Abstract
Objectives/hypothesis
The natural clinical progression of aspiration to eventual pulmonary compromise is not well understood. We hypothesized that dietary modification recommendations, penetration-aspiration scale (PAS) score, and dysphagia etiology would be associated with changes in time to first pulmonary event and overall survival for patients with documented aspiration on radiologic testing. This study identified a cohort of patients with detectable unsensed penetration or aspiration on videofluoroscopic swallowing study (VFSS) and followed this cohort over time for development of pulmonary events and death. We then evaluated the association of aspiration severity and dietary modification recommendations on incidence of these endpoints.
Study Type/Design
Retrospective chart review.
Methods
A total of 2616 VFSS exam reports were reviewed from our institution performed between January 1, 2009 and December 31, 2010. Aspiration or unsensed penetration (PAS of 5 or greater) was detected in 564 (21.5%) of these patients, who were then included in the study cohort. Medical records were reviewed retrospectively for development of pulmonary events (pneumonia, pneumonitis, or other life-threatening pulmonary illness) and all-cause mortality for up to 54 months after initial VFSS. Univariate Kaplan-Meier analysis and multivariate Cox regression were performed for time to first pulmonary event and survival predicted by recommended diet, PAS score, and dysphagia etiology.
Results
Dysphagia etiology was highly associated with increased development of pulmonary events for some patients, especially those with generalized nonspecific dysphagia due to deconditioning or frailty (hazard ratio [HR] versus stroke 2.95, 95% CI: 1.53–5.69, p=0.001) and esophageal dysphagia (HR 2.66, 95% CI: 1.17–6.02, p=0.019). Dysphagia etiology was also associated with increased mortality for patients with generalized nonspecific dysphagia due to deconditioning or frailty (HR 3.32, 95% CI: 2.0–5.52, p<0.001), post-surgical patients (HR 1.73, 95% CI: 1.05–2.86, p=0.032) and chronic neurologic disease (HR 1.87, 95% CI: 1.12–3.13, p=0.017). Dietary modification recommendations at the time of VFSS (prohibition of oral intake or modification of food consistency) had no significant impact on time to first pulmonary event (p=0.37) or survival (p=0.17), while PAS score was associated with decreased time to first pulmonary event on univariate but not multivariate analysis (hazard ratio for 1-point increase 1.6, 95% CI: 0.99–1.36, p=0.067). Kaplan-Meier estimate of overall 3-year mortality for this patient cohort was 39%.
Conclusions
Etiology of dysphagia is associated with a higher mortality rate and development of pulmonary events in patients with unsensed penetration or aspiration on VFSS, especially for those patients with generalized deconditioning and frailty or esophageal dysphagia. Severity of aspiration as defined by PAS was not associated with altered overall survival. Recommendations for dietary modification to an NPO status or modified food consistency had no statistically significant association with development of pulmonary events or survival in patients with detectable unsensed penetration or aspiration on VFSS compared to full diet recommendation.
Keywords: Dysphagia, aspiration, pneumonia, swallowing study, penetration aspiration scale, dietary modification, mortality
Introduction
Aspiration may be a direct consequence of altered pharyngoesophageal muscle coordination or mucosal sensation leading to failed airway protection. The oral phase of swallowing is swift, occurring in less than two seconds; once initiated it includes highly orchestrated voluntary and reflex-coordinated actions.1 The potential causes of pharyngoesophageal dysphagia are multiple and disparate and can include such etiologies as muscular weakness, generalized deconditioning and frailty, oropharyngeal or neurologic trauma, cancer treatment, head and neck surgery, neurologic injury, and mental status changes.2 Patients with pharyngoesophageal dysphagia are at high risk for aspiration of secretions, liquids or food particulates, with subsequent development of aspiration pneumonia.3 Prior studies have demonstrated a high risk for development of subsequent aspiration pneumonia and eventual death in those with documented presence of aspiration on swallowing studies.4 Healthy non-hospitalized patients are also known to have a baseline level of aspiration. Studies have shown that up to 1–3% of outpatients may demonstrate thin liquid aspiration on videofluoroscopic swallowing studies (VFSS) and 10–15% may show penetration without obvious apparent short or long-term consequences in regard to development of pneumonia or other life-threatening consequences.5,6 Trace aspiration and penetration of thin liquids has even been proposed as a normal finding in some healthy adults, although this is not widely agreed upon amongst experts.7 The complex connections between laryngeal penetration, aspiration, dysphagia etiology, prevalence of aspiration pneumonia, diet modification, and eventual mortality risk is poorly defined in the current literature.
Evaluation of aspiration is generally performed with a videofluoroscopic swallowing study (VFSS) involving radiologic evaluation of the swallowing mechanism via challenges with varying liquid and solid food consistencies.8 These studies allow for evaluation of laryngeal penetration to the vocal folds and below, as well as detection of silent or sensed aspiration. The Penetration-Aspiration Scale (PAS) was developed in 1996 by Rosenbeck et al9 and is commonly used in clinical practice. It defined an 8-point scale measuring the extent of penetration and aspiration on VFSS evaluation, with some authors defining aspiration as anything higher than 4 (material entering to the level of the vocal cords and not ejected, Table I).
Table I.
Penetration Aspiration Scale from Rosenbeck et. al.9
| Score | Description |
|---|---|
| 1 | Material does not enter the airway |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway |
| 6 | Material enters the airway, passes below the vocal folds and is ejected into the larynx or out of the airway |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort |
| 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject |
When penetration or aspiration is detected, speech language pathologists (SLP) and other dysphagia clinicians often recommend alteration of dietary regimens with thickened liquids to slow bolus transit, pureed or mechanical solid food consistencies, altered head positioning, or even restricting oral intake entirely.10 While studies have suggested that thickening liquids can decrease penetration and aspiration through slowed pharyngeal transit time, they have also demonstrated increased pharyngeal residues with risk for delayed aspiration of hypopharyngeal contents.11 Prior studies have also shown higher rates of pneumonia in patients that do not comply with dietary modification recommendations.12 Dietary alteration also has minimal impact on the ability of the patient to tolerate their own secretions, which may also have profound effect on these endpoints.13 The effect of these dietary modifications on eventual development of pulmonary events and patient survival is unknown, and studies in this area are challenging due to possible poor patient compliance with dietary recommendations and lack of longitudinal data.
