, low pressure amalgam lamps in the UV system are automatically dimmed when water flow drops or when the water quality changes, with UV doses varying mostly between 20 and 50 mWs/cm2. The effluent is discharged into a small river, which runs through a popular recreational park. WWTP3 is located near the coast and uses screening and vortex-type grit chambers to remove large particles in wastewater before the wastewater enters an integrated hydrolyzing pond. It uses the AAO process for biological treatment and chlorine dioxide for disinfection (the operational dosage was 8 ppm). The treated wastewater discharges into the sea and the seaside has become a tourist attraction, where visitors enjoy the beach and consume locally-harvested seafood. The three WWTPs studied use the Level 1-B or Level 2 discharge standard, with the limit of the fecal coliform count as 1,000 MPN/100 mL.
Sample collection and processing
A total of 50 grab samples (30 L per sample) of treated wastewater were collected during September 2014 to March 2015 from the three WWTPs, including 16 samples from WWTP1, 18 from WWTP2, and 16 from WWTP3. Four samples were collected from one WWTP at a time with a 10-min interval between each sample. Only one WWTP was visited each week, with the three WWTPs alternated among each other. Samples were transported to the laboratory in 10 L plastic containers and processed immediately upon arrival. The calcium carbonate flocculation (CCF) method was used for the concentration of pathogens in water samples as previously described (Vesey et al. 1993). The protists suspended in water were aggregated into the sediment after CCF. Half of the sediment (about 0.5 mL) was used in immunomagnetic separation (IMS) and immunofluorescence microscopy (IFA) of Cryptosporidium oocysts and Giardia cysts. The remaining part was divided into two: one was washed twice using phosphate buffered saline (PBS) by centrifugation and stored at −80 °C until DNA extraction, and the other received PMA treatment prior to DNA extraction.
Determination of fecal coliforms
For 38 of the 50 water samples, 10 mL of the mixed effluent was taken immediately for the determination of fecal coliforms upon their arrival in the laboratory. The Colilert Quanti-Tray/2000® (Idexx Laboratories, ME) was used in the enumeration of fecal coliforms with incubation at 44.5 °C and a dilution ratio of 1:100, as described recently (Staley et al. 2016).
IMS-IFA
Dynabeads® GC-combo kit (Idexx Laboratories), Easy-Stain™ (BTF, Australia), and 4′,6-diamidino-2-phenylindole (DAPI) were used for the IMS, IFA, and nuclear counter-staining of Cryptosporidium oocysts and Giardia cysts, respectively, following procedures of Method 1623 (Office of Water 2005). The stained oocysts and cysts with internal structures were counted under a fluorescence microscope equipped with the required epifluorescence filters.
PMA staining
The PMA (Biotium, USA) stock solution (50 mM) and working solution (150 μM) were prepared and stored as previously described (Brescia et al. 2009). Samples were incubated in the dark with an equal volume of PMA working solution and continuously stirred for 5 min. They were then placed on ice and exposed to an 800-W halogen light source 20 cm away. The total exposure time was 3 min, with a 1-min stirring after every 30-sec exposure. Treated samples were washed twice using PBS by centrifugation and stored at −80 °C until DNA extraction.
DNA extraction and molecular analysis
Genomic DNA was extracted from the sediment using the FastDNA SPIN Kit for Soil (MP Biomedicals, CA, USA), eluted into 100 μl of ultra-pure water, and stored at −20 °C before use (Jiang et al. 2005). Each sample was analyzed five times by nested PCR, described below, using 2 μL of the extracted DNA per PCR, with one positive control (DNA from target pathogens) and two negative controls (reagent-grade water for primary and secondary PCR reactions) in each PCR run. The secondary PCR products were examined by 1.5% agarose gel electrophoresis, and two positive PCR products per sample at each locus were sequenced in both directions by BioSune (Shanghai, China). The nucleotide sequences of each gene obtained were aligned with reference sequences downloaded from GenBank using ClustalX (http://www.clustal.org/).
Genotyping and subtyping of Cryptosporidium
A 587-bp fragment of the small-subunit (SSU) rRNA gene was amplified by nested PCR for the detection and genotyping of Cryptosporidium spp. (Ryan et al. 2003). Samples positive for C. hominis or C. parvum were further analyzed by PCR amplification of an approximately 400-bp fragment of the 60 kDa glycoprotein (gp60) gene (Sulaiman et al. 2005), whereas those positive for C. meleagridis were subtyped by a new PCR assay targeting a 900-bp fragment of the gp60 gene (Stensvold et al. 2014).
Genotyping of G. duodenalis
Giardia cysts in samples were detected and genotyped by nested PCR analysis of three genetic loci, including a 530-bp fragment of the triosephosphate isomerase (tpi) gene (Sulaiman et al. 2003a), a 599-bp fragment of the glutamate dehydrogenase (gdh) gene (Caccio et al. 2008), and a 511-bp fragment of the β-giardin gene (Lalle et al. 2005).
Detection and genotyping of E. bieneusi
A published nested PCR assay targeting a 392-bp fragment of the rRNA unit containing the entire internal transcribed spacer (ITS) was used for the detection and genotyping of E. bieneusi (Sulaiman et al. 2003b). The established nomenclature was used in naming the genotypes detected (Santin & Fayer 2011). The neighbor-joining analysis implemented in Mega 6.0 (http://www.megasoftware.net/) was used to determine the group of E. bieneusi genotypes detected in the study, based on genetic distances from the Kimura two-parameter model.
