Abstract
Objectives
This study aimed to investigate incorporation of polyacrylic acid (PAA) coated copper iodide (CuI) nanoparticles into dental adhesives, and to evaluate for the first time, their antibacterial properties, bond strength and cytotoxicity.
Methods
PAA-CuI nanoparticles were synthesized and incorporated into commercially available adhesives Optibond XTR (1.0 mg/ml) and XP Bond (0.5 and 1.0 mg/ml). The antibacterial properties of experimental and control specimens were evaluated (n = 8), after ageing for 18 h or 1 year, against Streptococcus mutans (1 × 108 cells/ml). Bond strength to human dentine of the control and experimental adhesives was evaluated by shear bond strength (n = 10). For cytotoxicity evaluation, HGF cells were cultured with gingival fibroblast media and exposed to control and experimental adhesive blends (n = 3). An MTT cell viability assay was used to assess cell metabolic function. A one-way analysis of variance followed by Tukey’s test was used for data analysis.
Results
Significantly greater antibacterial properties were demonstrated for PAA-CuI containing adhesives after ageing for 18 h or 1 year relative to all control groups. A reduction in Streptococcus mutans viable cell count of 99.99%, 99.99% and 79.65% was shown for XP Bond – 0.5 mg/ml, XP Bond – 1.0 mg/ml and Optibond XTR – 1.0 mg/ml PAA-CuI after ageing for 18 h, and 99.99% for both XP Bond – 0.5 mg/ml and XP Bond – 1.0 mg/ml PAA-CuI after ageing for 1 year. No significant variations in shear bond strength or cytotoxicity were detected between the experimental resins and their corresponding controls.
Conclusions
PAA-CuI nanoparticles are an effective additive to adhesive blends as it renders them antibacterial without adversely affecting their bond strength or cytotoxicity.
Clinical significance
The incorporation of PAA-coated copper iodide particles into adhesive resins renders the adhesive antibacterial to S. mutans for at least 1 year in vitro. This may prevent or delay bacterial invasion and the consequent development of caries lesions if the adhesive interface becomes defective.
Keywords: Antibacterial, Bond strength, Copper, Cytotoxicity, Dental adhesive, Nanoparticles
1. Introduction
In the last few decades, resin-based composites (RBC) have gradually become the most widely used dental restorative material, representing 65% of the restorations currently placed in the United States.1 Their wear resistance, life-like aesthetics and perceived ease of placement make them desirable for both patients and clinicians.2 The use of RBC is anticipated to continue to increase due to patient demands for aesthetic restorations.3–5 Despite its increased use and the significant developments taking place in the field of dental materials science, the durability of adhesive restorations remains a challenge.5–7 It is well accepted that current RBC cannot prevent recurrent caries.1–3,8–10 Its been reported that 50–70% of newly placed restorations are the result of failure of pre-existing restorations8,11 with the cost to replace defective restorations in the United States surpassing 5 billion dollars annually.12 While posterior high copper amalgam (HCA) restorations have an average lifespan of 11–12 years, the service life of posterior RBC restorations ranges between 6 and 7 years.1,13 A study showed that after 8 years, the failure rate for posterior RBC restorations was 50% greater than that of HCA restorations.14 The higher incidence of recurrent caries and consequently, the greater need for replacement, may explain their reduced lifespan.6,7,9,10,15
Silver and copper, present in amalgam, are known to have potent antimicrobial properties,16 which may play an essential role in the clinical success of amalgam restorations. Modern amalgams contain approximately 60% silver and more than 12% copper by weight17 making them potentially antimicrobial and delaying their failure from bacterial invasion.18 The incidence of recurrent caries is 3.5 times higher in RBC than HCA restorations.9 This notion is further supported by the observation that bacterial micro-leakage is the most frequent complication associated with RBC restorations, leading to recurrent caries as the primary cause of failure.19–21 A study evaluating interproximal restorations in 650 radiographs found that failure due to recurrent caries was 43% for RBC and only 8% for HCA.6 RBC micro-leakage and bacterial invasion was found to take place at the tooth-restoration interface. This adhesive interface, the so-called hybrid layer, is a biosynthetic layer composed of a mixture of type I collagen and adhesive resins,22 and has been reported to be the weakest link of RBC restorations.23 Continued developments in the field of dentine adhesion are still needed to improve the longevity of RBC restorations.
Recent research work has demonstrated that copper and silver nanoparticles incorporated into polymers have a broad spectrum of antimicrobial activity.24–27 We speculate that this may help decrease the incidence of recurrent caries. Silver ion-containing resin composites have shown to have an antibacterial effect on oral Streptococci.25 A study found that the incorporation of silver nanoparticles into experimental orthodontic resin adhesives decreased the attachment and slowed the growth of Streptococcus mutans and Streptococcus sobrinus relative to the control adhesive.28 Although incorporation of silver nanoparticles did not have a negative effect on the bond strength to dentine,29 it yielded a change in the colour of the resin to a degree that would limit its application to areas where aesthetics was not critical.28 Likewise, copper particles are also black in colour and have a similar aesthetic concern. Alternative copper particles with enhanced aesthetic properties are available. Copper chalcogenide or coppe halide (CuQ, where Q = chalcogens including oxygen or halogens) was found to have altered optical properties rendering them white (unpublished observations). Specifically, copper iodide particles are tooth coloured and were found to have minimal effect on the shade of the materials to which they were added (unpublished observations).
Copper particles are unique in that their antibacterial activity is derived from the localized leaching of copper ions, which generates an infinite electron sink that can alter the bacterial membrane charge causing lysis. These antimicrobial properties are further optimized by the high surface-to-mass ratio achieved from the structure of the nanosphere.30 Copper particles are also significantly less expensive than silver, easily incorporated into polymers and relatively stable with regards to their chemical and physical properties.24 Roselen et al. found that the addition of copper to sucrose significantly reduced the incidence of caries in rats when compared to sucrose diet alone.31
This study describes the development of an adhesive resin based on the incorporation of polyacrylic acid (PAA) coated copper iodide (CuI) nanoparticles that renders the adhesive resistant to the major processes of adhesive failure, bond degradation and destruction of the resin inter-diffusion zone from bacterial proliferation and invasion. The use of such antibacterial adhesives is anticipated to limit the recurrence of caries and failure of RBC restorations. The aims of this study were to develop an antibacterial adhesive resin incorporating PAA-CuI nanoparticles and to investigate for the first time, its antibacterial activity, bond strength and potential cytotoxicity. The null hypotheses evaluated were: (1) incorporation of PAA-coated CuI particles into adhesive resins would have no effect on their antibacterial properties; (2) incorporation of PAA-coated CuI particles into adhesive resins would have no effect on their shear bond strength to dentine; and (3) PAA-coated CuI particles would demonstrate no cytotoxicity to mammalian cells compared to control adhesives.