This study was designed to document the natural clinical progression of patients with proven unsensed penetration or aspiration on VFSS. This included assessment of the effects of dietary modifications, aspiration severity as determined by PAS, and etiology of dysphagia on time to development of pneumonia and all-cause mortality. We hypothesized that dietary modification recommendation would prolong the amount of time to the development of first pulmonary event and overall survival in patients with documented unsensed penetration or aspiration on VFSS. This knowledge would allow clinicians to make stronger recommendations regarding possible dietary changes for patient with documented aspiration. We further hypothesized that determination of the major cause of dysphagia prior to VFSS would allow for stratification of risk profiles amongst patients with detectable aspiration, and facilitate discussion with patients regarding prognosis and possible need for prohibition of oral intake.
Materials and Methods
Patient selection and data collection
Our Institutional Review Board approved this study. All inpatient and outpatient VFSS completed at our institution over two consecutive years were reviewed, from January 1, 2009 to December 31, 2010. VFSS were performed according to standard protocols14 with patients challenged with thin, honey, nectar, pureed, and cookie barium swallows. Not all patients were given all types of consistencies depending on severity of aspiration on initial liquid consumption screening. Patients whose VFSS result demonstrated a PAS score ≥ 5 on any food or liquid consistency according to Rosenbeck et al were selected for our study, with the highest score from all challenges being used for analysis. Further data were collected from the medical record for each patient, including patient age, gender, and dietary instruction given at time of initial swallow study by the SLP (regular diet, PO; modified diet, MPO; nothing by mouth or nil per os, NPO). Any recommendation for diet consistency modification was listed in this study as MPO. Etiologies of dysphagia were assigned in retrospective fashion for each patient by chart review based on admitting diagnosis or reason for VFSS referral according to one of the following groups presented in Table II. In cases where multiple possible dysphagia etiologies were possible, the most severe or recent cause of hospitalization and/or referral for VFSS was selected. We did not stratify patients based on frailty score or illness severity at time of VFSS due to the routine lack of measurable and comparable data in the medical record in this regard.
Table II.
Assigned Dysphagia Etiologies
| GROUP | Description |
|---|---|
| STROKE | Post-stroke patients, all types |
| NEURO | Chronic neurologic disease (ALS, Parkinson’s, neuropathy, dementia) |
| DYSPHAGIA NOS | Generalized weakness, frailty, deconditioning without specific diagnosis, presentation with aspiration pneumonia without other known cause. |
| LARYNX | Laryngeal non-cancer etiology including stenosis, paralysis |
| TRAUMA | Neurologic trauma, brain injury, neck trauma |
| SURGERY | Post-operative head and neck surgery/cancer, thoracic surgery, cervical spine surgery, or other post-operative cause |
| ESOPH | Cricopharyngeal or esophageal dysphagia |
Patient charts were then reviewed in retrospective fashion for documented pulmonary events at any time in their medical chart after the initial swallow study until June 30th 2013, a period of up to 54 months. Pulmonary events were defined as a documented pneumonia, pneumonitis, or other life-threatening pulmonary infection based on clinical imaging, ICD-9 diagnosis, or physician notes. Date of death was also noted within the data collection period from January 1, 2009 through June 30, 2013.
Statistical evaluation
The effects of dietary recommendation, PAS score, and dysphagia etiology were investigated for the outcomes of time to first pulmonary event and overall survival. All patients were censored at death or last follow-up. Covariates of interest were compared between outcome groups using chi-squared analysis. Probabilities for survival and time to first pulmonary event were calculated using the Kaplan-Meier estimator, and study groups were compared via the log-rank test. Multivariable Cox proportional hazards regression measured associations between the variables of interest (etiology, PAS score and diet modification) and pulmonary events and mortality separately. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Patient demographics
A total of 2616 inpatient and outpatient swallowing studies were performed during the two-year study period at our institution. A total of 564 patients (21.5%) were assigned a PAS score >5 during this time period and were therefore included in the study. A small number of patients (varying between 1 to 6 total patients) were excluded from data analysis for each statistical evaluation if pertinent clinical data was lacking from their medical record. This varied for each calculation depending on the specific clinical parameter missing. Patient age ranged from 18 to 101 years. The mean age in the study population was 67.5 years, with a median age of 69.0 years and a mode of 59 years. The majority of patients were male (345, or 61.3%) with a mean age of 67.1 years, and a mean female patient age of 68.1 years. There were no statistically significant differences between male and female study populations based on mean age. There were no statistically significant differences found for overall study parameters of pneumonia incidence or survival based on patient gender or age (data not shown).
PAS scores distribution is shown in Table III with final score assignment selected as the highest score for any tested food or liquid consistency. Patient gender and age distribution were not statistically different between PAS score groups (P > 0.19). Dietary modification recommendations based on dysphagia etiology made for each patient at the time of VFSS by the evaluating SLP are demonstrated in Table IV. The largest group of patients (47.1%) was recommended to follow a modified diet (MPO) following their VFSS evaluation and dietary recommendations varied significantly depending on the etiology of dysphagia. PO diet was recommended at the highest rate for the Larynx group, while the Stroke group had the lowest rate of recommendation of full PO diet, with the Trauma group closely behind. MPO was recommended most for the Stroke group, and at lowest rate for the Larynx group. NPO diet was most common for Trauma patients, while the Larynx group had the lowest rate of this diet. Chi-squared analysis on diet recommendations between etiology groups showed a statistically significant difference (P < 0.0001).
Table III.
Patient Penetration-Aspiration Scale Score Distribution
| PAS Score | Frequency | Percent | Age (Average) |
|---|---|---|---|
| 5 | 109 | 19.53 | 69.2 |
| 6 | 67 | 12.01 | 67.0 |
| 7 | 139 | 24.91 | 67.4 |
| 8 | 243 | 43.55 | 66.8 |
Table IV.