Statistical analysis
Data on pathogen occurrence were analyzed using SPSS 19.0 (http://www-01.ibm.com/software/analytics/spss/). The paired T test was used to evaluate the effect of PMA, whereas the χ2 test and ANOVA (analysis of variance) were used to compare differences in the occurrence of parasites and the concentration of Giardia cysts among three WWTPs, respectively (α = 0.05). A bivariate correlation analysis was used to assess the correlation between numbers of Cryptosporidium oocysts or Giardia cysts in IFA and fecal coliform counts.
Nucleotide sequence accession numbers
Unique nucleotide sequences generated in this study were deposited in GenBank under accession numbers KR902350 to KR902360.
RESULTS
Occurrence of Cryptosporidium oocysts and Giardia cysts by IFA
By microscopy, Cryptosporidium oocysts were detected in 31 (62%) of the 50 samples at concentrations of 0–0.93 oocyst/L. Among the three WWTPs, WWTP2 had a significantly lower Cryptosporidium oocyst occurrence than the other two WWTPs (P = 0.012); oocysts were detected in 33% of samples from WWTP2 compared to 81% of samples from WWTP1 and 75% of samples from WWTP3 (Table 1). In contrast, Giardia cysts were detected in 48 (96%) samples at concentration of 0–49 cysts/L. There were no significant differences in Giardia cyst occurrence (P = 0.109) or concentration (P = 0.059) among the three WWTPs (Table 2). As the sampling was done over a 6-month period, no effort was made to evaluate possible seasonal differences in the occurrence and concentration of these pathogens.
Table 1.
Occurrence and distribution of Cryptosporidium species in WWTP effluents in Shanghai, China
| Sampling site | No. of samples | IMS-IFA | Nested PCRa | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. of positive samples (%) | Mean ± SD oocysts/L (range) | No. of positive samples (%) | Species (No. of positive samples) | ||
| WWTP1 | 16 | 13 (81) | 0.30 ± 0.37 (0–0.93) | 10 (62) | C. muris (9), rat genotype I (2), C. hominis (1), C. meleagridis (1), C. canis (1), C. baileyi (1), rat genotype IV (1) |
| WWTP2 | 18 | 6 (33) | 0.093 ± 0.16 (0–0.47) | 4 (22) | C. suis-like (2), C. meleagridis (3), C. parvum (1), C. muris (1) |
| WWTP3 | 16 | 12 (75) | 0.088 ± 0.083 (0–0.27) | 6 (38) | C. muris (3), C. parvum (2), C. meleagridis (2), C. felis (1), C. suis-like (1) |
Data from PCR and PMA-PCR combined.
Table 2.
Occurrence and distribution of Giardia duodenalis genotypes in WWTP effluents in Shanghai, China
| Sampling site | No. of samples | IMS-IFA | Nested PCRa | MLG analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| No. of positive samples (%) | Mean ± SD cysts/L (range) | tpi gene | gdh gene | β-giardin gene | No. of samples | MLG types | |||||
|
|
|
|
|||||||||
| No. of positive samples (%) | Genotypes (No. of positive samples) | No. of positive samples (%) | Genotypes (No. of positive samples) | No. of positive samples (%) | Genotypes (No. of positive samples) | ||||||
| WWTP1 | 16 | 16 (100) | 7.3 ± 6.7 (0.07–17.77) | 16 (100) | A2 (16), A (KJ888992) (2), A2-ESHtpi01 (1) | 14 (88) | A2 (14), B-ESHgdh01 (1) | 15 (94) | A2 (15), A3 (5) | 14 | AII-1 (14) |
| WWTP2 | 18 | 18 (100) | 6.6 ± 7.8 (0.87–26.27) | 18 (100) | A2 (18), A (KJ888992) (9), A2-ESHtpi02 (1), B-ESHtpi (1) | 17 (94) | A2 (17), B-sh01 (JX994231) (1) | 17 (94) | A2 (17), A3 (3), A2-ESHbg (1) | 17 | AII-1 (17) |
| WWTP3 | 16 | 14 (88) | 14.4 ± 14.2 (0–48.80) | 16 (100) | A2 (15), A (KJ888992) (3) | 14 (88) | A2 (14), B-ESHgdh02 (1) | 14 (88) | A2 (14), A3 (4), A5 (1) | 13 | AII-1 (13) |
Data from PCR and PMA-PCR combined.
Occurrence of Cryptosporidium oocysts and Giardia cysts by PCR and PMA-PCR
By PCR analysis of the SSU rRNA gene, 15 of the 50 effluent samples were positive for Cryptosporidium spp. compared to 16 of the samples positive for Cryptosporidium by PMA-PCR (Table 3). Likewise, by PCR analysis, 44, 43, and 42 of the 50 effluent samples were positive for Giardia at the tpi, gdh, and β-giardin loci, respectively, compared to 49, 44, and 46 samples for Giardia by PMA-PCR at these loci, respectively. As most of the positive samples were positive by both PCR and PMA-PCR, there were no significant differences in pathogen detection rates between PCR and PMA-PCR (Table 3).
Table 3.
