Abstract
Background
Hypertension is more common in patients with rheumatoid arthritis (RA) than in the general population. It is unknown whether hypertension is due to RA-related medications or the disease itself. Therefore, we sought to investigate associations between RA-related autoantibodies, specifically, antibodies to citrullinated protein antigens (ACPA) and systolic (SBP) and diastolic (DBP) blood pressure in first-degree relatives of RA patients, who were free of RA and RA-related medications. We hypothesized that greater number of detectable ACPA would be associated with high SBP and DBP, independent of other risk factors in these first-degree relatives.
Methods
We evaluated associations between ACPA and SBP and DBP in a cross-sectional study of 72 first-degree relatives [defined as parent, child, or sibling] of RA patients. Fifteen ACPA were measured using a Bio-Plex bead-based assay; each was dichotomized as positive/negative based on pre-specified cut-points. ANCOVA was used to evaluate associations between ACPA positivity and SBP and DBP, adjusting for age, sex, race, body mass index (BMI), pack-years of smoking, C-reactive protein (CRP), and current use of anti-hypertensive medications.
Results
Average age was 51, and 69% were women. Mean SBP was 119±18 and DBP was 74±9mmHg. Thirty-three (46%) first-degree relatives were positive for ≥1 ACPA; and were younger, had lower BMI, more pack-years of smoking, and higher hsCRP concentrations compared to ACPA negative first-degree relatives. For each additional positive ACPA, SBP was 0.98±0.5mmHg (p=0.05) higher, and DBP was 0.66±0.3mmHg (p=0.04) higher. Anti-cit-fibrinogen A (211-230) positive and anti-cit-filaggrin positive first-degree relatives had 11.5 and 13.9mmHg higher SBP (p=0.02), respectively. Anti-cit-clusterin, cit-filaggrin, and cit-vimentin positive first-degree relatives had 7-8mmHg higher DBP (p=0.03, 0.05, 0.05, respectively), compared to being negative for these individual ACPA. Consistent with associations between ACPA, SBP, and DBP, anti-CCP2 positive first-degree relatives had 16.4± (p=0.03) higher SBP and 12.1± mmHg (p=0.01) higher DBP than anti-CCP2 negative first-degree relatives.
Conclusion
In first-degree relatives without RA, ACPA positivity is associated with higher SBP and DBP. Subclinical autoimmune processes and ACPA may play a role in the vascular changes potentially leading to hypertension prior to RA onset.
Keywords: Epidemiology, blood pressure, autoimmunity
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that is characterized by immune dysregulation. Cardiovascular disease (CVD) risk is 48% higher in RA patients compared to the general population, even after adjusting for traditional CVD risk factors.1 Hypertension is also more common in patients with RA than in the general population (34% versus 23.4%),2 and may contribute to a higher CVD event rate.1, 3 It is unclear, however, whether hypertension is due to RA-related medications or the disease process itself. We hypothesize that immune dysregulation, inflammation, and vascular damage may occur in parallel, and prior to a formal diagnosis and articular manifestation of RA. It is known that RA-related autoantibodies are detectable years before the onset of RA.4 In particular, antibodies to citrullinated protein antigens (ACPA), have been shown to correlate with subclinical atherosclerosis and prevalent coronary artery calcification,5 even in the absence of RA,6 suggesting that subclinical autoimmune processes are associated with CVD. It is unknown, however, whether ACPA are associated with higher blood pressure. Furthermore, it is uncertain whether ACPA may be related to higher blood pressure in persons without clinically apparent RA. Here, we examined whether ACPA were associated with blood pressure in community-dwelling individuals who are first-degree relatives of RA patients, but who do not have RA themselves.
METHODS
The Studies of the Etiology of Rheumatoid Arthritis (SERA) is a multi-center prospective study that includes first-degree relatives [defined as a parent, child, or sibling] of probands with RA, and is designed to examine the effect of genetic and environmental factors on the development and progression of RA-related autoimmunity. SERA is also designed to explore the preclinical immunological changes and pathophysiological processes in the absence of medications and complications associated with active RA.7, 8 For this ancillary study, first-degree relatives were recruited at the Denver and Los Angeles sites, and were eligible if they were positive for rheumatoid factor and/or anti-cyclic citrullinated peptides (anti-CCP2) for at least 2 clinic visits (n=41), frequency matched on age and sex to first-degree relatives who were rheumatoid factor and anti-CCP2 negative for all of their clinic visits (n=45); and did not meet the 1987 ACR Criteria or 2010 EULAR/ACR Criteria for RA. The Institutional Review Boards approved this study, and all participants provided written informed consent.