Comparison of Dysphagia Etiology by Diet Type with Chi-Squared test.
| NPO | PO | MPO | P-value | |
|---|---|---|---|---|
| Etiology (total) | <0.0001 | |||
| Stroke (122) | 44 (36.1%) | 11 (9.0%) | 67 (54.9%) | |
| Trauma (60) | 25 (41.7%) | 6 (10.0%) | 29 (48.3%) | |
| Neuro (112) | 29 (25.9%) | 24 (21.4%) | 59 (52.7%) | |
| Surgery (133) | 51 (38.4%) | 34 (25.6%) | 48 (36.0%) | |
| Dysphagia NOS (92) | 34 (37.0%) | 14 (15.2%) | 44 (47.8%) | |
| Esoph (35) | 7 (20%) | 13 (37.1%) | 15 (42.9%) | |
| Larynx (9) | 1 (11.1%) | 5 (55.6%) | 3 (33.3%) | |
| Overall (563) | 191 (33.9%) | 107 (19.0%) | 265 (47.1%) |
Dietary recommendation at the time of VFSS was compared directly to PAS score, with data shown in Table V. Patients with PAS score of 8 had the highest recommendation to remain NPO after VFSS, while patients with a score of 5 had the highest recommendation to follow an MPO diet. PO diet recommendation was highest for PAS score of 6. Chi-Squared analysis for dietary recommendations based on PAS score was statistically significant (P = 0.0203). This confirms that patients with greater amounts of aspiration based on PAS score were statistically more likely to have recommendation for NPO or MPO diets.
Table V.
Comparing PAS Score by Diet Type with Chi-Squared test.
| NPO | PO | MPO | P-value | |
|---|---|---|---|---|
| Score | 0.0203 | |||
| 5 | 28 (25.7%) | 21 (19.3%) | 60 (55%) | |
| 6 | 16 (23.9%) | 21 (31.3%) | 30 (44.8%) | |
| 7 | 53 (38.1%) | 22 (15.8%) | 64 (46.1%) | |
| 8 | 93 (38.3%) | 40 (16.5%) | 110 (45.3%) |
Pulmonary event analysis
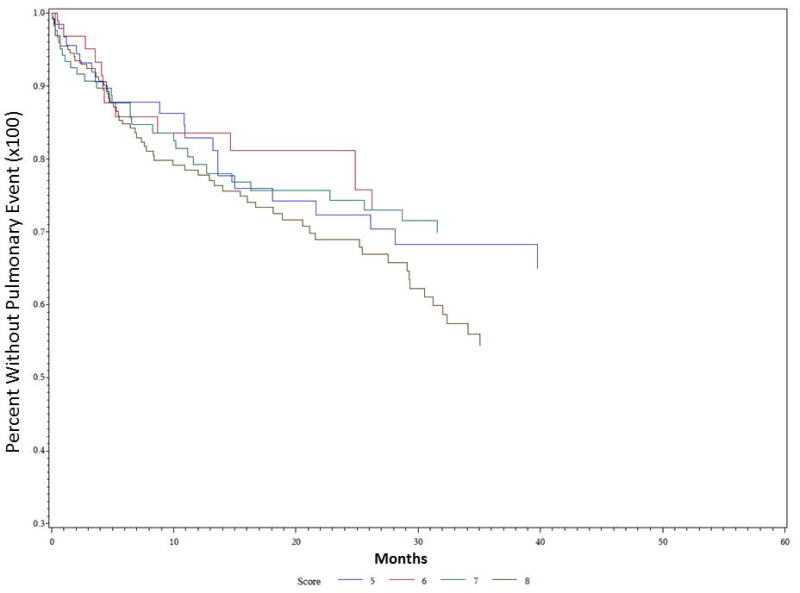
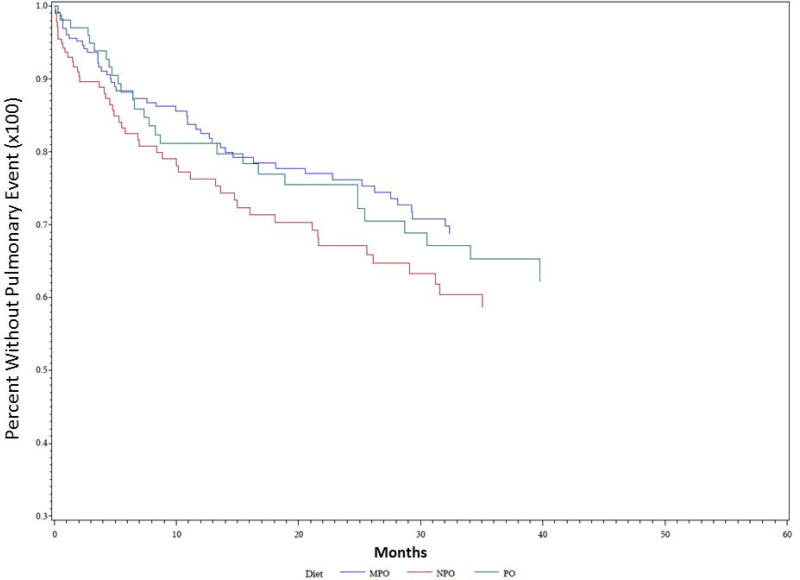
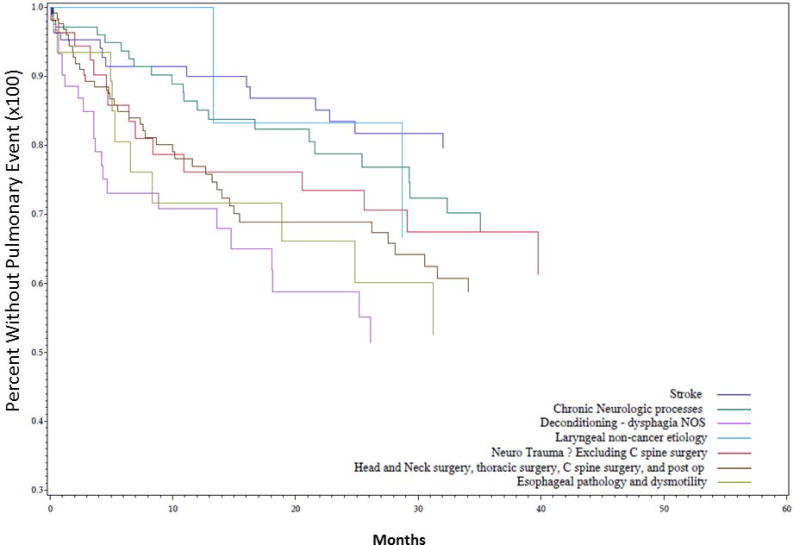
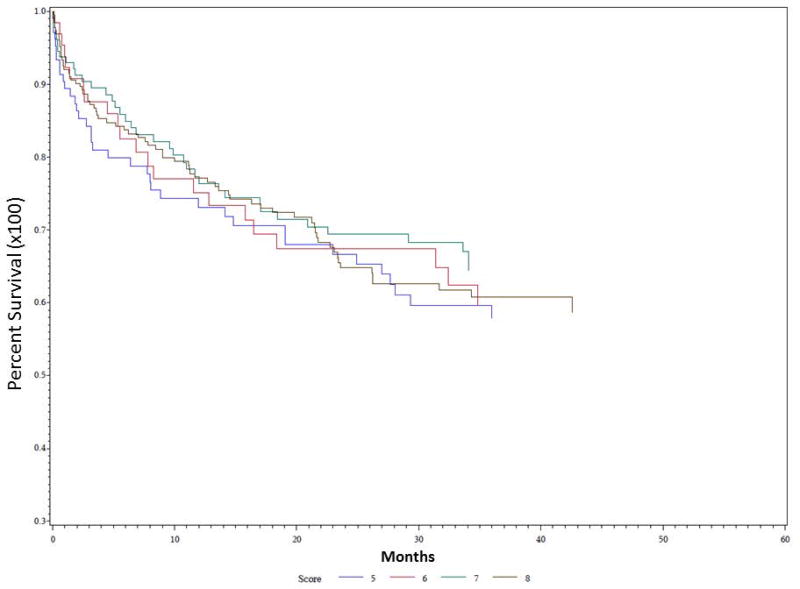
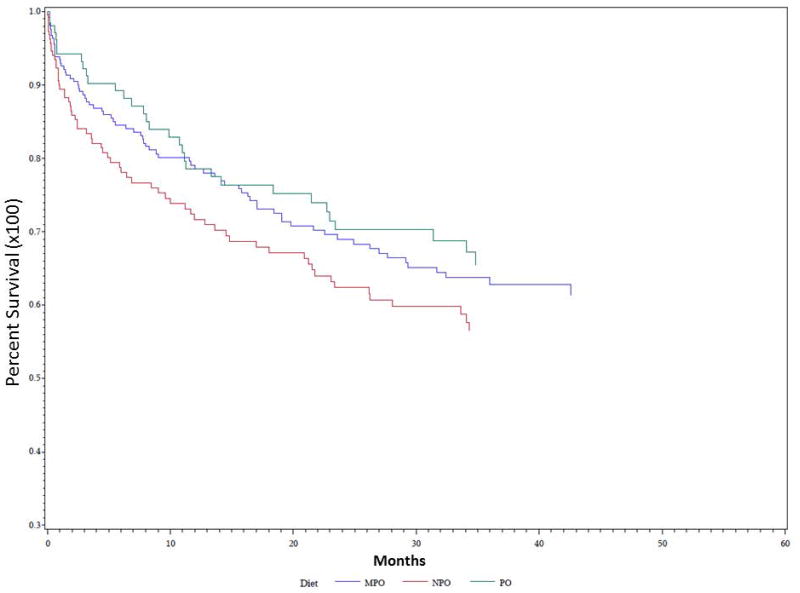
Univariate Kaplan-Meier analysis was performed for the duration of the study period evaluating time to first pulmonary event for multiple different parameters, including PAS score, dietary recommendation after VFSS, and etiology of dysphagia. Data demonstrating analysis for PAS score is demonstrated in Figure 1. PAS score differences between groups for time to first pulmonary event were statistically significant on univariate analysis (P = 0.035). PAS score of 8 showed the highest rate of development of pulmonary events over time, followed by PAS score