Abstract
Purpose: The aim of this study was to investigate antimicrobial and biofilm removal potential of Zataria multiflora essential oil (ZEO) and silver nanoparticle (SNP) alone and in combination on Staphylococcus aureus and Salmonella Typhimurium and evaluate the mechanism of action.
Methods: The minimum inhibitory concentration (MIC), and optimal inhibitory combination (OIC) of ZEO and SNP were determined according to fractional inhibitory concentration (FIC) method. Biofilm removal potential and leakage pattern of 260-nm absorbing material from the bacterial cell during exposure to the compounds were also investigated.
Results: MICs of SNP for both bacteria were the same as 25 μg/ mL. The MICs and MBCs values of ZEO were 2500 and 1250 μg/mL, respectively. The most effective OIC value for SNP and ZEO against Salm. Typhimurium and Staph. aureus were 12.5, 625 and 0.78, 1250 μg/ mL, respectively. ZEO and SNP at MIC and OIC concentrations represented a strong removal ability (>70%) on biofilm. Moreover, ZEO at MIC and OIC concentrations did a 6-log reduction of primary inoculated bacteria during 15 min contact time. The effect of ZEO on the loss of 260-nm material from the cell was faster than SNP during 15 and 60 min.
Conclusion: Combination of ZEO and SNP had significant sanitizing activity on examined bacteria which may be suitable for disinfecting the surfaces.
Keywords: Antimicrobial, Sanitizing, Biofilm, Essential oil, Silver nanoparticle, Combination
Introduction
Nanotechnology, the science of study and use of structures at nanoscale, is a promising tool for producing novel materials with biomedical applications. Silver nanoparticles (SNPs) has been extensively investigated in various scientific disciplines. Silver in the form of silver nitrate and silver sulfadiazine has been widely used to cure bacterial infections associated with burns. SNPs are considered as a potent antimicrobial, antifungal, antiviral and antiprotozoal compound and it is also reported to have anti-inflammatory activities.1 Synthesis and use of SNPs as a new generation of antimicrobial agents would be a fascinating and economical tool to solve drug resistance. Microorganisms display different responses to nanoparticles which is related to differences in the bacterial structure and the composition of the cell wall.1,2
Essential oils (EOs), as a plant secondary metabolites, are volatile aromatic compounds extracted from different parts of plants. For centuries, EOs have been used in medicine, perfumery, cosmetics and food. They are primarily used in medicine, but in the nineteenth-century, EOs have found their importance to impart aroma and taste ingredients.3 Lamiaceae is a family of plant with more than 230 genera, which distributed nearly worldwide. It contains many well- known species with fairly similar properties in botanical characteristics and applications, including Thymus vulgaris, Thymus caramanicus, Zataria multiflora, Ziziphora clinopodioides and Ziziphora tenuior.3-5Zataria multiflora Boiss is an important medicinal plant, distributed in Iran, Afghanistan, and Pakistan. The main antimicrobial compounds of Zataria multiflora Boiss essential oil (ZEO) are thymol, carvacrol, and p-cymene. The plant also contains tannins, flavonoids, saponins and some bitter substances.6 Among the phenolic compounds, thymol is the most characteristic chemical substance of ZEO which founds in leaves, flowers, and roots at various amounts.6,7 ZEO displays inhibitory activity on both gram-negative and gram-positive bacteria.8
The practical advantage of antimicrobial combinations has been comprehended for over 50 years. Combinations of two antimicrobial agents with a different mechanism of action, may enhance antimicrobial activity especially where resistance to a single agent develops by bacteria. Also, due to synergy or additive interaction, the combination of drugs allows utilizing lower antimicrobial concentration, reducing the harmful side effects and increasing treatment efficacy.9 Tackling public health issues occur by the growing number of multidrug-resistant bacteria, proposes new antimicrobial formulation based on the combination of older antimicrobials with a rich source of new agents, such as natural products.10 Simultaneous use of EOs and other antimicrobial compounds with great disinfectant properties has a high priority since using EOs in high concentrations make some unpleasant organoleptic changes in food and also on food contact surface when used as a sanitizing compound. Owing to the possible synergistic properties, combined use of EO with SNPs as a sanitizing mixture for food plant sanitation has been proposed.11 The combined application of nanoparticles with EO has been reported.2,12 To understand the combined antimicrobial and biofilm removal properties of SNP and ZEO, their effects on two important bacterial pathogens, Staph aureus and Salm. Typhimurium was investigated as a guideline for a possible application of formulated solution in sanitation schedule.