Antibodies to 15 citrullinated protein antigens (ACPA) were measured in serum using a custom Bio-PlexTM (BioRad, Hercules, CA) bead-based autoantibody assay as described previously.9 ACPA positivity cutoffs were developed using receiver operating characteristic (ROC) curves of data from 200 RA probands and 98 blood donor controls. Cutoffs were defined by values that gave greater than 90% specificity for RA.9
During research clinic visits, first-degree relatives completed disease and exposure assessment questionnaires as well as anthropometric measurements (i.e., height, weight, waist circumference), underwent a standardized interview and 68-count joint examination by a trained health care practitioner, and had blood drawn. Systolic (SBP) and diastolic (DBP) blood pressure was measured during this clinic visit using a hand held sphygmanomometer. Two measurements were made in the same arm with a 30-second rest between measurements, consistent with previous validated methods.10 We used the average of these measurements as our blood pressure variable. Prevalent hypertension was defined as either SBP ≥ 140, DBP ≥ 90, or currently taking anti-hypertensive medications.
For the current cross-sectional study, the timing of ACPA measurements were within 2 years of blood pressure measurements; 14 first-degree relatives lacked ACPA measurements, and were thus excluded. Among the remaining 72 first-degree relatives, we utilized generalized linear models and analysis of covariance (ANCOVA); and adjusted for age, sex, race, body mass index (BMI) kg/m2, pack-years of smoking, high sensitivity C-reactive protein (CRP), and current use of anti-hypertensive medications. Our primary analyses evaluated the number of positive ACPA as the predictor variable of interest, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) as the outcome variables of interest. Subsequently, we evaluated the associations of individual ACPA measurements with SBP and DBP. Given that non-steroidal anti-inflammatory medications (NSAIDs) can contribute to hypertension, we performed a sensitivity analysis excluding 15%, or 11 total, first-degree relatives who were taking NSAIDs. In addition, although our main analysis adjusted for 18%, or 13 total, first-degree relatives who were currently taking anti-hypertensive medications, we performed a second sensitivity analysis excluding these 13 individuals. Given our a priori objective to evaluate associations between individual ACPA and SBP and DBP, we did not adjust for multiple comparisons. All analyses were conducted in SAS (SAS Version 9.4, Cary, North Carolina), and p-values < 0.05 were considered statistically significant.
RESULTS
The mean age of the 72 person study sample of first-degree relatives was 51± 14 years; 69% were women, and 28% had prevalent hypertension. Thirty-three (46%) were positive for at least one ACPA, and among these, the median number of ACPA that were positive was 1 (interquartile range 1 to 6). Study participants who were positive for at least one ACPA were younger, had a lower BMI, lower concentration of CRP, and lower frequency of anti-hypertensive medication use. ACPA positive and negative first-degree relatives had similar distributions of sex, race, pack-years of smoking (Table 1).
Table 1.