2. Materials and methods
2.1. Synthesis of poly-acryic acid (PAA) coated copper iodide (CuI) particles and generation of PAA-CuI adhesives
Copper (II) sulfate (CuSO4, Puratonic, 99.999% metal bases, Alfa-Aesar, Ward Hill, MA), potassium iodide (PI, 99.995%, Acros, Waltham, MA) and poly (acrylic) acid (PAA, 50 wt% in water, MW = 5000, Acros) were used as received. All solutions were prepared using deionized 18.2 MΩ water (Milli-Q, Milipore, Billerica, MA). In a typical reaction, 78.8 ml of 0.2 M CuSO4 was combined with 7.8 ml of 20 wt% PAA followed by the addition of 100 ml of 400 mM KI. Subsequently, more potassium iodide (50 ml of 400 mM) was added to drive the reaction to completion. The resulting white precipitate was washed with deionized water four times by centrifugation and dried under vacuum at 50 °C. The powder was characterized by field emission scanning electron microscope (FE-SEM) and energy dispersive X-ray (EDX) analysis (4800 SEM, Hitachi, Wallingford, CT).
Generation of PAA-CuI adhesives was done in two stages. First, 10 mg of PAA-CuI powder was admixed with 1 ml of one of two commercial adhesive resins, XP Bond (Dentsply, York, PA) and Optibond XTR (Kerr, Orange, CA) to yield a concentrated solution (10 mg/ml) of PAA-CuI adhesives. The PAA-CuI particles were added to the adhesive of etch-and-rinse XP Bond and to both, primer and adhesive, of self-etch Optibond XTR in order to maximize its therapeutic benefit. To ensure uniform dissolution of the PAA-CuI particles, a probe tip sonicator (Sonic Dismebrator 100, Fisher Scientific, Waltham, MA) was used for 15 s under dark conditions in an iced-water bath. Immediately after, 50 µl or 100 µl of the concentrated solution (10 mg/ml) of PAA-CuI adhesives was micro-pipetted into amber vials containing either 950 µl or 900 µl, respectively of the appropriate stock adhesive to yield a final working concentration of 0.5 mg/ml or 1.0 mg/ml of PAA-CuI adhesive. A total of three PAA-CuI containing blends were obtained, XP Bond – 0.5 mg/ml PAA-CuI, XP Bond – 1.0 mg/ml PAA-CuI and Optibond XTR – 1.0 mg/ml PAA-CuI. Optibond XTR – 0.5 mg/ml PAA-CuI was excluded from testing since adequate dispersion of the particles into the mix was not attainable (unpublished observations).
To ensure uniform distribution of the particles into the adhesive solution, representative discs of XP bond adhesive modified with 0.5 mg/ml PAA-CuI particles were observed by transmission electron microscopy (TEM). The adhesive blend was inserted in a disc shaped mould and polymerized with an LED light-curing unit (VALO, Ultradent Products, South Jordan, UT, USA) at a minimum intensity of 1000 mW/cm2 for 10 s. The resulting resin disc was mounted in Spurr’s resin and sectioned using a Reichert-Jung Ultracut E microtome with a Diatome 45° diamond knife to obtain sections approximately 70 nm thick. The sections were observed in high-resolution TEM (JEM1010, JEOL Peabody, MA) at 80 kV with direct magnifications ranging from 25 kX to 300 kX.
2.2. Assessment of the antibacterial activity
To evaluate the antibacterial activity of the adhesives, the methods by Imazato et al.32 were adopted. Briefly, discs of 10 mm in diameter and 2 mm in thickness were fabricated with resin composite (Filtek Supreme Ultra, 3M ESPE, St. Paul, MN) and either left uncoated (control) or coated with adhesives, XP Bond, Optibond XTR, Clearfil SE Protect (Kuraray America, New York, NY), or the equivalent experimental adhesive blends of XP Bond and Optibond XTR mixed with 0.5 mg/ml or 1.0 mg/mlPAA-CuI and polymerized according to manufacturers’ recommendations for 10 s with an LED light curing unit (VALO, Ultradent Products) and a power density of 1000 mW/cm2. For Optibond XTR and Clearfil SE Protect, equal parts of primer and adhesive were mixed together and the resultant mixture was used to coat the discs before polymerization. Clearfil SE Protect (Kuraray, Medical Inc., Okayama, Japan), which consists of a self-etching primer containing antibacterial monomer MDPB and a fluoride-releasing bonding resin, was used as a positive control. Amalgam discs (Contour, Kerr, Orange, CA) were fabricated as previously described and also used as a positive control. A total of eight study groups with eight specimens per group (n = 8) were evaluated in this part of the experiment as follows: (1) Control; (2) Amalgam; (3) XP Bond; (4) Optibond XTR; (5) Clearfil SE Protect; (6) XP Bond – 0.5 mg/ml PAA-CuI; (7) XP Bond – 1.0 mg/ml PAA-CuI and (8) Optibond XTR – 1.0 mg/ml PAA-CuI. The study materials, including their composition and application protocol, as recommended by their corresponding manufacturer, are listed in Table 1. To ensure removal of unpolymerized monomer, the discs were then immersed in 10 ml of sterile deionized distilled water and agitated for 2 h at 200 rpm at 37 °C, after which the discs were left to dry at room temperature for at least 24 h. The discs were sterilized by incubation in 70% ethanol for 10 min and then aseptically dried for a minimum of 48 h.
Table 1.
Study materials, including composition and application protocol as per manufacturers’ recommendations.
| Material | Category | Lot # | Composition (%) | Application protocol |
|---|---|---|---|---|
| Filtek Supreme Ultra (3M ESPE) | Resin composite | – | Bis-GMA, UDMA, TEGDMA, Bis-EMA, non-agglomerated/non-aggregated 20 nm silica filler, non-agglomerated/non-aggregated 4–11 nm zirconia filler, and aggregated zirconia/silica cluster filler (comprised of 20 nm silica and 4–11 nm zirconia particles) |
|
| XP Bond (Dentsply) | Etch-and-rinse adhesive | 111202 | TCB resin, PENTA, UDMA, TEGDMA, HEMA, butylated benzenediol (stabilizer), ethyl-4-dimethylaminobenzoate, CQ, functionalized amorphous silica, t-butanol |
|
| Optibond XTR (Kerr Corp.) | Self-etch adhesive | 3793304 |
|
|
| Clearfil SE Protect (Kuraray Medical Inc.) | Self-etch adhesive | 061159 |
|
|
| Contour (Kerr Corp.) | Amalgam | – | Silver (41), Tin (31), Copper (28), Mercury: alloy ratio (47:53) |
|
CQ, camphorquinone; HEMA, 2-hydroxyethylmethacrylate; MDP, 10-methacryloyloxydecyl dihydrogen phosphate; MDPB, 12-methacryloylox- ydodecylpyridinium bromide; PENTA, phosphoric acid modified acrylate resin; TEGDMA, triethyleneglycol dimethacrylate; TCB resin, carboxylic acid modified dimethacrylate; UDMA, urethane dimethacrylate.