Assessment of viability of Cryptosporidium oocysts and Giardia cysts in WWTP effluents in Shanghai, China by PMA-PCR
| Gene | Cryptosporidium SSU-rRNA | Giardia tpi | Giardia gdh | Giardia β-giardin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||
| Sampling site | WWTP1 | WWTP2 | WWTP3 | WWTP1 | WWTP2 | WWTP3 | WWTP1 | WWTP2 | WWTP3 | WWTP1 | WWTP2 | WWTP3 |
| No. of samples analyzed | 16 | 18 | 16 | 16 | 18 | 16 | 16 | 18 | 16 | 16 | 18 | 16 |
| No. of samples positive in both PCR and PMA-PCR | 7 | 1 | 3 | 15 | 15 | 13 | 13 | 16 | 14 | 14 | 15 | 13 |
| No. of samples positive in PMA-PCR only | 1 | 2 | 2 | 1 | 3 | 2 | 1 | 1 | 0 | 1 | 2 | 1 |
| No. of samples positive in PCR only | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| P value a | 0.411 | 0.805 | 0.718 | 0.203 | 0.717 | 0.096 | 0.102 | 0.331 | 0.261 | 0.287 | 0.805 | 0.261 |
P values in paired T test for pathogen detection between PCR and PMA PCR methods.
Cryptosporidium species/subtypes
Altogether 20 (40%) of the 50 effluent samples were positive for Cryptosporidium spp. by PCR or PMA-PCR. Among the three WWTPs, Cryptosporidium spp. were detected in 62% of samples from WWTP1, 22% of samples from WWTP2, and 38% of samples from WWTP3 (P = 0.055; Table 1). DNA sequencing of PCR products revealed the presence of 10 Cryptosporidium species or genotypes, including C. muris, C. meleagridis, C. parvum, C. hominis, C. canis, C. felis, C. baileyi, C. suis-like, rat genotype I, and rat genotype IV. The most common species was C. muris, which was detected in 13 samples. This was followed by C. meleagridis, C. parvum, and C. suis-like, being detected in six, three, and three samples, respectively. Others were detected in only one or two samples (Table 1). Subtyping of C. hominis and C. parvum in the samples was unsuccessful. One of the C. meleagridis samples from WWTP2 was identified as the IIIbA22G1R1c subtype (KJ210607).
G. duodenalis genotypes and subtypes
PCR and PMA-PCR analyses of the tpi gene showed that all samples from the three WWTPs were positive for G. duodenalis. DNA sequence analysis of the PCR products identified the subtype A2 of assemblage A in 49 samples and another related assemblage A subtype (KJ888992) in 14 samples. This subtype (KJ888992) has been reported in humans (Karim et al. 2015). Moreover, three new subtypes, A2-ESHtpi01 (one SNP from A2), A2-ESHtpi02 (one SNP from A2), and B-ESHtpi (four SNPs from B) were found in this study.
Analysis of the gdh locus showed that 90% (45/50) of the samples were positive for G. duodenalis. Subtype A2 of assemblage A was identified in 45 samples and subtype B-sh01 (JX994231) of assemblage B in one sample. Two new assemblage B subtypes, B-ESHgdh01 and B-ESHgdh02, each with two SNPs from the B-sh03 subtype (JX994233), were also detected.
Analysis of the β-giardin locus showed that 92% (46/90) of the samples were positive for G duodenalis. Subtypes A2, A3, and A5 were detected in 46,12, and one sample, respectively. One new subtype A2-ESHbg (one SNP from A2) of assemblage A was found (Table 2).
Altogether, 44 samples were positive at all three genetic loci by PCR or PMA-PCR. Multilocus genotype (MLG) analysis (Feng & Xiao 2011) indicated that AII-1 was the dominant type in this study, being detected in all 44 samples (Table 2). The other assemblage A subtypes and assemblage B in samples with partial data occurred in a few samples mostly together with AII-1.
E. bieneusi genotypes
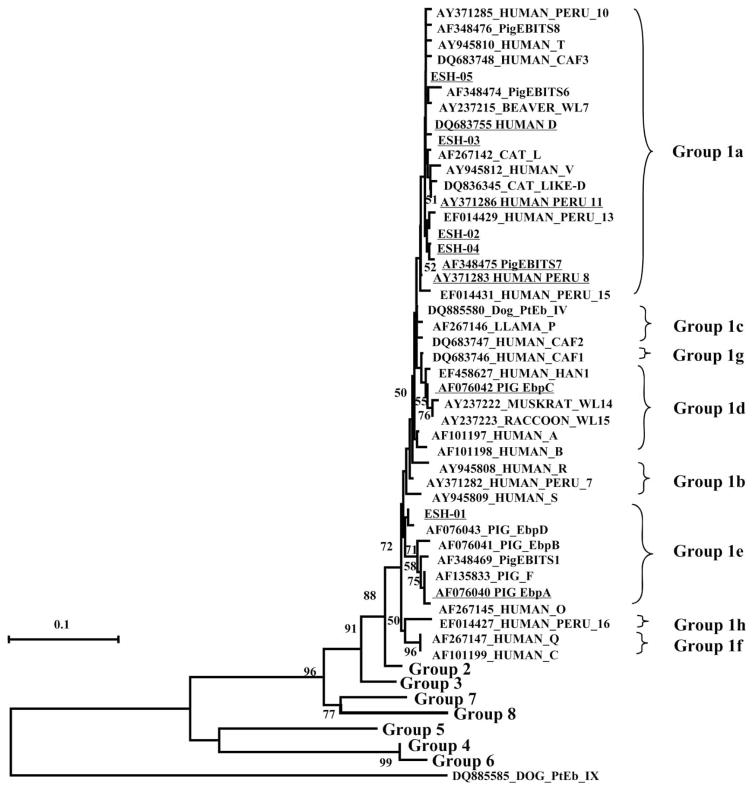
By PCR analysis of the ITS, 35 (70%) of the 50 samples were positive for E. bieneusi. The occurrence rates of E. bieneusi in WWTP1, WWTP2, and WWTP3 were 81%, 78%, and 50%, respectively (P = 0.104). Eleven genotypes of E. bieneusi were identified in this study, including six known ones (D, EbpC, PigEBITS7, Peru11, Peru8, and EbpA) and five new ones (ESH-01 to ESH-05). The predominant genotype was D, being detected in 31 samples. This was followed by ESH-03 in six samples (Table 4). A neighbor-joining analysis of the ITS sequences revealed that all E. bieneusi genotypes found in this study belonged to the zoonotic Group 1. The majority of these genotypes were in subgroup 1a, except for EbpC in subgroup 1d and ESH-01 and EbpA in subgroup 1e (Figure 1).