Characteristics of First-Degree Relatives by ACPA Positivity; and ACPA Distribution Among ACPA Positive First-Degree Relatives in the Studies of the Etiology of Rheumatoid Arthritis (SERA)
| ACPA (+) (n=33) | ACPA (−) (n=39) | p-value | |
|---|---|---|---|
| Mean age, years (SD) | 49.8 (14) | 52.5 (14) | 0.41 |
| Female, % | 70 | 69 | 0.97 |
| Non-Hispanic White race, % | 91 | 87 | 0.62 |
| Mean BMI, kg/m (SD) | 26.4 (5) | 29.2 (7) | 0.06 |
| Median pack-years smoking (25th, 75th percentile) | 2.0 (1, 2) | 2.0 (1, 2) | 0.58 |
| Median hsCRP (25th, 75th percentile) | 1.2 (0.7, 6) | 2.1 (0.6, 4) | 0.96 |
| Anti-hypertensive medications, % | 12 | 23 | 0.35 |
| Mean Systolic BP, mmHg (SD) | 119.3 (17) | 119.6 (19) | 0.95 |
| Mean Diastolic BP, mmHg (SD) | 74.4 (8) | 73.0 (9) | 0.53 |
| Rheumatoid factor positive, % | 15 | 8 | 0.32 |
| Anti-CCP2 positive, % | 9 | 3 | 0.33 |
|
| |||
| ACPA Distribution among ACPA (+) FDRs | N (%)* | ||
|
| |||
| Histone 2A (1-20) cit cyclic | 13 (18.1) | – | |
| Histone 2B (62-81) cit cyclic | 13 (18.1) | – | |
| Fibrinogen A (556-575) cit cyclic | 11 (15.3) | – | |
| Fibrinogen A cit | 11 (15.3) | – | |
| Apolipo E (277-296) cit2 cyclic | 10 (13.9) | – | |
| Fibrinogen A (211-230) cit cyclic | 10 (13.9) | – | |
| Fibrinogen A (41-60) cit3 cyclic | 10 (13.9) | – | |
| Biglycan (247-266) cit cyclic | 9 (12.5) | – | |
| Clusterin (221-240) cit | 8 (11.1) | – | |
| Enolase (5-21) cit | 8 (11.1) | – | |
| Vimentin cit | 8 (11.1) | – | |
| Filaggrin (48-65) cit2 cyclic | 7 (9.7) | – | |
| Fibrinogen A (616-635) cit3 cyclic | 5 (6.9) | – | |
| Histone 2B-cit | 5 (6.9) | – | |
| Vimentin (58-77) cit3 cyclic | 3 (4.2) | – | |
first-degree relatives may be positive for multiple ACPA. Thus, these proportions do not add to 100%.
Adjusting for age and gender, posivity for each additional ACPA was associated with 1 ± 0.55 mmHg higher SBP (p=0.07) and a 0.60 ± 0.31 mmHg higher DBP (p=0.06). When evaluated in a fully adjusted model, each additional ACPA was significantly associated with 0.98 ± 0.50 mmHg higher SBP (p=0.05) and 0.66 ± 0.30 mmHg higher DBP (p=0.04) (Table 2). When evaluating individuals with any positive ACPA vs. none, associations were not significantly different in unadjusted or fully adjusted models, but those with any ACPA had higher mean SBP and DBP measurements as indicated by the point estimates (Table 2).
Table 2.
The Difference in Systolic Blood Pressure and Diastolic Blood Pressure for Each Additional Positive ACPA; and for ACPA Positive versus ACPA Negative First-Degree Relatives in the Studies of the Etiology of Rheumatoid Arthritis (SERA)
| SBP mmHg | DBP mmHg | |||
|---|---|---|---|---|
|
|
||||
| β (SD) | p-value | β (SD) | p-value | |
| Number of ACPA | ||||
| Adjusted for age, sex, race | 1.02 (0.55) | 0.07 | 0.60 (0.31) | 0.06 |
| Fully adjusted* | 0.98 (0.48) | 0.05 | 0.66 (0.30) | 0.04 |
| Positive for Any ACPA | ||||
| Adjusted for age, sex, race | 1.65 (3.83) | 0.67 | 1.62 (2.17) | 0.46 |
| Fully adjusted* | 4.79 (3.40) | 0.16 | 3.79 (2.17) | 0.09 |
Number of ACPA ranged from 0 to 15. There were 33 first-degree relatives who were positive for ≥1 ACPA, and 39 first-degree relatives who were negative for any ACPA. Fully adjusted analyses accounted for age, sex, race, BMI, pack-years of smoking, hsCRP, and current use of anti-hypertensive medications
We next evaluated associations of rheumatoid factor, anti-CCP2, and individual ACPA with SBP and DBP. First-degree relatives who were positive for antibodies to Filaggrin (48-65) cit2 cyclic had SBP 14 mmHg higher (p=0.017) than first-degree relatives who were negative for this ACPA. Similarly, first-degree relatives who were positive for antibodies to Fibrinogen A (211-230) cit cyclic had a 12 mmHg higher SBP (p=0.022) than first-degree relatives who were negative. Associations between anti-CCP2 and SBP were similar, where first-degree relatives positive for anti-CCP2 had 16 mmHg higher SBP (p=0.027) than anti-CCP2 negative first-degree relatives. There was no association between rheumatoid factor and SBP. Notably, regardless of which individual ACPA was evaluated, and their individual statistical significance, positivity for each ACPA was associated with higher SBP than in those who were negative across the panel of ACPA measurements (Figure 1).