The surface of each disc was inoculated with 100 µl of a defined viable concentration of 1 × 108 cells/ml of anaerobically grown Streptococcus mutans, ATCC® 25175 in Brain Heart Infusion (BHI, 211059, Becton, Dickinson and Company, Franklin Lakes, NJ) broth. To analyze the viable cell concentration for each treatment group in colony forming units per ml (CFU/ml), the inoculated discs were anaerobically incubated in distilled water at 37 °C for 18 h. After incubation, each disc was transferred to individual tubes containing 9.9 ml of sterile BHI broth, which were then vortexed for 3 min to ensure uniform liberation of bacteria from the discs into solution. The suspension was then serially diluted and plated onto brain heart infusion (BHI, 221570, Becton, Dickinson and Company) agar for subsequent anaerobic incubation for 48 h at 37 °C, after which the total number of recovered CFU was determined.
XP Bond was selected as the representative adhesive blend to further evaluate antibacterial effectiveness after 1 year of ageing since this blend demonstrated both the best dispersion of PAA-CuI particles into solution and antimicrobial results. Additional discs were fabricated following the same procedures described above, for evaluation of the antibacterial properties of representative blends of XP Bond after 1 year of ageing in water. Discs corresponding to the following groups were evaluated: (1) control; (2) Clearfil SE Protect; (3) XP Bond; (4) XP Bond – 0.5 mg/ml PAA-CuI and (5) XP Bond – 1.0 mg/ml PAA-CuI. The discs were stored in individual wells containing 10 ml of distilled water (pH of 7.0) at room temperature for 1 year. The water was replaced once per week. After 1 year, the discs were re-evaluated following the same methods described above to determine antibacterial effectiveness.
2.3. Evaluation of the shear bond strength
Fifty non-carious human molars were obtained under a protocol approved by the MUSC Institutional Review Board. The teeth were obtained no more than ten days prior to the start of the experiment and stored in 1% chloramine solution until ready to be used. The crowns were separated from the roots with a slow-speed diamond saw (Isomet 1000, Buehler, Lake Bluff, IL) and embedded in a chemically polymerized methacrylate (Hygenic Hygon Tray Material, Coltène Whale-dent, Cuyahoga Falls, OH) with the facial surfaces exposed and ground flat on a model grinder (EcoMet 250, Buehler, Lake Bluff, IL) under running water to reveal superficial dentine, which was finished to 600-grit silicon carbide abrasive paper.
The specimens were equally and randomly assigned to five study groups with ten specimens per group (n = 10) to receive the following treatments: (1) XP Bond, (2) XP Bond – 0.5 mg/ml PAA-CuI, (3) XP Bond – 1.0 mg/ml PAA-CuI, (4) Optibond XTR and (5) Optibond XTR – 1.0 mg/ml PAA-CuI. All bonding procedures were performed according to manufacturers’ instructions (Table 1). The adhesives were applied and polymerized for 10 s with an LED light curing unit (VALO, Ultradent Products) and a power density of 1000 mW/cm2. After polymerization of the adhesives, resin composite cylinders were fabricated by use of a bonding jig (Ultradent Products) with a cylindrical mould of standardized dimensions (2.38 mm in diameter and 2 mm in height). The composite material (Filtek Supreme Ultra, 3M ESPE) was inserted into the mould in a single increment no greater than 2 mm and polymerized for 20 s. The specimens were then stored in distilled water at 37 °C for 24 h, after which shear bond strengths were determined using a calibrated testing device (UltraTester, Ultradent Products) loaded at a crosshead test speed of 1 mm/min and a load cell of 1000 lbs (453.6 kg). A shearing assembly with a notched-edge crosshead matching the diameter of the bonded cylinder was used to apply the testing load. The load required to debond the specimen was recorded and expressed in megapascals (MPa) and descriptive statistics determined.
Representative scanning electron microscope images were obtained to assess the different hybridization patterns observed in each of the failure modes. The fractured samples were sectioned longitudinally with a diamond saw (Isomet, Buehler) with copious water. The sectioned surface was polished with increasingly finer SiC paper up to 4000-grit (Buehler), and then treated with 50% phosphoric acid for 30 s and 5% NaOCl for 10 min. After air-drying the samples, they were sputter-coated with gold palladium (100 Å, Denton Vacuum Desk V) and examined with a scanning electron microscope (JEOL, 5600LV, Peabody, MA).
2.4. Evaluation of the cell viability of human gingival fibroblasts
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay (R&D Systems Inc., Minneapolis, MN) was used to assess cell metabolic function based on mitochondrial dehydrogenase activity. This colorimetric assay measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan.33 XP Bond was used as the representative adhesive blend to evaluate cytotoxicity since this blend showed both the best dispersion of PAA-CuI particles into solution and antimicrobial results. Representative adhesive blends of XP Bond modified with CuI particles were compared with unmodified XP Bond adhesive, which served as control and whose survival rates were set to represent 100% viability. Discs of 8 mm in diameter and 1 mm in thickness were fabricated, under aseptic conditions, by micropipeting XP Bond and XP Bond modified with 0.5 mg/ml PAA-CuI or 1.0 mg/ml PAA-CuI into a custom mould against microscope glass slabs and polymerized for 20 s from both top and bottom with LED light curing unit (VALO, Ultradent Products). The discs were sterilized by suspension in 70% ethanol for 10 min before the experiment. The following groups were evaluated in this part of the study (n = 3): (1) XP Bond, (2) XP Bond – 0.5 mg/ml PAA-CuI, (3) XP Bond – 1.0 mg/ml PAA-CuI, and (4) serum free media (SFM).
Human gingival fibroblast cells (HGF; ScienCell Inc., Cedro Carlsbad, CA, USA) were cultured with gingival fibroblast medium (500 ml of basal medium, 10 ml of foetal bovine serum, 5 ml of fibroblast growth supplement, and 5 ml of penicillin/streptomycin solution; pH 7.4; ScienCell Inc.) at 37 °C in humidified 5% CO2 and grown to 80% confluence. A seeding density of 100,000 cells/well was used in 6 well plates of 9.6cm2. This media was used to incubate discs corresponding to groups 1, 2 and 3. Group 4 was incubated using media without serum to determine cell viability in the absence of nutrients provided by serum. After 24 h of incubation, the media was filtered through sterile 0.2 µm syringe filters (Luer-Lock, Thomas Scientific Inc., Swedesboro, NJ, USA) and added to cell culture wells for 36-h incubation. The cells were then incubated for4 h in 1 ml of MTT solution at 37 °C in 5% CO2 in an incubator. After removal of the MTT solution, the cells were washed 3 times with PBS (pH 7.4). The insoluble formazan product was dissolved by incubation with 1 ml of a developing solution (10% sodium dodecyl sulfate (SDS)/0.01 M HCl), which was added to the attached cells and kept for 30 min on a shaker. Absorbance values were spectrophotometrically measured using an excitation filter of 485 nm and emission filter of 612 nm. These experiments were repeated in triplicate and normalized to the XP Bond control to determine percent viability for each group relative to the control.