Table 4.
Occurrence and distribution of Enterocytozoon bieneusi genotypes in WWTP effluents in Shanghai, China
| Sampling site | No. of samples | No. of positive samples (%) | Genotypes (No. of samples) |
|---|---|---|---|
| WWTP1 | 16 | 13 (81) | D (11), ESH-03 (6), EbpC (2) |
| WWTP2 | 18 | 14 (78) | D (13), PigEBITS7 (2), Peru 11 (1), ESH-04 (2) |
| WWTP3 | 16 | 8 (50) | D (7), Peru 8 (1), EbpA (1), ESH-02(1), ESH-05 (1), ESH-01 (1) |
Figure 1.
Phylogenetic relationship of Enterocytozoon bieneusi genotypes as indicated by a neighbor-joining analysis of ITS sequences from this study and reference sequences from GenBank, based on genetic distances calculated with the Kimura two-parameter model. Bootstrap values greater than 50% from 1,000 replicate analyses are shown on nodes of the tree. Genotypes underlined are those identified in this study.
Fecal coliform counts and correlation between pathogen and fecal coliform levels
The concentrations of fecal coliforms were 3.36 × 104 ± 3.27 × 104 (mean ± SD) MPN/100 mL and 2.23 × 104 ± 1.59 × 104 (mean ± SD) MPN/100 mL for WWTP1 and WWTP3 (the two plants that use chlorine dioxide for disinfection), respectively. In contrast, the level of fecal coliforms in WWTP2 (the plant that uses UV for disinfection) was above the detection threshold for only three samples (100, 100, and 310 MPN/100 mL). By fecal coliform concentrations, one of the 14 samples from WWTP1, all 11 samples from WWTP2 and two of the 12 samples from WWTP3 met the level 1-B or level 2 of the Discharge Standard of Pollutants for Municipal WWTP, China (GB18918-2002).
There was a weak correlation between numbers of Cryptosporidium oocysts detected by IFA and fecal coliform counts (r = 0.432; P = 0.008). There was no such correlation between numbers of Giardia cysts and fecal coliforms (r = 0.234; P = 0.162).
DISCUSSION
Effluents from municipal WWTPs play important roles in the transport of parasites into water. Previous studies have demonstrated that the occurrence and concentration of Cryptosporidium oocysts and Giardia cysts in surface water near outlets of WWTPs are higher than those at other sites (Van Dyke et al. 2012). Moreover, Cryptosporidium oocysts and Giardia cysts are environmentally resistant (Fayer & Nerad 1996; Olson et al. 1999), thus retain viability and infectivity in the environment for a long period of time. In addition, the ingestion of as few as 10 cysts or 30 oocysts can cause giardiasis and cryptosporidiosis in humans (Adam 1991; DuPont et al. 1995). As a result, if raw wastewater is not fully treated, the reuse of WWTP effluents for irrigation, recreation, or surface water recharge can be a potential public health problem.
In this study, three different techniques were used in the detection of pathogens in WWTP effluents, including IFA, PCR, and PMA-PCR. The IFA microscopy implemented in Method 1623 has the advantage of providing quantitative assessment of the concentrations of Cryptosporidium oocysts and Giardia cysts in WWTP effluent samples, but cannot differentiate human-pathogenic species or genotypes from animal-specific species or genotypes, or viable oocysts or cysts from dead oocysts or cysts (Weintraub 2006). PCR along with DNA sequencing of the PCR products, in contrast, allows not only the assessment of source and human-infective potential of oocysts and cysts in WWTP effluents but also the detection of other pathogens, such as E. bieneusi (Kothavade 2012). However, it cannot accurately assess the level of pathogen contamination. PMA-PCR is intended to assess the viability of Cryptosporidium oocysts and Giardia cysts, as cross-linking of PMA to DNA through the damaged external structure obstructs the amplification of target DNA by PCR (Brescia et al. 2009). Thus, only DNA from viable oocysts and cysts is expected to be amplified by PMA-PCR. Both PCR and PMA-PCR are likely to be affected by UV irradiation, which induces cyclobutylthymine dimer formation and strand breakage in DNA (Al-Adhami et al. 2007), although no studies have been conducted thus far to evaluate the effect of UV treatment on PCR detection of waterborne pathogens in water samples.