Figure 1. The Difference in Diastolic Blood Pressure and Systolic Blood Pressure in ACPA Positive versus ACPA Negative First-Degree Relatives in the Studies of the Etiology of Rheumatoid Arthritis (SERA).
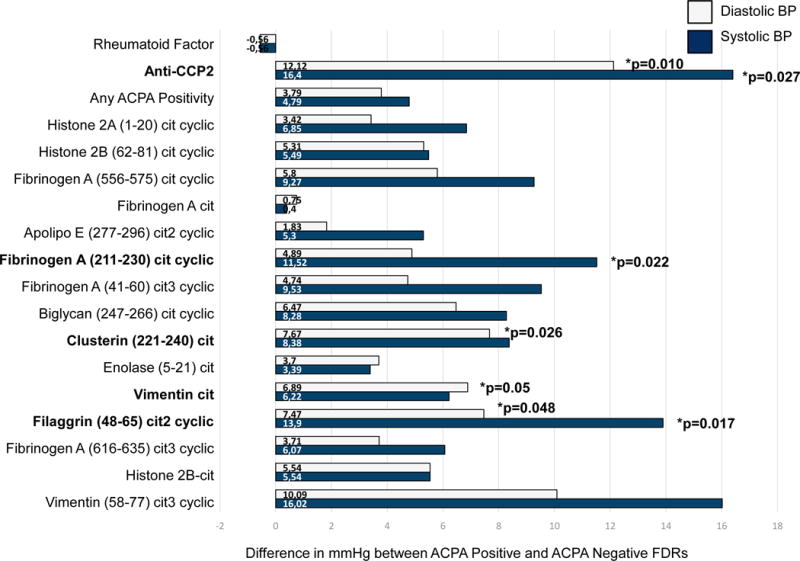
This figure presents the difference in diastolic blood pressure and systolic blood pressure (in mmHg) in ACPA positive first-degree relatives compared to ACPA negative first-degree relatives. The proportion of first-degree relatives who were positive for each individual ACPA are presented in Table 1. Differences in diastolic and systolic blood pressure for rheumatoid factor and anti-CCP2 positive first-degree relatives versus rheumatoid factor and anti-CCP2 negative first-degree relatives are included as well. Analyses were adjusted for age, sex, race, BMI, CRP, pack-years of smoking, current use of anti-hypertensive medications. The bars and p-values represent the difference in blood pressure in mmHg, where 0mmHg indicates no difference in diastolic BP or systolic BP between ACPA positive and ACPA negative first-degree relatives.
First-degree relatives who were positive for antibodies to citrullintated vimentin had a 7 mmHg higher DBP compared to first-degree relatives who were negative. Similarly, first-degree relatives who were positive for antibodies to Filaggrin (48-65) cit2 cyclic and Clusterin (221-240) cit had an 8 mmHg higher DBP compared to first-degree relatives who were negative. DBP was 3 mmHg higher in first-degree relatives who were positive for any ACPA (p=0.09), and higher overall in all ACPA positive first-degree relatives, although not all of the associations were statistically significant. A similar observation was observed with anti-CCP2, where in first-degree relatives positive for anti-CCP2, DBP was 12 mmHg higher than in anti-CCP2 negative first-degree relatives. There was no association between rheumatoid factor and DBP. As with SBP, regardless of which individual ACPA was evaluated, and their individual statistical significance, positivity for each ACPA was associated with higher DBP than in those who were negative across the panel of ACPA measurements (Figure 1).
Of the first-degree relatives studied, 15%, or 11 total, had taken NSAIDs. Although the frequency, dose, and timing of the medications were unknown, our sensitivity analysis for associations between ACPA and blood pressure, excluding individuals currently using NSAIDs, found that associations between Fibrinogen A (211-230) cit cyclic, Filaggrin (48-65) cit2 cyclic and SBP remained statistically significant while associations between Clusterin (221-240), Filaggrin (48-65) cit2 cyclic, citrullinated vimentin, and DBP were slightly attenuated. Further, associations between anti-CCP and SBP and DBP remained statistically significant as well (p=0.02 and p=0.04, respectively) (data not shown).