2.5. Statistical analyses
The sample size for the evaluation of the antibacterial activity and shear bond strength of PAA-CuI adhesives was determined following a priori power analysis based on Imazato et al.32 and Duarte et al.34 For assessment of the antibacterial efficacy of dental adhesives, since the limit of detection for cell counts was 1000 CFU/ml, all discs that exhibited cell counts below the detection limits were set to 1000. The resultant cell count was transformed to a logarithmic scale (log) prior to the analysis to create a normal distribution of the data. Both bond strength and cell viability data were normally distributed. A one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test for pairwise multiple comparisons among group means were used for analysis of the antibacterial, bond strength and cell viability data. All analyses were conducted with statistical software SAS v. 9.3 (SAS Institute, Cary, NC) with a significance level of p < 0.05.
3. Results
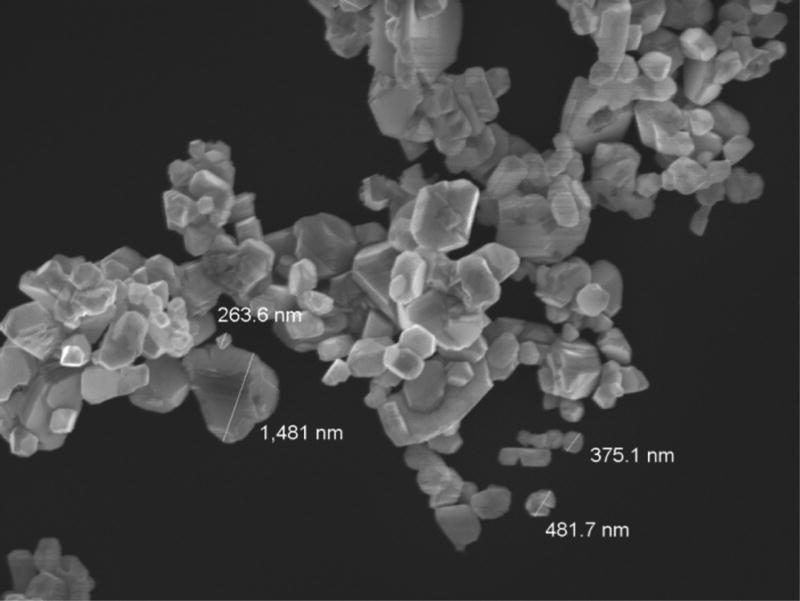
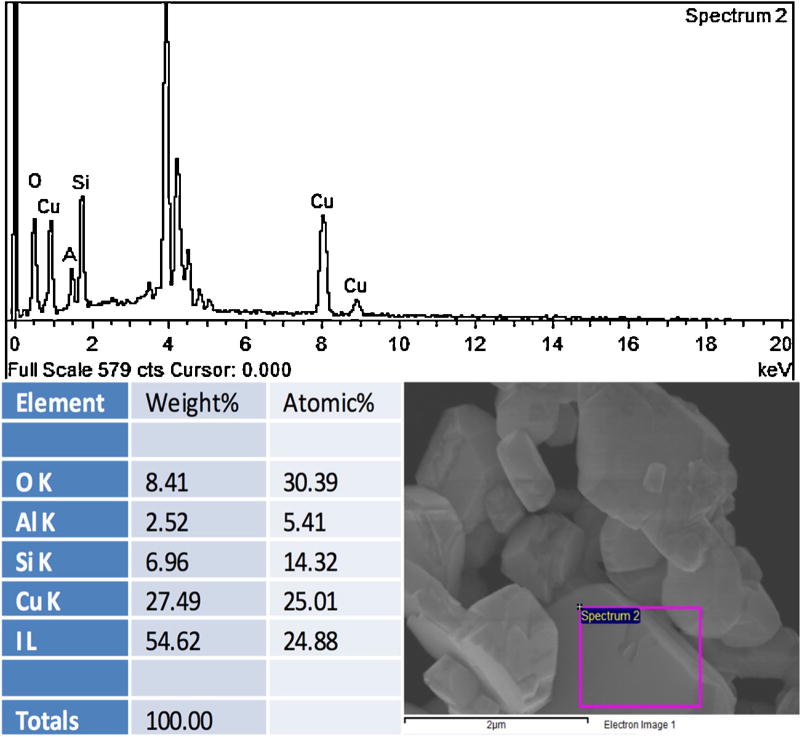
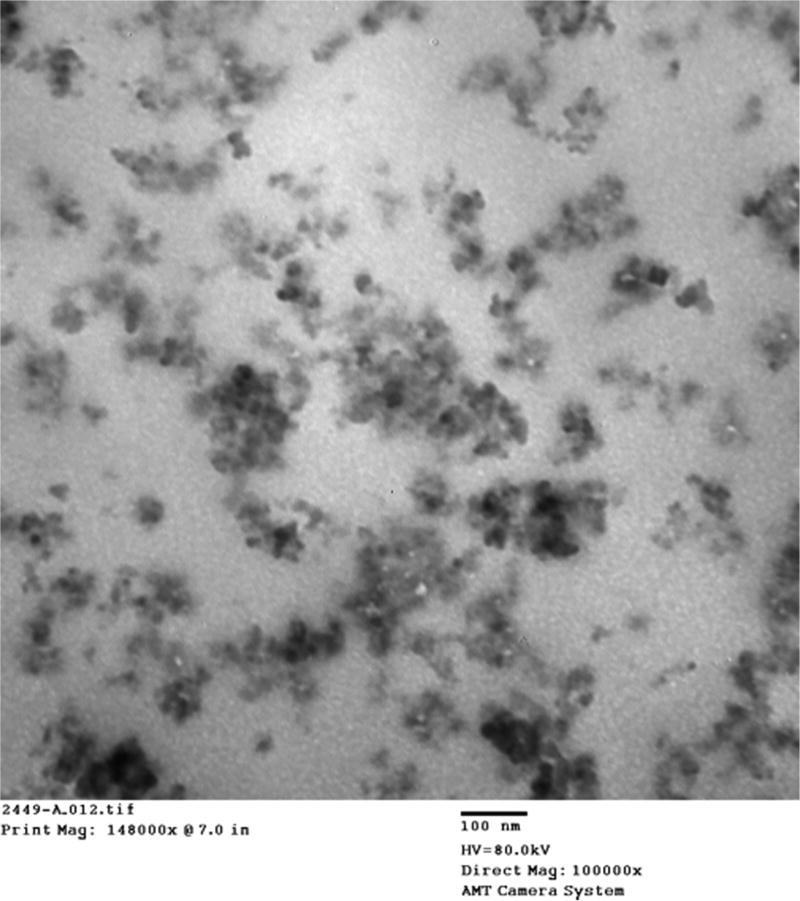
The FE-SEM micrograph depicted in Fig. 1 reveals the crystallinity and size poly-dispersity of the synthesized PAA-CuI particles. This poly-dispersity is characteristic of the synthetic method used in this work. The particle sizes depicted in Fig. 1 ranged from 20 nm to 1.5 µm. As demonstrated in the EDX spectrum of a representative specimen (Fig. 2), the ratio of Cu (K-line) to I (L-line) close to 1:1 confirmed the composition of these particles as copper iodide. The observed small EDX signals from oxygen, silicon and aluminium were most likely originated from impurities and/or underlying substrate. Successful incorporation of PAA-CuI particles into the adhesive blends was demonstrated in the TEM micrograph of a representative sample of XP Bond – 0.5 mg/ml PAA-CuI (Fig. 3). The micrograph shows adequate dispersion of the particles with some agglomeration. Most agglomerations were only 100 nm in diameter.