Among the three detection techniques, PCR and IFA were used as the primary techniques in the detection of Cryptosporidium spp. and G. duodenalis in WWTP effluents. Some previous studies have shown that PCR appears to be more sensitive than IFA in the detection of Cryptosporidium oocysts and Giardia cysts in water samples (Mayer & Palmer 1996). However, in this study, the IFA-positive rates of Cryptosporidium oocysts are slightly higher than PCR-positive rates. Similar results have been reported previously (Robertson et al. 2006; Lobo et al. 2009; Kitajima et al. 2014). The existence of PCR inhibitors in water samples, uneven distribution of oocysts in sample concentrates, UV treatment of wastewater in one WWTP, and a smaller proportion of water concentrates (the entire 0.5 mL of concentrate for IFA versus 10% DNA from 0.5 mL of concentrate for PCR) analyzed by PCR may have contributed to the lower detection rate by PCR. In the case of Giardia spp., similar detection rates were obtained by the two approaches, probably because of the much higher concentrations of cysts in WWTP effluents.
We attempted to use PMA-PCR in assessing the viability of Cryptosporidium oocysts and Giardia cysts in WWTP effluents. However, the effect of the PMA treatment prior to PCR on pathogen detection was insignificant in this study. Since the UV disinfection in WWTP2 can destroy the internal DNA without affecting the integrity of the oocyst/cyst wall, we expected to see similar pathogen detection rates between PCR and PMA-PCR for WWTP2 samples. On the other hand, chlorine dioxide kills Cryptosporidium oocysts and Giardia cysts through damage to the oocyst and cyst wall and the parasite membranes. As damage in oocysts and cysts would allow the incorporation of PMA into DNA, which prevents the amplification of target genes by PCR, only potentially viable organisms are expected to be detected by PMA-PCR. Data from this study suggest that at least some pathogens detected in samples from the WWTPs 1 and 3 are potentially viable, as similar Cryptosporidium and Giardia detection rates were obtained between regular PCR and PMA-PCR. The potential viability of oocysts and cysts was also supported by the results of the fecal coliform detection, as the chlorine dioxide treatment in these two WWTPs has failed in reducing the fecal coliform level to the accepted threshold. As quantitative PCR was not used, we do not know the proportion of viable Cryptosporidium oocysts and Giardia cysts in WWTP samples. We also cannot exclude the possibility of insufficient cross-linking of PMA to pathogen DNA due to the high turbidity of sample concentrates.
Ten species or genotypes of Cryptosporidium were detected in WWTP effluents in this study, including C. muris, C parvum, C hominis, C meleagridis, C felis, and C. canis, among which, C. muris was the most prevalent species. C. parvum and C. hominis are two species responsible for most cryptosporidiosis cases in humans and C. meleagridis. C felis, and C. canis, traditionally regarded as host-adapted species, have also been found in a number of human patients (Ryan et al. 2014). The C. meleagridis subtype IIIbA22G1R1c seen in this study has been reported in humans in India (Stensvold et al. 2014). A recent study in healthy volunteers indicates that humans are susceptible to C. muris (Chappell et al. 2015). The occurrence of human-pathogenic Cryptosporidium spp. in WWTP effluents in this study supports recent calls for more stringent treatment of municipal wastewater in China.
The occurrence rate of G. duodenalis in this study was very high. Of the 50 samples collected, 48 (96%) were positive by IFA and all positive by PCR analysis of the tpi gene. DNA sequence analysis of PCR products demonstrated that most positive samples had assemblage A. Results of MLG analysis suggest that almost all belonged to AII-1. Sub-assemblage AII is a common G. duodenalis pathogen in humans and has been only occasionally found in animals (Feng & Xiao 2011). The high concentration of G. duodenalis cysts and common occurrence of sub-assemblage AII in WWTP effluents highlight the potential of treated wastewater as a source of G. duodenalis contamination in watersheds in China.
E. bieneusi was found in more than half of the samples, including six known genotypes, D, EbpC, PigEBITS7, Peru11, Peru8, and EbpA. The most prevalent genotype was D, which was detected in 31 samples. The known genotypes in this study have been commonly reported in human patients (Matos et al. 2012). Five new genotypes were found in this study. Among them, ESH-02, ESH-03, ESH-04, and ESH-05 belong to the subgroup 1a, which is known to primarily infect humans (Thellier & Breton 2008). ESH-01 is in the subgroup 1e, which although commonly found in pigs, has been reported in humans (Leelayoova et al. 2006). The existence of human-pathogenic E. bieneusi genotypes in WWTP effluents is consistent with our findings on Cryptosporidium spp. and G. duodenalis.
In the study areas, WWTP effluents discharge into agricultural lands, recreational parks, and the coast, thus microbial levels above allowable thresholds can raise public health concerns. Previous studies have also demonstrated the potential of pathogen contaminations in recreational water by WWTP effluents (Castro-Hermida et al. 2008; Betancourt et al. 2014). Moreover, Cryptosporidium spp. and G. duodenalis in WWTP effluents may contaminate shellfish or fresh produce, causing foodborne outbreaks of diarrheal illnesses (Thurston-Enriquez et al. 2002; Willis et al. 2013). A previous report on WWTP effluents for irrigation also described the occurrence of both Cryptosporidium oocysts and Giardia cysts (Lubello et al. 2004).