of 5. The effect of dietary recommendation on time to first pulmonary event is shown in Figure 2. There were no statistically significant differences between time to first pulmonary event and dietary recommendation at time of VFSS on univariate analysis (P = 0.159). Additional univariate analysis was performed evaluating time to first pulmonary event by etiology of dysphagia, demonstrated in Figure 3. Time to first pulmonary event varied significantly between dysphagia etiology groups (P = 0.01). Patients in the Stroke group had the longest time to first pulmonary event, followed by Neuro group. Shortest time to first pneumonia was seen in the Dysphagia NOS group, followed by the Esoph group.
Figure 1. Time to first pulmonary event predicted by PAS score.
Univariate Kaplan Meier analysis of demonstrated significant differences between score groups, with patients scored at 8 with highest risk of pulmonary events over time (in months), and patients scored at 6 at lowest risk. P = 0.035.
Figure 2. Time to first pulmonary event predicted by dietary recommendation.
Univariate Kaplan Meier analysis of dysphagia patients grouped into regular diet (PO), modified diet (MPO), and nothing by mouth (NPO) groups showed trends without statistical significance (P= 0.159).
Figure 3. Time to first pulmonary event predicted by dysphagia etiology.
Univariate Kaplan Meier analysis of dysphagia patients grouped into seven etiologies (according to table II) and assessed for time to first pulmonary event (in months) showed statistically significant differences between groups, with patients in the Stroke group at lowest risk, and patients in the Dysphagia NOS, Esoph, and Surgery groups at highest risk (P = 0.01).
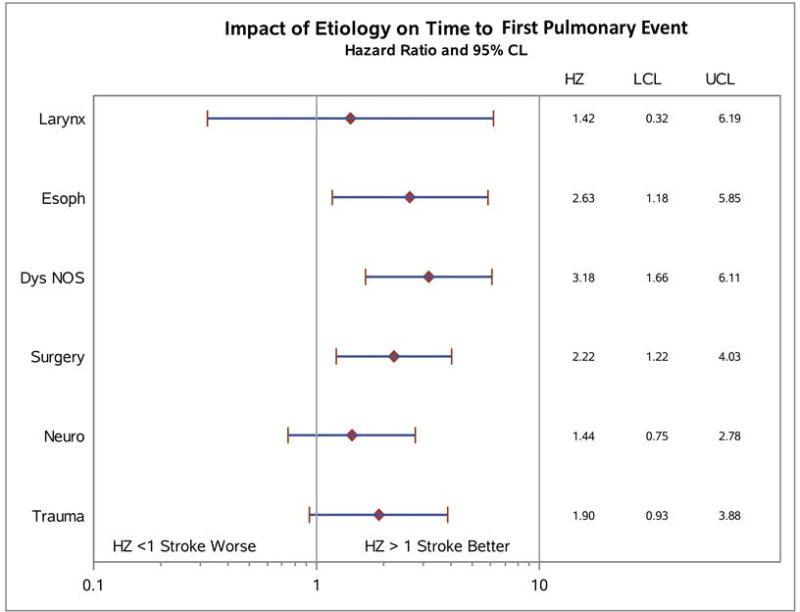
Univariate hazard ratios were calculated for risk of pulmonary event, shown in Figure 4. Analyses were performed in comparison to the group with longest time to first pulmonary event, the Stroke group. The impact of etiology on time to first pulmonary event was statistically significant for Dysphagia NOS, Esoph, and Surgery groups (P = 0.01). Dysphagia NOS patients had a hazard ratio 3.18 times that of Stroke patients, Esoph patients 2.63, and Surgery patients 2.22. Other groups had elevated hazard ratios compared to Stroke that did not meet statistical significance.