Materials and Methods
Silver nanoparticles solution (4000 µg/ mL with the particle size of 35 nm) was purchased from Pars Nano Nasb Co (Tehran, Iran) and sterilized by filtration through 0.22 µm filters before use. Peptone water, phosphate buffered saline (PBS), Luria-Bertani (LB) broth and agar, Agar-agar, and resazurin sodium were obtained from Sigma Chemical Co (St. Louis, MO., USA). All other chemicals were purchased from Merck (Darmstadt, Germany). The plant, Zataria multiflora Boiss, was purchased from local groceries. Staph. aureus ATCC 25923 and Salm. Typhimurium ATCC 14028 were obtained from the Department of Food Hygiene and Quality Control, Urmia University, Urmia, Iran.
Preparation of bacterial suspension
Staph. aureus and Salm. Typhimurium were grown at 37±1 °C for 18 h by transferring 10 microliters of frozen stocks (at -20 °C) into 10 mL of LB broth. Bacterial suspensions were adjusted to ~8 log10 CFU mL-1 using visible-ultraviolet spectrophotometer (Amersham Pharmacia Biotech Inc., Buckinghamshire, UK) at 600 nm (optical density: ~0.1) and confirmed by plating and counting on LB agar after 24 h incubation at 37±1 °C.
Essential oil extraction and quantification
The EO of Zataria multiflora Boiss (100 g) was extracted from dried aerial parts of plant using a Clevenger apparatus based on hydrodistillation procedure for 3 hours. The collected ZEO was dried over anhydrous Na2SO4, then filtrated and stored at 4 °C. The chemical composition of ZEOs was analyzed using a gas chromatograph (GC), as explained previously.6
Determination of Minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC)
The MICs and MBC of ZEO and SNP against both bacteria were determined in LB using broth microdilution assay in 96-wells polystyrene flat-bottomed microtitre plates based on CLSI guidelines.13 Two-fold serial dilutions of ZEO were prepared in 0.1% peptone water containing 0.15% agar (to make a stable emulsion of EO in peptone water), whereas dilutions of SNP were made in 0.1% peptone water. The wells of a microplates with U-bottom were poured by 160 μL of LB and 20 μL of the bacterial suspension with OD600 = 0.1 to reach a suspension with 106 CFU/ mL in each well. Then, 20 μL either ZEO or SNP concentrations were added into the desired wells to achieve final concentrations of 312 to 5000 μg/ mL for ZEO and 62.5 to 2000 μg/mL for SNP. For every experiment, three controls, including LB alone, LB with bacteria and LB containing treatment agents without bacteria were used. The plate was mixed on a plate shaker at 250 rpm for 20 s and incubated at 37 ± 1 °C for 24 h. MICs were determined visually and by using 0.01% (w/v) resazurin sodium salt solution as explained previously. To determine MBCs, 10 µL from each well was inoculated into LB agar at 37 ± 1 °C for 24 h. The MBC was determined as the lowest concentration of antimicrobial agents that produces 99.99% inhibition in the initial population of microorganism. MBC: MIC ratio, which describes the relationship between the minimum in vitro bactericidal concentration and the MIC of antimicrobial agents, were also investigated.