Fig. 1.
Scanning electron micrograph of the synthesized PAA-CuI powder demonstrating poly-dispersity of particles ranging from nano to micron size.
Fig. 2.
EDX spectrum (a) from selected area of the PAA-CuI powder sample outlined by a magenta rectangle in (b). The figure table summarizes the elemental composition of the sample area outlined.
Fig. 3.
Transmission electron micrograph of a representative sample of XP Bond – 0.5 mg/ml PAA-CuI demonstrating adequate dispersion of particles with agglomeration.
3.1. Assessment of the antibacterial activity
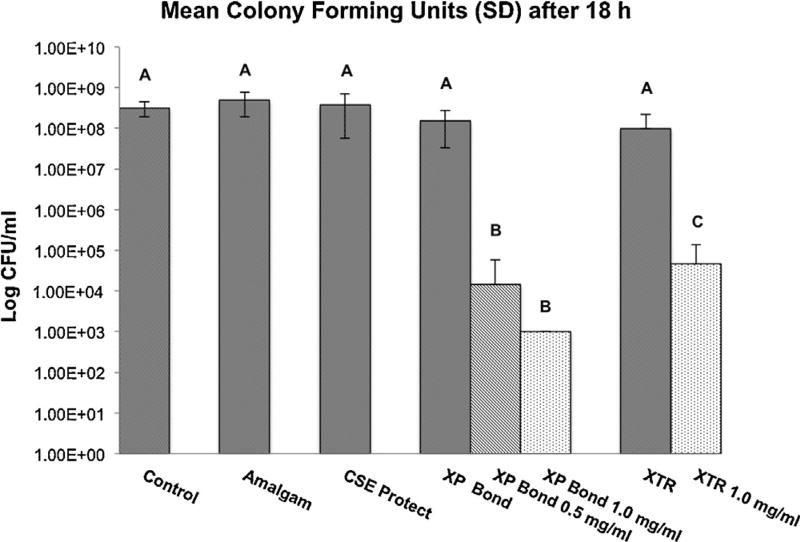
The mean log viable Streptococcus mutans cell concentration for each treatment is summarized in Fig. 4. No significant differences were demonstrated in the log viable cell concentration recovered from the control, amalgam, Clearfil SE Protect, XP Bond and Optibond XTR groups. Significantly lower mean log viable cell concentration was observed for experimental groups, XP Bond – 0.5 mg/ml PAA-CuI, XP Bond – 1.0 mg/ml PAA-CuI and Optibond XTR – 1.0 mg/ml PAA-CuI (p < 0.001) relative to all the control groups. Relative to the control group, discs treated with XP Bond – 0.5 mg/ml PAA-CuI, XP Bond – 1.0 mg/ml PAA-CuI and Optibond XTR – 1.0 mg/ml PAA-CuI demonstrated a reduction in viable cell count of 99.99%, 99.99% and 79.65%, respectively (Fig. 4). Both XP Bond – 0.5 mg/ml PAA-CuI and XP Bond – 1.0 mg/ml PAA-CuI, in turn, demonstrated significantly lower mean log viable cell concentration relative to Optibond XTR – 1.0 mg/ml PAA-CuI (p = 0.0253 and p = 0.0114, respectively).
Fig. 4.
Mean log viable cell concentration of the Streptococcus mutans biofilms in colony-forming units (CFU) after 18 h of anaerobic incubation of control and experimental discs in water (mean ± SD; n = 8). The experimental resin discs were coated with PAA-CuI nanoparticles containing-adhesives XP Bond and Optibond XTR. All discs were inoculated with a defined viable concentration of 1 × 108 cells/ml of Streptococcus mutans prior to incubation. Different letters indicate groups that are significantly different from each other (Tukey’s test; p < 0.05).
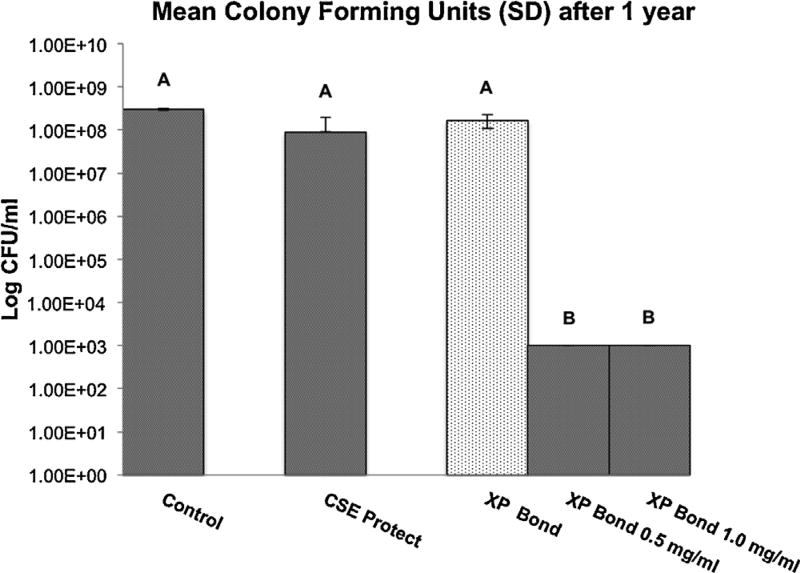
Fig. 5 shows log viable Streptococcus mutans cell concentration recovered after 1 year of incubation of the resin discs in distilled water. No differences in log viable cell concentration were shown between the control, XP Bond and Clearfil SE Protect groups. Discs treated with XP Bond – 0.5 mg/ml PAA-CuI and XP Bond – 1.0 mg/ml PAA-CuI both demonstrated a significantly lower mean log viable cell concentration relative to the three control groups (p < 0.001), with a 99.99% reduction in viable cell count for both groups relative to the control groups.