Figure 4. Univariate hazard ratio analysis for time to first pulmonary event predicted by dysphagia etiology.
Dysphagia etiologies were compared directly to patients in the Stroke group and hazard ratios calculated with 95% confidence limit. Patients in the Dysphagia NOS, Esoph, and Surgery groups all had significantly elevated hazard ratios. HZ = hazard ratio, LCL = lower confidence limit, UCL = upper confidence limit. P = 0.01.
Multivariate analysis of time to first pulmonary event also showed significant differences in risk stratified by dysphagia etiology (Table VI, P = 0.0215). Patients in the Dysphagia NOS (HZ 2.95, P = 0.0012), Esoph (HZ 2.66, P = 0.019), and Surgery (HZ 2.08, P = 0.0178) groups all had statistically increased hazard ratios for time to first pneumonia when compared to the Stroke group. Diet modification to MPO or NPO did not show statistical significance when compared to PO for time to first pulmonary event. PAS score effect on time to first pulmonary event also did not meet statistical significance on multivariate analysis.
Table VI.
Multivariate Analysis Model for Time to First Pulmonary Event.
| Hazard Ratio | 95% Confidence | P-value | |
|---|---|---|---|
| Etiology (vs Stroke) | 0.0215 | ||
| Dysphagia NOS | 2.95 | 1.53, 5.69 | 0.0012 |
| ESOPH | 2.66 | 1.17, 6.02 | 0.0190 |
| LARYNX | 1.29 | 0.29, 5.77 | 0.7376 |
| NEURO | 1.33 | 0.69, 2.59 | 0.3945 |
| SURGERY | 2.08 | 1.14, 3.82 | 0.0178 |
| TRAUMA | 1.80 | 0.88, 3.70 | 0.1092 |
| Diet (vs PO) | 0.3681 | ||
| MPO | 0.96 | 0.99, 1.36 | 0.8665 |
| NPO | 1.26 | 0.59, 1.56 | 0.3540 |
| PAS Score, 1 point | 1.16 | 0.99, 1.36 | 0.0666 |
Mortality Analysis
392 patients remained alive and 171 patients were deceased at the completion of the study period. Overall 3-year mortality for patients with a PAS score of 5 or greater was 39%. Univariate Kaplan-Meier analysis was performed to evaluate overall survival predicted by PAS score, dietary recommendation after VFSS, and etiology of dysphagia. Survival predicted by PAS score is shown in Figure 5. Patients in all PAS score groups had similar mortality over the study period, without any statistically significant differences between score groups (P = 0.92). Further analysis of survival predicted by diet recommendation after initial VFSS also did not show any significant differences between treatment groups (Figure 6, P = 0.198).
Figure 5. Survival predicted by PAS score.
Univariate Kaplan Meier analysis of overall survival (in months) demonstrated no significant differences between PAS score groups. Overall 3-year mortality for all patients with PAS > 5 was 39%. P = 0.9206.
Figure 6. Survival predicted by dietary recommendation.
Univariate Kaplan Meier analysis of dysphagia patients grouped into regular diet (PO), modified diet (MPO), and nothing by mouth (NPO) groups showed that patients in the NPO group had the highest mortality over time (in months), but differences between groups did not meet statistical significance (P= 0.198)
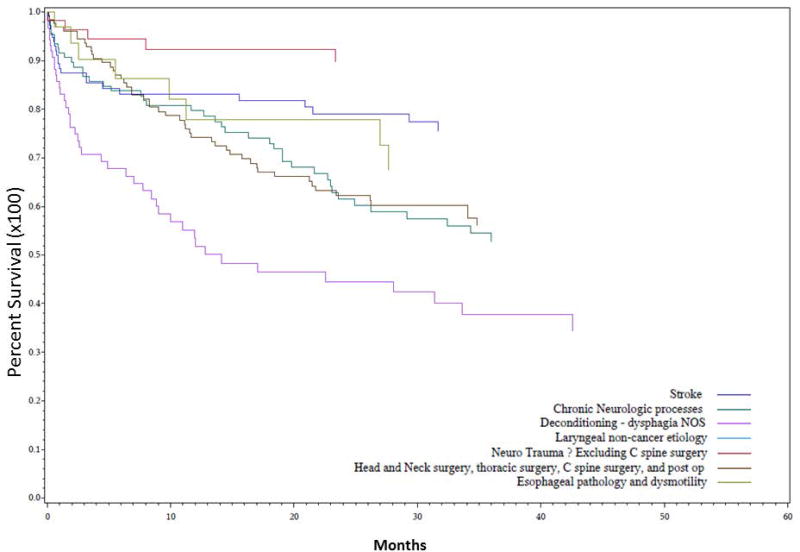
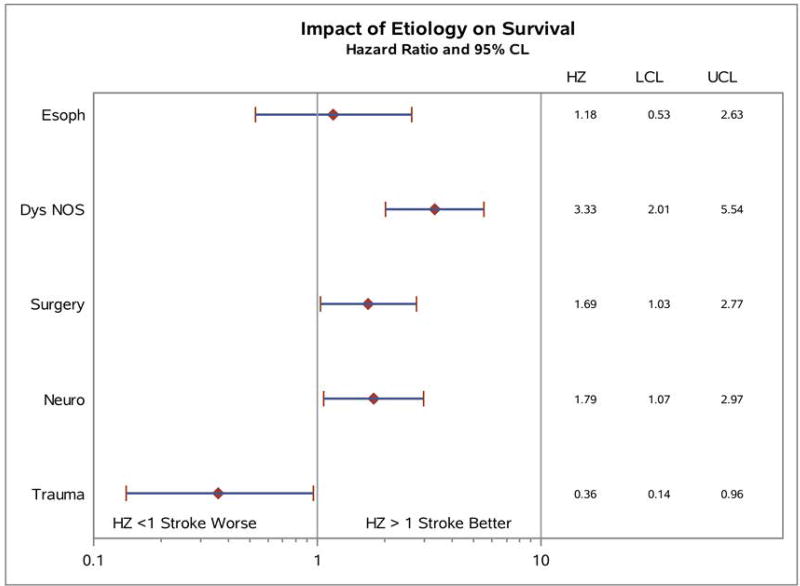
Survival analysis predicted by dysphagia etiology is shown in Figure 7. Significant differences were noted between various etiologies over time, with patients in the Dysphagia NOS, Neuro and Surgery groups at highest risk for mortality. Patients in the Trauma group had lower mortality over time, and there were no deaths among the 9 patients in the Larynx group (P < 0.0001). Further analysis of univariate hazard ratios for impact of etiology of dysphagia on mortality is shown in Figure 8, with all etiologies compared again to the Stroke group. Survival hazard ratio for Dysphagia NOS etiology compared to Stroke patients was 3.33, 1.69 for Surgery, and 1.79 for Neuro patients; Trauma patients had significantly lower risk for mortality with a HR of 0.36 (P < 0.0001).