Our second sensitivity analysis excluding the 18%, or 13 total, first-degree relatives currently taking anti-hypertensive medications revealed similar associations between individual ACPA and SBP and DBP; and further added several other significant associations between individual ACPA and blood pressure, including anti-CCP with SBP and DBP (p=0.02 and p=0.01, respectively) (data not shown).
DISCUSSION
We demonstrated that the number of positive ACPA was associated with higher systolic and diastolic blood pressure in first-degree relatives of persons with RA. Among individual ACPA, first-degree relatives positive for antibodies to various forms of citrullinated Filaggrin, Fibrinogen, Vimentin, and Clusterin had systolic and diastolic blood pressures that were significantly higher than in those who were ACPA negative, which was consistent with the findings for anti-CCP2, a clinical test for ACPA. To our knowledge, this is the first study to evaluate associations of ACPA with systolic and diastolic blood pressure. These findings give new insights into mechanisms linking RA to CVD, and may also provide insights to mechanisms driving hypertension in the general, non-RA population.
Several anti-rheumatic medications that can lead to hypertension in RA include traditional/non-selective NSAIDs (e.g., ibuprofen, indomethacin), coxibs (e.g., rofecoxib), cyclosporine, and biologics, (e.g. tocilizumab, certolizumab pergol, golimumab). Of the FDRs studied, 15% had taken NSAIDs. No first-degree relatives had taken any of the other anti-rheumatic medications. Because of the potential contribution of NSAIDs to higher blood pressure, we excluded 15%, or 11 total, first-degree relatives currently using NSAIDs in a sensitivity analysis, and found that the associations between ACPA and blood pressure presented in our main analysis remained.
Although our main analysis adjusted for 18%, or 13 total, first-degree relatives currently taking anti-hypertensive medications, our second sensitivity analysis excluded them to determine whether associations between ACPA and blood pressure were affected by these medications. This sensitivity analysis was congruent with our main study results, supports our current findings, and further adds several other significant associations between individual ACPA and blood pressure. Thus, we have chosen to present results from the original analysis as these results remain consistent with both sensitivity analyses and are the most conservative findings.
Other potential mechanisms that may contribute to hypertension in RA include the occurrence of specific genetic polymorphisms,1 and inflammatory pathway activation subsequently leading to increased peripheral vascular resistance.1, 11 Because the first-degree relatives in our study did not meet the 1987 ACR or 2010 EULAR/ACR criteria for RA, we were able to investigate associations between ACPA and blood pressure in the absence of clinically apparent RA and in the absence of anti-rheumatic medications. Prior work in the SERA cohort among first-degree relatives has shown that positivity for rheumatoid factor and/or anti-cyclic citrullinated peptides are associated with inflammatory cytokines7 and with markers of endothelial dysfunction.12 The findings on systolic and diastolic blood pressure presented here, in combination with this prior work, leads us to hypothesize that vascular changes and subsequent peripheral vascular resistance may occur before clinically apparent RA develops.
The pathogenesis of essential hypertension is not well understood, but it is thought that it results from combinations of genetic and environmental effects on cardiovascular and kidney function. Only recently has hypertension been proposed as an ‘autoimmune disese’ where hypertension is an autoimmune response to the modified self.13, 14 The major histocompatibility complex (MHC) is one recognized immune response gene that controls the magnitude and specificity of antibody production. In humans, the MHC HLA-DRB1 shared epitope alleles predisposes for ACPA, where citrullinated peptides bind tightly into the antigen binding groove, and elicit a more robust T cell response.15 In the setting of hypertension in the absence of RA, these RA-related immune processes may offer novel insights into the observed associations between ACPA and systolic and diastolic blood pressure observed in our study. Morevoer, the associations demonstrated here may provide information into mechanisms contributing to hypertension in the general population. Future studies are needed to evaluate the associations of ACPA with blood pressure and CVD in general population cohorts to address this important hypothesis.