In the Discharge Standard of Pollutants for Municipal WWTP, China (GB18918-2002), the fecal coliform level is the only microbiological indicator, and WWTP effluents can be discharged into surface water or the ocean when they have met the standard. The three WWTPs studied use the Level 1-B or Level 2 standard, which has the upper limit of the fecal coliform count as 1,000 MPN/100 mL. Fewer than half of the effluent samples in WWTP1 or WWTP3 met the standard in this small-scale study, and both of them use chlorine dioxide as the disinfectant for secondary effluents. Previously in China, chlorine dioxide was shown to be effective in controlling fecal coliform levels in wastewater at the dosage of 2.8 ppm and contact time of 13 min (Zhang et al. 2009). The designed dosage was 5 ppm for WWTP1 and the operational dosage was 8 ppm in WWTP3 during the study, with a designed contact time of 30 min for both WWTPs. In controlled settings, chlorine dioxide treatment at 10 ppm can lead to the loss of excystation ability of Giardia cysts within 1 min at room temperature and treatment at 1.3 ppm for 1 hour can results in 90% inactivation of Cryptosporidium oocysts (Korich et al. 1990; Winiecka-Krusnell & Linder 1998). However, the effectiveness of chlorine dioxide in inactivating fecal coliforms in wastewater is known to be affected significantly by the level of organic matter, and in Italy, its use as a disinfectant is not sufficient to obtain wastewater that meets the national quality standard for irrigation (De Luca et al. 2008). More studies are needed to evaluate the effectiveness of chlorine dioxide in reducing fecal coliforms and pathogen levels in wastewater effluents.
In contrast to the poor quality of effluents in WWTP1 and WWTP3, all effluent samples from WWTP2, which uses UV as the disinfectant for secondary effluents, had fecal coliform concentrations below the acceptable level for discharge. Previously, UV was shown to be much more effective in reducing fecal coliform levels in wastewater than chlorine dioxide (Carrasco & Turner 2006). A much lower occurrence of Cryptosporidium oocysts was also seen in WWTP2, although we did not see any major differences among the three WWTPs in the occurrence of Giardia cysts. The occurrence of Cryptosporidium oocysts and Giardia cysts in surface water correlated with fecal coliform or E. coli counts in some studies (LeChevallier et al. 1991; Yang et al. 2008; Xiao et al. 2013) but not in others (Wu et al. 2011; Pachepsky et al. 2015).
CONCLUSION
We have demonstrated that human pathogens Cryptosporidium spp., G duodenalis, and E. bieneusi are present at significant concentrations in municipal WWTP effluents in China. The chlorine dioxide disinfection employed in some of the WWTPs is not effective in inactivating the residual pathogens and fecal coliforms. These findings raise concerns regarding the implementation of the current Chinese regulation on WWTP effluents. More studies in other settings are needed to determine whether there is a need for the inclusion of major waterborne pathogens in the national standard of WWTP effluents in China and other countries.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31425025 and 31229005), Water Special Project of the Ministry of Science and Technology, China (2014ZX07104006), and Fundamental Research Funds for the Central Universities, China. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the US Environmental Protection Agency. The work has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Contributor Information
Jiawen Ma, State Key Laboratory of Bioreactor Engineering, School of Resources and Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China.
Yaoyu Feng, State Key Laboratory of Bioreactor Engineering, School of Resources and Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China.
Yue Hu, State Key Laboratory of Bioreactor Engineering, School of Resources and Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China.
Eric N. Villegas, National Exposure Research Laboratory, US Environmental Protection Agency, Cincinnati, OH 45268, USA
Lihua Xiao, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
References
- Adam RD. The biology of Giardia spp. Microbiol Rev. 1991;55(4):706–732. doi: 10.1128/mr.55.4.706-732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Adhami BH, Nichols RA, Kusel JR, O’Grady J, Smith HV. Detection of UV-induced thymine dimers in individual Cryptosporidium parvum and Cryptosporidium hominis oocysts by immunofluorescence microscopy. Appl Environ Microbiol. 2007;73:947–955. doi: 10.1128/AEM.01251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JL, Amoros I, Guy RA. Quantification of viable Giardia cysts and Cryptosporidium oocysts in wastewater using propidium monoazide quantitative real-time PCR. Parasitol Res. 2014;113(7):2671–2678. doi: 10.1007/s00436-014-3922-9. [DOI] [PubMed] [Google Scholar]
- Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks–an update 2004–2010. Water Res. 2011;45(20):6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Betancourt WQ, Duarte DC, Vasquez RC, Gurian PL. Cryptosporidium and Giardia in tropical recreational marine waters contaminated with domestic sewage: estimation of bathing-associated disease risks. Mar Pollut Bull. 2014;85(1):268–273. doi: 10.1016/j.marpolbul.2014.05.059. [DOI] [PubMed] [Google Scholar]