Figure 7. Survival predicted by dysphagia etiology.
Univariate Kaplan Meier analysis of dysphagia patients grouped into seven etiologies (according to table II) and assessed for overall survival (in months) compared to Stroke patients showed statistically significant differences between groups, with patients in the Larynx group at lowest risk, and patients in the Dysphagia NOS, Neuro, and, and Surgery groups at highest risk of mortality (P < 0.0001).
Figure 8. Univariate hazard ratio analysis for impact of dysphagia etiology on survival.
Dysphagia etiologies were compared directly to patients in the Stroke group and hazard ratios calculated with 95% confidence limit. Patients in the Dysphagia NOS, Surgery and Neuro groups all had significantly elevated hazard ratios compared to Stroke, while the Trauma group had significantly lower mortality compared to Stroke patients. HZ = hazard ratio, LCL = lower confidence limit, UCL = upper confidence limit. P < 0.0001.
Multivariate analysis of survival also showed significant differences in survival stratified by dysphagia etiology (Table VII, P < 0.0001). Patients in the Dysphagia NOS (HZ 3.32, P <0.001), Neuro (HZ 1.871, P = 0.017), Surgery (HZ 1.73, P = 0.0319), and Trauma (HZ 0.354, P = 0.0357) groups all had statistically altered hazard ratios for survival when compared to the Stroke group. Diet modification to MPO or NPO did not show statistically significant effect on survival when compared to PO. PAS score effect on survival also did not meet statistical significance on multivariate analysis.
Table VII.
Multivariate Analysis Model for Survival
| Hazard Ratio | 95% Confidence | P-value | |
|---|---|---|---|
| Etiology (vs Stroke) | <0.0001 | ||
| Dysphagia NOS | 3.32 | 2.0, 5.52 | <0.0001 |
| ESOPH | 1.249 | 0.56, 2.18 | 0.5911 |
| LARYNX | 0 | 0, 0 | 0.9753 |
| NEURO | 1.871 | 1.12, 3.13 | 0.0171 |
| SURGERY | 1.733 | 1.05, 2.86 | 0.0319 |
| TRAUMA | 0.354 | 0.14, 0.93 | 0.0357 |
| Diet (vs PO) | 0.1747 | ||
| MPO | 1.161 | 0.76, 1.79 | 0.4971 |
| NPO | 1.474 | 0.95, 2.30 | 0.0866 |
| Score | 0.97 | 0.86, 1.09 | 0.6114 |
Discussion
This study followed a group of 564 patients with documented unsensed penetration or aspiration on VFSS and followed these patients for development of pulmonary events and mortality for up to 54 months. Little prior data exists to demonstrate the predictive power of VFSS to alter clinical course.15,16 Current recommendations for dietary alteration following VFSS include various changes in dietary bolus viscosity to thickened liquids, mechanical consistencies, or full regular diet based on findings on oropharyngeal swallowing evaluation, but data supporting this practice is limited.11,17 Studies have also evaluated the ability of PAS score to predict the ability of the patient to tolerate specific types of diet, but again this data is limited in scope.18 The ability of our clinical interventions (dietary modification, NPO status, and/or gastrostomy tube placement) to reduce pneumonia incidence and mortality is relatively unknown.4,19 Recent reviews suggest that dietary modification and NPO status may have no effect on overall survival, and this is further supported by our data set.20 A relatively high mortality risk of aspiration is demonstrated by our data, with an overall all-cause 3-year mortality rate of 39% for patients in our cohort with a PAS of 5 or greater. To our knowledge, no other study has shown this type of long term follow-up of patients with unsensed aspiration and penetration. This study was designed to evaluate the ability of VFSS to predict clinical outcomes for these patients on with these concerns in mind.
Many studies in this area of dysphagia research suffer from challenges in defining aspiration and pneumonia. The repercussions of pulmonary infection can be clinically varied in severity, and pulmonary insults are wide-ranging between mild pneumonitis and fulminate pneumonia with sepsis.21 Studies have shown prevalence of aspiration on VFSS to be extremely variable, and mortality rates can be as high as 70% in selected patient populations.3 The penetration aspiration scale was designed in 1996 by Rosenbeck to attempt to categorize the severity of aspiration seen on VFSS exams, and allow clinicians to not only score severity but also evaluate changes in swallowing function over time.9 There continue to be controversies in the application and interpretation of parameters such as the PAS for evaluating and tracking dysphagia, with testing showing poor inter and intra-subject reproducibility.22,23 VFSS maneuvers may be good at detecting large volume tracheal aspiration but may not adequately stratify patients with borderline swallowing function.8 Some have recommended a brief liquid swallow test to document aspiration with reasonable efficacy.24 Rosenbeck was able to define PAS score distributions for normal patients and those with known dysphagia, and since the development of this scale it has spread widely into clinical use.25 Prior work has shown that rates of aspiration generally remain stable over time in patients with known silent penetration and aspiration on VFSS without acute health insults,7 and our study results demonstrate the long-term sequelae of such documented aspiration. Multivariate model analysis from this study did not show statistical significance for PAS score to predict development of pulmonary event. Our data would suggest that severity of aspiration on VFSS as measured by PAS has no significant effect on overall mortality. Altering diets to restrictive consistencies or even NPO status has a profound effect on patient quality of life.26 Our data would suggest that these prohibitions may have minimal effect on the natural clinical course of the dysphagia patient. Therefore, a discussion of more aggressive feeding regimens with the aspirating patient is perhaps warranted, with potential avoidance of NPO regimens whenever possible under the direction of a dysphagia care team.