Fig. 5.
Mean log viable cell concentration of the Streptococcus mutans biofilms in colony-forming units (CFU) after 1 year of anaerobic incubation of control and experimental discs in water (mean ± SD; n = 8). The experimental resin discs were coated with PAA-CuI nanoparticles containing-adhesives XP Bond. All discs were inoculated with a defined viable concentration of 1 × 108 cells/ml of Streptococcus mutans prior to incubation. Different letters indicate groups that are significantly different from each other (Tukey’s test; p < 0.05).
3.2. Evaluation of the shear bond strength
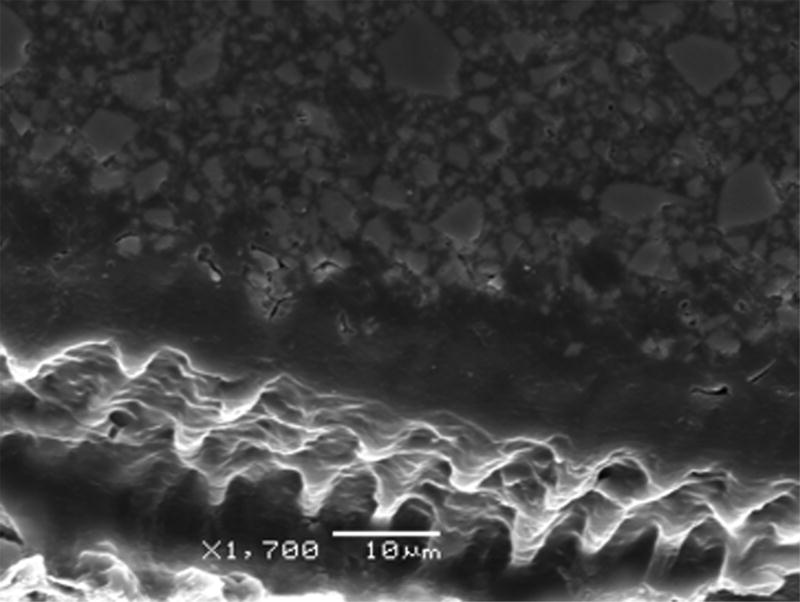
Mean bond strength values for the study groups are summarized in Table 2. A significant effect of the treatment on bond strength was demonstrated (p = 0.006). Post hoc multiple comparisons with Tukey’s test, however, revealed no differences between each of the control adhesive blends, XP Bond and Optibond XTR, and their corresponding experimental blends modified with PAA-CuI particles (Table 2). Significant differences were only observed between the control adhesive Optibond XTR and experimental blends of XP Bond, XP Bond – 0.5 mg/ml PAA-CuI and XP Bond – 1.0 mg/ml PAA-CuI, both of which demonstrated significantly lower bond strengths than Optibond XTR (p = 0.032 and p = 0.024, respectively). The scanning electron micrograph depicted in Fig. 6 shows hybridization with resin tag formation at the interdiffusion zone after demineralization and infiltration with experimental XP Bond – 1.0 mg/ml PAA-CuI.
Table 2.
Mean shear bond strength values in MPa (SD) for the study groups at 24 h (n = 10).
| Treatment group | Mean bond strength (MPa ± SD) |
|---|---|
| XP Bond | 20.8 (6.8)a,b |
| XP Bond – 0.5 mg/ml PAA-CuI | 18.7 (2.2)b |
| XP Bond – 1.0 mg/ml PAA-CuI | 18.4 (2.9)b |
| Optibond XTR | 25.6 (4.9)a |
| Optibond XTR-1.0 mg/ml PAA-CuI | 24.5 (7.0)a,b |
Different superscript letters indicate significant differences between groups (Tukey’s test, p < 0.05).
Fig. 6.
Scanning electron micrograph showing hybridization with resin tag formation at the interdiffusion zone after demineralization and infiltration with experimental XP Bond – 1.0 mg/ml PAA-CuI.
3.3. Evaluation of the cell viability of human gingival fibroblasts
Fig. 7 summarizes the MTT assay results of cytotoxicity against human gingival fibroblast-like cells. One-way ANOVA demonstrated no significant differences in cell viability among the groups evaluated, XP Bond, XP Bond – 0.5 mg/ml PAA-CuI, XP Bond – 1.0 mg/ml PAA-CuI, and serum free media (SFM).
Fig. 7.
MTT assay results of cytotoxicity against human gingival fibroblast-like cells obtained from cured resin eluates. Blends of XP Bond containing PAA-CuI nanoparticles were compared with unmodified XP Bond (control), whose survival rates were set to represent 100% viability. Results are expressed in percentage of viable cells. Bars represent mean ± SD (n = 3). Different letters indicate groups that are significantly different from each other (Tukey’s test; p < 0.05).
4. Discussion
The present study evaluated the incorporation of PAA-coated CuI particles into adhesive resin blends, and investigated for the first time, the effect of PAA-CuI modified adhesives on antibacterial activity against Streptococcus mutans, dentine bond strength and cytotoxicity to mammalian cells. The first null hypothesis was rejected since the adhesive blends containing the two different concentrations of PAA-coated CuI particles, demonstrated significant antibacterial activity against Streptococcus mutans. Incorporation of the PAA-CuI particles caused bacterial cell death in the range of 79.65–99.99% compared to the controls. XP Bond – 1.0 mg/ml PAA-CuI had a dramatic effect on the viability of Streptococcus mutans, essentially dropping viable cell count from over one and a half million to less than one thousand. Similar results were obtained after storage of the resin discs for one year in distilled water. The second null hypothesis was accepted since incorporation of PAA-CuI particles did not affect the bond strength to dentine relative to their corresponding control adhesives. The third null hypothesis was also accepted since incorporation of PAA-coated CuI particles into adhesive resins yielded no appreciable effect on fibroblast cytotoxicity compared to the control adhesives. Our results provide an in vitro proof-of-principal demonstrating that incorporation of copper iodide particles into two commercially available dental adhesives can provide strong antibacterial properties without adversely affecting their immediate bond strength to dentine or cytotoxicity.