There are several important limitations in this study. We lack measures of kidney function, such as estimated glomerular filtration rate and proteinuria, which would indicate renal contribution to hypertension. Our study had a cross-sectional study design, where ACPA and blood pressure were not measured in the same moment, which limits our ability to determine temporal relationships between ACPA positivity and blood pressure. Nonetheless, the careful clinical phenotyping excluded clinically apparent RA in study participants, and allowed us to demonstrate that ACPA are associated with higher blood pressure even among persons without clinically apparent RA. The study had a small sample size, which may have led us to miss associations of some ACPA with higher blood pressure. In light of this, the uniform direction of associations wherein any positive ACPA, regardless of which one, was associated with higher mean blood pressure, suggests a consistent association between ACPA and blood pressure.
In conclusion, we demonstrate for the first time that detectable ACPA are associated with higher blood pressure in a population of first-degree relatives without clinically apparent RA. These findings suggest that vascular injury and peripheral vascular resistance may occur before clinical development of RA and that ACPA may play a role in the vascular changes leading to hypertension prior to RA. Future studies are required to determine if ACPA may also contribute to hypertension in the general population.
Acknowledgments
The authors thank the investigators, staff, and participants of the SERA study for their valuable contributions.
Funding
Funding for this research was made possible by grants from the National Heart Lung and Blood Institute (K01 HL122394), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR051394), and National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK110427) at the National Institutes of Health, the ACR Research and Education Foundation Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign and the Career Development Bridge Funding Award: K Bridge, as well as the Foundation for Physical Therapy Miami-Marquette Challenge Research Grant, and the Autoimmunity Prevention Center (U19 AI050864-10) at the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol. 2015;11:693–704. doi: 10.1038/nrrheum.2015.112. [DOI] [PubMed] [Google Scholar]
- 2.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–72. [PubMed] [Google Scholar]
- 3.Radner H, Lesperance T, Accortt NA, Solomon DH. Incidence and Prevalence of Cardiovascular Risk Factors Among Patients With Rheumatoid Arthritis, Psoriasis, or Psoriatic Arthritis. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23171. [DOI] [PubMed] [Google Scholar]
- 4.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Barbary AM, Kassem EM, El-Sergany MA, Essa SA, Eltomey MA. Association of anti-modified citrullinated vimentin with subclinical atherosclerosis in early rheumatoid arthritis compared with anti-cyclic citrullinated peptide. J Rheumatol. 2011;38:828–34. doi: 10.3899/jrheum.101143. [DOI] [PubMed] [Google Scholar]
- 6.Majka DS, Vu TT, Pope RM, Teodorescu M, Karlson EW, Liu K, Chang RW. Association of Rheumatoid Factors With Subclinical and Clinical Atherosclerosis in African American Women: The Multiethnic Study of Atherosclerosis. Arthritis Care Res (Hoboken) 2017;69:166–174. doi: 10.1002/acr.22930. [DOI] [PubMed] [Google Scholar]
- 7.Hughes-Austin JM, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolove J, Lahey LJ, Weisman MH, Buckner JH, Mikuls TR, O’Dell JR, Keating RM, Gregersen PK, Robinson WH, Holers VM, Norris JM. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA) Ann Rheum Dis. 2013;72:901–7. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, Gersuk VH, Wei S, Mikuls TR, O’Dell J, Gregersen PK, Keating RM, Norris JM, Holers VM. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61:1735–42. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young K, Deane K, Derber L, Hughes-Austin J, Wagner C, Sokolove J, Weisman M, Buckner J, Mikuls T, O’Dell J, Keating R, Gregersen P, Robinson W, Holers V, Norris J. Relatives Without Rheumatoid Arthritis Show Reactivity to Anti-Citrullinated Protein/Peptide Antibodies Which are Associated with Arthritis-Related Traits: Studies of the Etiology of Rheumatoid Arthritis. 2013 doi: 10.1002/art.38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D’Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–10. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Wong M, Toh L, Wilson A, Rowley K, Karschimkus C, Prior D, Romas E, Clemens L, Dragicevic G, Harianto H, Wicks I, McColl G, Best J, Jenkins A. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48:81–9. doi: 10.1002/art.10748. [DOI] [PubMed] [Google Scholar]
- 12.Hughes-Austin JM. The investigation of early indicators of atherogenesis, inflammation, adiponectin and rheumatoid arthritis-related autoimmunity. Colorado School of Public Health; 2011. Doctor of Philosophy. [Google Scholar]
- 13.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pober JS. Is hypertension an autoimmune disease? J Clin Invest. 2014;124:4234–6. doi: 10.1172/JCI77766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8:573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]