A standard clinical recommendation for patients with airway penetration or subtle aspiration on VFSS is to modify the patients diet to various degrees. This may include altered food consistency, thickened liquids, head positioning maneuvers, or even NPO status and gastrostomy tube placement. Alteration of food consistency has been shown to change pharyngeal transit rates and thereby facilitate ease of swallow.27 Data from our study demonstrates no statistically significant association of recommendations for dietary modification with incidence of pulmonary events or survival over time. It should be noted that this study was not adequately structured or designed to do a thorough analysis of this topic due to the observational nature of this type of research. We also were not able to evaluate adherence to dietary recommendations in our study, and it is possible that documented strict adherence to modified diets may have improved outcomes.12 Careful coaching of modification strategies and swallowing therapy recommendations has shown benefit in improving patient adherence.28 Little is known about the amount of swallowing therapy that is necessary to produce sufficient swallowing improvement to reduce pneumonia and mortality.29 A recent systematic review has shown that thickened liquids do seem to reduce risk of penetration and aspiration, but are also associated with higher pyriform residuals.17 Food hardness, cohesiveness (scatter potential), and viscosity are also highly associated with ease of transit through the hypopharynx. There is noted to be a lack of uniformity in studies in the details of dietary modification recommendation.11 Dietary modifications such as thickening of liquids or maintenance of NPO status also have no effect on a patient’s ability to handle their own secretions. This is mostly affected by a patient’s individual strength and vigor including muscle strength and pulmonary reserve, and may in fact have as much or more impact on the development of pulmonary infections in an aspirating patient population than food or liquid aspiration.30
Prior studies have shown marked differences between dysphagia etiologies and overall risk of pneumonia and mortality.2,30,31 The vital protective role of the larynx in swallowing can be compromised through multiple factors, including chronic neurologic disease, surgical alteration, altered consciousness, frailty, deconditioning, tube feeding, and perhaps even reflux.3 Dysphagia risk may become additive or even synergistic in many patients when multiple risk factors are present. Dysphagia is known to be a strong risk factor for aspiration pneumonia, but some studies have shown that dysphagia alone is not sufficient to generally lead to aspiration pneumonia, and other compromising diagnoses must also be present.30 Our data show that patients with deconditioning and generalized dysphagia due to frailty and dementia have substantially increased risk of pneumonia and overall mortality. Goldberg and Altman directly addressed this population in a systematic review of patients with dementia and showed no long-term survival benefit of PEG placement for these patients.20 Early PEG placement may be indicated for these patients to maintain quality of life, hydration, nutrition, and ease of medication administration. We also demonstrated increased pneumonia risk in patients with esophageal dysphagia when compared to stroke patients. It is likely that patients with stroke will improve markedly as they recover from their acute injury, whereas esophageal dysphagia is likely only to worsen with time. Indeed, prior investigations have shown significant effects of swallowing interventions on the stroke population, and our data suggest that these patients may do well compared to other causes of dysphagia.28,32–35 Our data also show that progressive neurologic disease patients (ALS, Parkinson’s Disease) have a high rate of mortality after documented aspiration, likely again due to the relentless nature of these conditions.36 It should be noted, however, that the findings of this study do not usurp individual personalized clinical evaluations for the dysphagia patient, nor do these data suggest specific dietary recommendations for individual patients. These plans need to be made based on a highly personalized evaluation of the patient’s current swallowing status and illness data available to the treating clinician. In the end, our data provide some possible prognostic information that may be of use in these challenging conversations.
There are several strong limitations of this study which should be clearly stated. There are some clinical data that would add significantly to the evaluation in this study which were not available, including lack of frailty scores or graded illness severity at the time of initial VFSS as well as adherence to dietary recommendations. This study is also a single institutional experience at a tertiary academic facility, and these data may not in fact be widely generalizable to a wider population. This study was also performed in a retrospective manner and further prospective evaluations including analysis of these clinical data points would further clarify the ability of VFSS to predict patient outcomes. A carefully controlled prospective study would allow for increased confidence and improved ability to make clinical care recommendations based on VFSS results.
Conclusion
Detectable unsensed penetration or aspiration on VFSS (PAS > 5) was present in 21.5% of 2616 evaluations during a two-year time window at our institution. Dietary modification recommendation had no impact on time to first pulmonary event or mortality over a 54-month follow-up period in this observational study cohort. PAS score was associated with shorter time to first pneumonia on univariate but not multivariate analysis, and had no association with overall mortality. Dysphagia etiology, especially generalized dysphagia due to deconditioning, was highly predictive of decreased time to first pneumonia and overall mortality in this patient cohort. Overall three-year mortality for this cohort of patients with a PAS of 5 or above was 39%.
Acknowledgments
The authors would like to acknowledge Anika Szabo, PhD, and Alexis Vistocky, MS for assistance with the biostatistics on this project, and the Clinical and Translational Science Institute at the Medical College of Wisconsin for providing financial support for this biostatistics analysis. We also appreciate the assistance of Brian Chen-Yung Feng, MD for help with chart review, and lastly Robert Toohill, MD for initial guidance and vision for this project.
Financial Disclosures: This work was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 5UL1TR001436-02 at the Medical College of Wisconsin. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Submitted as a Triological Thesis for Dr. Bock, recipient of the 2017 Harris P. Mosher award for best clinical research thesis, and accepted for presentation at 2017 Triological Society Meeting, COSM, San Diego, CA, April 28, 2017
Conflicts of Interest: Dr. Blumin has a consulting agreement with Olympus. All other authors have nothing to disclose.