It is generally accepted that copper’s mechanism of action is through the collective disruption of the bacterial membrane proteins and DNA, through the generation of free radicals, manifested by an electrical imbalance introduced as a consequence of the conductivity and proximity of bacteria to copper.35–38 At a concentration of 0.5 mg/ml, PAA-CuI particles were shown to be bactericidal to Streptococcus mutans, but did not show any cell cytotoxicity relative to the control. The antibacterial effects of PAA-coated CuI particles in polymerized resin discs may be due to a slow release of CuI into the medium. However, since these particles were completely enveloped by polymerized resin, it is unknown whether CuI particles were actually able to leach into the media. Future experiments will include placing PAA-CuI particles in resin discs on agar plates covered with test bacterial colonies to see if bacteria are killed only by contact with the discs or if there is a zone of inhibition around the discs suggesting leaching of the antibacterial agent from the discs.
Agents such as quaternary ammonium compounds and fluoride may have a limited antimicrobial lifespan in situ due to leaching or inactivation during polymerization.39 The use of leachable agents may thus provide a potent effect initially, but this effect is expected to decrease over time eventually becoming insufficient. The ultimate goal is to develop agents that will be able to provide sustained antibacterial benefits over long time periods (years). By providing a profound antibacterial effect at the dentine-resin interface several years after placement, we speculate that one may be able to contribute to an overall increase in the longevity of adhesive restorations. The long-term efficacy of PAA CuI-containing adhesive resins was demonstrated with the one-year data, which showed similar significant antibacterial activity against Streptococcus mutans to the initial assay after 18 h.
The SEM and TEM micrographs demonstrate the polydispersity and wide range of particle sizes used in the experimental adhesive blends ranging from nano to micron size. Ideally, the particles should be able to fit within the intercollagen spaces that are approximately 20 nm in size.39 This would allow PAA-CuI particles to become integrated within the hybrid layer making it easier to exert their antibacterial properties in this extremely vulnerable space. Polyacrylic acid was used to coat the particles in the present study. This coating was intended to prevent clumping of the particles and optimize their mixing in hydrophilic media such as the monomer 2-hydroxyethyl methacrylate found in dental adhesive primers. One wonders what the particle size of CuI would be without the PAA coating, and whether the depth of infiltration into the nanospaces of the interfibrillar collagen varies considerably with and without the PAA coating. Future experiments will use a nanoparticle size analyzer to investigate the particle size distribution with and without PAA.
In our study, Clearfil SE Protect, which contains 12-methacryloyloxydodecylpyridinium bromide (MDPB), a known quaternary ammonium methacrylate with antibacterial properties, did not demonstrate effective antibacterial properties against Streptococcus mutans after polymerization. Most of the available studies have reported that MDPB is a highly effective antimicrobial agent prior to polymerization, and that it does not interfere with the mechanical properties or colour of the resin when incorporated into adhesive blends.40–43 Not enough evidence, however, is available on the long-term antibacterial efficacy of MDPB once it has been polymerized, and it has been suggested to be short lived possibly due to the decreased antimicrobial activity after light polymerization.39 A study evaluating the antibacterial properties of different adhesives long-term found that MDPD lost its antibacterial properties after 14 days.44 Our results of no appreciable bactericidal activity for MDPB after 1 year of ageing in water support these findings. However, the absence of appreciable antibacterial activity for MDPB after 18 h found in our study, differ from the results reported by Imazato et al.,32 and may be the result of a myriad of factors. First, the concentration of Streptococcus mutans used to inoculate the discs varied between studies. While Imazato et al. inoculated the discs with Streptococcus mutans at a concentration of 1 × 105 CFU/ml,32 we used a concentration of 1 × 108 ells/ml. This higher concentration of Streptococcus mutans appears to have completely overcome the effectiveness of polymerized MDPB.45 Our study, different from most studies reporting on the antibacterial effectiveness of MDPB, reported on the antibacterial properties of different adhesive blends after polymerization. It has been reported that light polymerization significantly reduces its antibacterial activity.46
The present work was intended to investigate, for the first time, incorporation of PAA-CuI as a possible antibacterial additive to adhesive resins. Effective antibacterial properties without cytotoxicity or adverse effects in the initial bond strength were demonstrated in this study. Further testing is necessary to determine if the use of PAA-CuI modified adhesive resins can prevent recurrent caries in vivo. Future studies should be undertaken to evaluate other bacterial strains also known to play a role in the carious process such as Actinomyces spp. and Lactobacillus, and to investigate the antibacterial properties of PAA-CuI modified adhesives across all bacterial types. In an attempt to reproduce, as closely as possible, true oral conditions observed at the dentine- adhesive interface, we elected to conduct the present study under anaerobic conditions. Future bacterial testing under aerobic conditions should also be undertaken as these could provide valuable information as to the behaviour of CuI modified resin blends at the interface between the restoration and the oral cavity. Eventually, tests utilizing a split mouth design in an animal model should be performed to determine the effectiveness of these antibacterial modified blends in an oral environment. The development of adhesive systems with sustained anti-bacterial properties may considerably increase the longevity of adhesive restorations, limiting the frequency with which they require replacement and thus contributing to increasing the overall lifespan of the individual tooth.
5. Conclusions
Within the limitations of this study, it can be concluded that dental adhesives incorporating PAA-CuI particles not only demonstrated effective antibacterial properties against Streptococcus mutans, but also equivalent shear bond strength and no cytotoxicity relative to the control adhesive blends. The antibacterial benefits that can be derived from the incorporation of PAA-CuI particles into the adhesive blends without adversely affecting the adhesives bond strength and biocompatibility may contribute to an increase in the longevity of adhesive restorations by preventing microbial invasion at the restorative tooth interface.
Acknowledgments
The authors gratefully acknowledge the financial support from SC COBRE for Oral Health Research P20RR017696 and the Medical University of South Carolina Center for Oral Health Research. Thanks to Dr. David Sentelle for his invaluable collaboration with the cytotoxicity analysis, Dr. Michael Schmidt for his assistance with the antimicrobial experiments and Dr. George Chumanov for his significant contributions with the chemical synthesis and imaging of the PAA-CuI nanoparticles.
Footnotes
The authors declare no other conflicts.