- Brescia CC, Griffin SM, Ware MW, Varughese EA, Egorov AI, Villegas EN. Cryptosporidium propidium monoazide-PCR, a molecular biology-based technique for genotyping of viable Cryptosporidium oocysts. Appl Environ Microbiol. 2009;75(21):6856–6863. doi: 10.1128/AEM.00540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò SM, Sprong H. Giardia. Springer-Verlag/Wien; New York: 2011. [Google Scholar]
- Cacciò SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. IntJ Parasitol. 2008;38(13):1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Carr RM, Blumenthal UJ, Mara DD. Guidelines for the safe use of wastewater in agriculture. Water Sci Technol. 2004;50(2):31–38. [PubMed] [Google Scholar]
- Carrasco L, Turner CD. Evaluation of disinfection techniques in the treatment of advanced primary treated wastewater for Ciudad Juarez, Mexico. Water Environ Res. 2006;78(1):49–58. doi: 10.2175/106143005x84512. [DOI] [PubMed] [Google Scholar]
- Castro-Hermida JA, Garcia-Presedo I, Almeida A, Gonzalez-Warleta M, Correia Da Costa JM, Mezo M. Contribution of treated wastewater to the contamination of recreational river areas with Cryptosporidium spp. and Giardia duodenalis. Water Res. 2008;42(13):3528–3538. doi: 10.1016/j.watres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124(1):138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Chappell CL, Okhuysen PC, Langer-Curry RC, Lupo PJ, Widmer G, Tzipori S. Cryptosporidium muris: infectivity and illness in healthy adult volunteers. Am J Trop Med Hyg. 2015;92(1):50–55. doi: 10.4269/ajtmh.14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca G, Sacchetti R, Zanetti F, Leoni E. Comparative study on the efficiency of peracetic acid and chlorine dioxide at low doses in the disinfection of urban wastewaters. Ann Agric Environ Med. 2008;15(2):217–224. [PubMed] [Google Scholar]
- Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis. 2011;24(5):490–495. doi: 10.1097/QCO.0b013e32834aa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Gerba CP, Pepper IL. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol. 1998;64(9):3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332(13):855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- Fayer R, Nerad T. Effects of low temperatures on viability of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1996;62(4):1431–1433. doi: 10.1128/aem.62.4.1431-1433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24(1):110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S, Liguory O, Santillana-Hayat M, Guillot E, Sarfati C, Dumoutier N, Molina J, Derouin F. Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol Med Microbiol. 2000;29(2):95–100. doi: 10.1111/j.1574-695X.2000.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Hachich EM, Galvani AT, Padula JA, Stoppe NC, Garcia SC, Bonanno VM, Barbosa MR, Sato MI. Pathogenic parasites and enteroviruses in wastewater: support for a regulation on water reuse. Water Sci Technol. 2013;67(7):1512–1518. doi: 10.2166/wst.2013.019. [DOI] [PubMed] [Google Scholar]
- Jiang J, Alderisio KA, Singh A, Xiao L. Development of procedures for direct extraction of Cryptosporidium DNA from water concentrates and for relief of PCR inhibitors. Appl Environ Microbiol. 2005;71(3):1135–1141. doi: 10.1128/AEM.71.3.1135-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Wang R, Dong H, Zhang L, Li J, Zhang S, Rume FI, Qi M, Jian F, Sun M, Yang G, Zou F, Ning C, Xiao L. Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Appl Environ Microbiol. 2014;80(6):1893–1898. doi: 10.1128/AEM.03845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Wang R, Yu F, Li T, Dong H, Li D, Zhang L, Li J, Jian F, Zhang S, Rume FI, Ning C, Xiao L. Multi-locus analysis of Giardia duodenalis from nonhuman primates kept in zoos in China: geographical segregation and host-adaptation of assemblage B isolates. Infect Genet Evol. 2015;30:82–88. doi: 10.1016/j.meegid.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Haramoto E, Iker BC, Gerba CP. Occurrence of Cryptosporidium, Giardia, and Cyclospora in influent and effluent water at wastewater treatment plants in Arizona. Sci Total Environ. 2014;484:129–136. doi: 10.1016/j.scitotenv.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990;56(5):1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothavade RJ. Potential molecular tools for assessing the public health risk associated with waterborne Cryptosporidium oocysts. J Med Microbiol. 2012;61(8):1039–1051. doi: 10.1099/jmm.0.043158-0. [DOI] [PubMed] [Google Scholar]
- Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Caccio SM. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35(2):207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- LeChevallier MW, Norton WD, Lee RG. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl Environ Microbiol. 1991;57(9):2610–2616. doi: 10.1128/aem.57.9.2610-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelayoova S, Subrungruang I, Suputtamongkol Y, Worapong J, Petmitr PC, Mungthin M. Identification of genotypes of Enterocytozoon bieneusi from stool samples from human immunodeficiency virus-infected patients in Thailand. J Clin Microbiol. 2006;44(8):3001–3004. doi: 10.1128/JCM.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo ML, Xiao L, Antunes F, Matos O. Occurrence of Cryptosporidium and Giardia genotypes and subtypes in raw and treated water in Portugal. Lett Appl Microbiol. 2009;48(6):732–737. doi: 10.1111/j.1472-765X.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- Lubello C, Gori R, Nicese FP, Ferrini F. Municipal-treated wastewater reuse for plant nurseries irrigation. Water Res. 2004;38(12):2939–2947. doi: 10.1016/j.watres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Matos O, Lobo ML, Xiao L. Epidemiology of Enterocytozoon bieneusi infection in humans. J Parasitol Res. 2012;2012:981424. doi: 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CL, Palmer CJ. Evaluation of PCR, nested PCR, and fluorescent antibodies for detection of Giardia and Cryptosporidium species in wastewater. Appl Environ Microbiol. 1996;62(6):2081–2085. doi: 10.1128/aem.62.6.2081-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Water. USEPA Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. US Environmental Protection Agency; Washington, DC: 2005. [Google Scholar]