Bibilography
- 1.Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol. 2007;21:563–573. doi: 10.1016/j.bpg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Hoy M, Domer A, Plowman EK, Loch R, Belafsky P. Causes of dysphagia in a tertiary-care swallowing center. Ann Otol Rhinol Laryngol. 2013;122:335–338. doi: 10.1177/000348941312200508. [DOI] [PubMed] [Google Scholar]
- 3.DeLegge MH. Aspiration pneumonia: incidence, mortality, and at-risk populations. JPEN J Parenter Enteral Nutr. 2002;26:S19–24. doi: 10.1177/014860710202600604. discussion S24-15. [DOI] [PubMed] [Google Scholar]
- 4.McClave SA, DeMeo MT, DeLegge MH, et al. North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr. 2002;26:S80–85. doi: 10.1177/014860710202600613. [DOI] [PubMed] [Google Scholar]
- 5.Butler SG, Stuart A, Markley L, Rees C. Penetration and aspiration in healthy older adults as assessed during endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2009;118:190–198. doi: 10.1177/000348940911800306. [DOI] [PubMed] [Google Scholar]
- 6.Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol Head Neck Surg. 2010;142:208–213. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Todd JT, Stuart A, Lintzenich CR, Wallin J, Grace-Martin K, Butler SG. Stability of aspiration status in healthy adults. Ann Otol Rhinol Laryngol. 2013;122:289–293. doi: 10.1177/000348941312200501. [DOI] [PubMed] [Google Scholar]
- 8.McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J Speech Lang Hear Res. 2005;48:1280–1293. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- 9.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 10.Via MA, Mechanick JI. Malnutrition, dehydration, and ancillary feeding options in dysphagia patients. Otolaryngol Clin North Am. 2013;46:1059–1071. doi: 10.1016/j.otc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30:2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia. 2001;16:123–127. doi: 10.1007/s004550011002. [DOI] [PubMed] [Google Scholar]
- 13.Ponfick M, Linden R, Nowak DA. Dysphagia--a common, transient symptom in critical illness polyneuropathy: a fiberoptic endoscopic evaluation of swallowing study. Crit Care Med. 2015;43:365–372. doi: 10.1097/CCM.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 14.Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg. 1997;116:335–338. doi: 10.1016/S0194-59989770269-9. [DOI] [PubMed] [Google Scholar]
- 15.Pitts T. Airway protective mechanisms. Lung. 2014;192:27–31. doi: 10.1007/s00408-013-9540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman KW. Understanding dysphagia: a rapidly emerging problem. Otolaryngol Clin North Am. 2013;46:xiii–xvi. doi: 10.1016/j.otc.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Barbon CE, Steele CM. Efficacy of thickened liquids for eliminating aspiration in head and neck cancer: a systematic review. Otolaryngol Head Neck Surg. 2015;152:211–218. doi: 10.1177/0194599814556239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler SG, Stuart A, Case LD, Rees C, Vitolins M, Kritchevsky SB. Effects of liquid type, delivery method, and bolus volume on penetration-aspiration scores in healthy older adults during flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2011;120:288–295. doi: 10.1177/000348941112000502. [DOI] [PubMed] [Google Scholar]
- 19.Ickenstein GW, Riecker A, Hohlig C, et al. Pneumonia and in-hospital mortality in the context of neurogenic oropharyngeal dysphagia (NOD) in stroke and a new NOD step-wise concept. J Neurol. 2010;257:1492–1499. doi: 10.1007/s00415-010-5558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733–1739. doi: 10.2147/CIA.S53153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman KW. Oropharyngeal dysphagia pathophysiology, complications and science-based interventions. Nestle Nutr Inst Workshop Ser. 2012;72:119–126. doi: 10.1159/000340000. [DOI] [PubMed] [Google Scholar]
- 22.McCullough GH, Wertz RT, Rosenbek JC, Mills RH, Ross KB, Ashford JR. Inter- and intrajudge reliability of a clinical examination of swallowing in adults. Dysphagia. 2000;15:58–67. doi: 10.1007/s004550010002. [DOI] [PubMed] [Google Scholar]
- 23.Altman KW. Dysphagia evaluation and care in the hospital setting: the need for protocolization. Otolaryngol Head Neck Surg. 2011;145:895–898. doi: 10.1177/0194599811415803. [DOI] [PubMed] [Google Scholar]
- 24.Leder SB, Suiter DM, Warner HL, Acton LM, Swainson BA. Success of recommending oral diets in acute stroke patients based on passing a 90-cc water swallow challenge protocol. Top Stroke Rehabil. 2012;19:40–44. doi: 10.1310/tsr1901-40. [DOI] [PubMed] [Google Scholar]
- 25.Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 26.Paleri V, Patterson J. Use of gastrostomy in head and neck cancer: a systematic review to identify areas for future research. Clin Otolaryngol. 2010;35:177–189. doi: 10.1111/j.1749-4486.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi KH, Ryu JS, Kim MY, Kang JY, Yoo SD. Kinematic analysis of dysphagia: significant parameters of aspiration related to bolus viscosity. Dysphagia. 2011;26:392–398. doi: 10.1007/s00455-011-9325-5. [DOI] [PubMed] [Google Scholar]
- 28.Rosenvinge SK, Starke ID. Improving care for patients with dysphagia. Age Ageing. 2005;34:587–593. doi: 10.1093/ageing/afi187. [DOI] [PubMed] [Google Scholar]
- 29.Logemann JA. Clinical efficacy and randomized clinical trials in dysphagia. Int J Speech Lang Pathol. 2012;14:443–446. doi: 10.3109/17549507.2012.717966. [DOI] [PubMed] [Google Scholar]
- 30.Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 31.Roden DF, Altman KW. Causes of dysphagia among different age groups: a systematic review of the literature. Otolaryngol Clin North Am. 2013;46:965–987. doi: 10.1016/j.otc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Dziewas R, Stogbauer F, Ludemann P. Risk factors for pneumonia in patients with acute stroke. Stroke. 2003;34:e105. doi: 10.1161/01.STR.0000083465.17164.AB. author reply e105. [DOI] [PubMed] [Google Scholar]
- 33.Altman KW, Schaefer SD, Yu GP, et al. The voice and laryngeal dysfunction in stroke: a report from the Neurolaryngology Subcommittee of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2007;136:873–881. doi: 10.1016/j.otohns.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Daniels SK, Anderson JA, Willson PC. Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43:892–897. doi: 10.1161/STROKEAHA.111.640946. [DOI] [PubMed] [Google Scholar]
- 35.Altman KW, Richards A, Goldberg L, Frucht S, McCabe DJ. Dysphagia in stroke, neurodegenerative disease, and advanced dementia. Otolaryngol Clin North Am. 2013;46:1137–1149. doi: 10.1016/j.otc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Saleem TZ, Higginson IJ, Chaudhuri KR, Martin A, Burman R, Leigh PN. Symptom prevalence, severity and palliative care needs assessment using the Palliative Outcome Scale: a cross-sectional study of patients with Parkinson's disease and related neurological conditions. Palliat Med. 2013;27:722–731. doi: 10.1177/0269216312465783. [DOI] [PubMed] [Google Scholar]