References
- 1.Christensen GJ. Should resin-based composite dominate restorative dentistry today? Journal of the American Dental Association. 2010;141:1490–3. doi: 10.14219/jada.archive.2010.0112. [DOI] [PubMed] [Google Scholar]
- 2.Khalichi P, Cvitkovitch DG, Santerre JP. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004;25:5467–72. doi: 10.1016/j.biomaterials.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Godoy F, Krämer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs in vivo. Dental Materials. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Kramer N, Garcia-Godoy F, Frankenberger R. Evaluation of resin composite materials Part II: In vivo investigations. American Journal of Dentistry. 2005;18:75–81. [PubMed] [Google Scholar]
- 5.Manhart J, Chen H, Hamm G, Hickel R. Buonocore Memorial Lecture Review of the clinical survival of direct and indirect restorations in posterior teeth of the permanent dentition. Operative Dentistry. 2004;29:481–508. [PubMed] [Google Scholar]
- 6.Levin L, Coval M, Geiger SB. Cross-sectional radiographic survey of amalgam and resin-based composite posterior restorations. Quintessence International. 2007;38:511–4. [PubMed] [Google Scholar]
- 7.Simecek JW, Diefenderfer KE, Cohen ME. An evaluation of replacement rates for posterior resin-based composite and amalgam restorations in U.S. Navy and marine corps recruits. Journal of the American Dental Association. 2009;140:200–9. doi: 10.14219/jada.archive.2009.0134. quiz 49. [DOI] [PubMed] [Google Scholar]
- 8.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Reports. 2007;122:657–63. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. Journal of the American Dental Association. 2007;138:775–83. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 10.DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitao J, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. Journal of the American Medical Association. 2006;295:1784–92. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 11.Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Critical Reviews in Oral Biology and Medicine. 2002;13:509–20. doi: 10.1177/154411130201300607. [DOI] [PubMed] [Google Scholar]
- 12.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations FDI Commission Project 2–95. International Dental Journal. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 13.Mjor IA, Dahl JE, Moorhead JE. Age of restorations at replacement in permanent teeth in general dental practice. Acta Odontologica Scandinavica. 2000;58:97–101. doi: 10.1080/000163500429208. [DOI] [PubMed] [Google Scholar]
- 14.Collins CJ, Bryant RW, Hodge KL. A clinical evaluation of posterior composite resin restorations: 8-year findings. Journal of Dentistry. 1998;26:311–7. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 15.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings from the New England Children’s Amalgam Trial. Journal of the American Dental Association. 2007;138:763–72. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 16.Mohan R, Shanmugharaj AM, Sung Hun R. An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;96:119–26. doi: 10.1002/jbm.b.31747. [DOI] [PubMed] [Google Scholar]
- 17.Shaini FJ, Fleming GJ, Shortall AC, Marquis PM. A comparison of the mechanical properties of a gallium-based alloy with a spherical high-copper amalgam. Dental Materials. 2001;17:142–8. doi: 10.1016/s0109-5641(00)00054-3. [DOI] [PubMed] [Google Scholar]
- 18.Orstavik D. Antibacterial properties of and element release from some dental amalgams. Acta Odontologica Scandinavica. 1985;43:231–9. doi: 10.3109/00016358509046503. [DOI] [PubMed] [Google Scholar]
- 19.Hickel R, Manhart J. Longevity of restorations in posterior teeth and reasons for failure. Journal of Adhesive Dentistry. 2001;3:45–64. [PubMed] [Google Scholar]
- 20.Hicks J, Garcia-Godoy F, Donly K, Flaitz C. Fluoride-releasing restorative materials and secondary caries. Journal of the California Dental Association. 2003;31:229–45. [PubMed] [Google Scholar]
- 21.Murray PE, Hafez AA, Smith AJ, Cox CF. Bacterial microleakage and pulp inflammation associated with various restorative materials. Dental Materials. 2002;18:470–8. doi: 10.1016/s0109-5641(01)00072-0. [DOI] [PubMed] [Google Scholar]
- 22.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, et al. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. Journal of Dentistry. 2011;39:238–48. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allaker RP. The use of nanoparticles to control oral biofilm formation. Journal of Dental Research. 2010;89:1175–86. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Lee DK, Cha HG, Kim CW, Kang YC, Kang YS. Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles. Journal of Physical Chemistry B. 2006;110:24923–8. doi: 10.1021/jp0656779. [DOI] [PubMed] [Google Scholar]
- 26.Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia. 2008;4:707–16. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Yoon KY, Byeon JH, Park J-H, Ji JH, Bae GN, Hwang J. Antimicrobial characteristics of silver aerosol nanoparticles against Bacillus subtilis bioaerosols. Environmental Engineering Science. 2008;25:289–94. [Google Scholar]
- 28.Yamamoto K, Ohashi S, Aono M, Kokubo T, Yamada I, Yamauchi J. Antibacterial activity of silver ions implanted in SiO2 filler on oral Streptococci. Dental Materials. 1996;12:227–9. doi: 10.1016/s0109-5641(96)80027-3. [DOI] [PubMed] [Google Scholar]
- 29.Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dental Materials. 2009;25:206–13. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 31.Rosalen PL, Bowen WH, Pearson SK. Effect of copper co-crystallized with sugar on caries development in desalivated rats. Caries Research. 1996;30:367–72. doi: 10.1159/000262344. [DOI] [PubMed] [Google Scholar]
- 32.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 33.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte S, Jr, Phark JH, Varjao FM, Sadan A. Nanoleakage, ultramorphological characteristics, and microtensile bond strengths of a new low-shrinkage composite to dentin after artificial aging. Dental Materials. 2009;25:589–600. doi: 10.1016/j.dental.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Espirito Santo C, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, et al. Bacterial killing by dry metallic copper surfaces. Applied and Environmental Microbiology. 2011;77:794–802. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Applied and Environmental Microbiology. 2011;77:1541–7. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santo CE, Quaranta D, Grass G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. MicrobiologyOpen. 2012;1:46–52. doi: 10.1002/mbo3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warnes SL, Caves V, Keevil CW. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environmental Microbiology. 2012;14:1730–43. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 39.da Silva BM, Franca FM, Florio FM, Basting RT. In situ anticariogenic effect of adhesive systems containing fluoride and MDPB. American Journal of Dentistry. 2010;23:75–80. [PubMed] [Google Scholar]
- 40.Giammanco GM, Cumbo EM, Luciani A, Gallina G, Mammina C, Pizzo G. In vitro evaluation of the antibacterial activity of cured dentin/enamel adhesive incorporating the antimicrobial agent MDPB. New Microbiologica. 2009;32:385–90. [PubMed] [Google Scholar]
- 41.Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Operative Dentistry. 2004;29:369–75. [PubMed] [Google Scholar]
- 42.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. Journal of Dental Research. 1997;76:768–72. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 43.Imazato S, Torii Y, Takatsuka T, Inoue K, Ebi N, Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. Journal of Oral Rehabilitation. 2001;28:314–9. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 44.Feuerstein O, Matalon S, Slutzky H, Weiss EI. Antibacterial properties of self-etching dental adhesive systems. Journal of the American Dental Association. 2007;138:349–54. doi: 10.14219/jada.archive.2007.0167. quiz 96–8. [DOI] [PubMed] [Google Scholar]
- 45.Thoden van Velzen SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. Journal of Clinical Periodontology. 1984;11:209–20. doi: 10.1111/j.1600-051x.1984.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 46.Gondim JO, Duque C, Hebling J, Giro EM. Influence of human dentine on the antibacterial activity of self-etching adhesive systems against cariogenic bacteria. Journal of Dentistry. 2008;36:241–8. doi: 10.1016/j.jdent.2007.12.007. [DOI] [PubMed] [Google Scholar]