- Olson ME, Goh J, Phillips M, Guselle N, McAllister TA. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J Environ Qual. 1999;28(6):1991–1996. [Google Scholar]
- Pachepsky Y, Shelton D, Dorner S, Whelan G. Can E. coli or thermotolerant coliform concentrations predict pathogen presence or prevalence in irrigation waters? Crit Rev Microbiol. 2015:1–10. doi: 10.3109/1040841X.2014.954524. (online) [DOI] [PubMed] [Google Scholar]
- Robertson LJ, Hermansen L, Gjerde BK. Occurrence of Cryptosporidium oocysts and Giardia cysts in sewage in Norway. Appl Environ Microbiol. 2006;72(8):5297–5303. doi: 10.1128/AEM.00464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U, Xiao L, Read C, Zhou L, Lal AA, Pavlasek I. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl Environ Microbiol. 2003;69(7):4302–4307. doi: 10.1128/AEM.69.7.4302-4307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141(13):1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- Santin M, Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. 2011;90(3):363–371. doi: 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- SEPA & AQSIQ. GB18918 – 2002 Discharge standard of pollutants for municipal wastewater treatment plant. Beijing, China: 2002. (in Chinese) [Google Scholar]
- Staley ZR, Robinson C, Edge TA. Comparison of the occurrence and survival of fecal indicator bacteria in recreational sand between urban beach, playground and sandbox settings in Toronto, Ontario. Sci Total Environ. 2016;541:520–527. doi: 10.1016/j.scitotenv.2015.09.088. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Beser J, Axen C, Lebbad M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J Clin Microbiol. 2014;52(7):2311–2319. doi: 10.1128/JCM.00598-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis. 2003a;9(11):1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, Xiao L. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. 2003b;69(8):4495–4501. doi: 10.1128/AEM.69.8.4495-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Hira PR, Zhou L, Al-Ali FM, Al-Shelahi FA, Shweiki HM, Iqbal J, Khalid N, Xiao L. Unique endemicity of cryptosporidiosis in children in Kuwait. J Clin Microbiol. 2005;43(6):2805–2809. doi: 10.1128/JCM.43.6.2805-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellier M, Breton J. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite. 2008;15(3):349–358. doi: 10.1051/parasite/2008153349. [DOI] [PubMed] [Google Scholar]
- Thurston-Enriquez JA, Watt P, Dowd SE, Enriquez J, Pepper IL, Gerba CP. Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J Food Prot. 2002;65(2):378–382. doi: 10.4315/0362-028x-65.2.378. [DOI] [PubMed] [Google Scholar]
- Van Dyke MI, Ong CS, Prystajecky NA, Isaac-Renton JL, Huck PM. Identifying host sources, human health risk and indicators of Cryptosporidium and Giardia in a Canadian watershed influenced by urban and rural activities. J Water Health. 2012;10(2):311–323. doi: 10.2166/wh.2012.131. [DOI] [PubMed] [Google Scholar]
- Vesey G, Slade JS, Byrne M, Shepherd K, Fricker CR. A new method for the concentration of Cryptosporidium oocysts from water. J Appl Bacteriol. 1993;75(1):82–86. doi: 10.1111/j.1365-2672.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Weintraub JM. Improving Cryptosporidium testing methods: a public health perspective. J Water Health. 2006;4(Suppl 1):23–26. [PubMed] [Google Scholar]
- Willis JE, McClure JT, Davidson J, McClure C, Greenwood SJ. Global occurrence of Cryptosporidium and Giardia in shellfish: should Canada take a closer look? Food Res Int. 2013;52(1):119–135. [Google Scholar]
- Winiecka-Krusnell J, Linder E. Cysticidal effect of chlorine dioxide on Giardia intestinalis cysts. Acta Trop. 1998;70(3):369–372. doi: 10.1016/s0001-706x(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Wu J, Long SC, Das D, Dorner SM. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health. 2011;9(2):265–278. doi: 10.2166/wh.2011.117. [DOI] [PubMed] [Google Scholar]
- Xiao G, Qiu Z, Qi J, Chen JA, Liu F, Liu W, Luo J, Shu W. Occurrence and potential health risk of Cryptosporidium and Giardia in the Three Gorges Reservoir, China. Water Res. 2013;47:2431–2445. doi: 10.1016/j.watres.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Yang W, Chen P, Villegas EN, Landy RB, Kanetsky C, Cama V, Dearen T, Schultz CL, Orndorff KG, Prelewicz GJ, Brown MH, Young KR, Xiao L. Cryptosporidium source tracking in the Potomac River watershed. Appl Environ Microbiol. 2008;74:6495–6504. doi: 10.1128/AEM.01345-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BX, Zhao X, Zhang YL, Gong XX. Comprehensive analysis on using chlorine dioxide as disinfectant for effluent from wastewater treatment plant. China Water Wastewater. 2009;25(23):69–71. [Google Scholar]