Antimicrobial combination and interaction
Broth checkerboard micro-assay was carried out to evaluate the antagonistic, indifferent, additive and synergistic interactions of ZEO and SNP using the fractional inhibitory concentration (FIC) index method.14 Eight different concentrations, 5 concentrations lower than MIC, two concentrations higher than MIC and one concentration as same as MIC of ZEO and SNP were used to design an 8 × 8 checkerboards of combinations. The microplates were prepared by dispensing 160 μL of LB and 20 μL of the logarithmic suspension of bacteria and 10 μL of different concentration of both antimicrobial agents into each well. Then, plates were kept in a plate shaker at 250 rpm for 20 s and incubated at 37 ± 1 °C for 24 h. MICs were determined visually and by resazurin reduction. MICs of the individual antimicrobials and all of the combinations were used to calculate Fractional inhibitory concentrations (FICs) of ZEO and SNP and FIC index using the following formula:
If the FICI is < 0.5, the interaction is synergistic, if the FICI= 0.5-1, the interaction is additive, if the FICI= 1-4, the interaction is indifferent, and an FICI >4 is considered antagonistic. Optimal inhibitory combinations (OIC) is defined as the combinations producing an inhibitory effect by using the lowest concentration of one antimicrobial in combination with the other.15
Determination of the release of 260-nm absorbing material
260-nm absorbing material released into the supernatant was estimated according to the method described previously,16 with some modifications. The bacterial suspension was prepared as described above. The procedure performed in 2 mL of harvested and washed cells (OD600nm = 0.45) to which SNP and ZEO were added at final concentrations equivalent to their MICs and OIC and incubated in a shaker incubator (250 rpm at 37 °C). Two samples were taken at 15 and 60 min time points and centrifuged at 4000 g for 15 min and the absorbance was determined at 260 nm using PBS as blank. Controls containing bacterial supernatant without treatment agents and antibacterial compounds without bacteria were also prepared and analyzed.
Biofilm removal
Biofilm removal potential of both agents alone and in combination was assessed using 24-well flat-bottomed polystyrene microtiter plates according to the method explained previously with some modifications.17 An aliquot of 200 μL of bacterial suspension with OD600 = 0.1 was dispensed into each well which was filled previously with 1800 μL of LB broth, using four repetitions per treatment to reach a suspension with 107 CFU/ mL per well. After incubation for 24 h at 37 ±1 °C, the planktonic cells in wells were then removed and the plates were washed three times with PBS and air-dried for 20 min at 23 ±2 °C. Then, 2000 μL of MIC, 2MIC and 4MIC concentrations of ZEO and SNP and their combinations (1/4 OIC, 1/2OIC, and OIC concentrations) were gently poured into the wells and incubated for 15 min at ambient temperature. The solutions were then removed and plates were washed three times with PBS and air-dried for 20 min at 23 ±2 °C. Following staining with 2 mL of 1% crystal violet (CV) (w/v) for 30 min, the contents of the wells were decanted and washed twice with tap water to remove the color excess and then allowed to air-dry for 30 min. The biomass of biofilms was quantified by solubilizing CV with 2 mL of acetic acid 33% and subsequent measuring optical absorbance (OD) at 540 nm. Wells containing LB broth and LB with bacteria without antibacterial were considered as negative and positive controls, respectively. Biofilm removal percentage was calculated as follow:
where C is OD540nm of control wells, B is OD540nm of negative controls and T is OD540nm of treated wells.
Combined sanitizing activity
Sanitizing effects of ZEO and SNP combination were investigated by determining the growth of the microorganism in LB broth supplemented with different concentration of antimicrobials (1/4OIC, 1/2OIC, and OIC) to obtain optimal concentration for both compounds according to the method explained before with some modifications.18 Bacterial cultures (1×107 CFU/ mL) (0.5 mL) were added to tubes containing 4.5 mL of antimicrobial agents in combination and then, tubes were incubated at 23± 2 °C for 15 min and bacterial growth was monitored by sampling (1mL) and counting the viable cells. After sampling, 1 mL aliquot were dispersed in a 9 mL neutralizing solution containing an equal volume of sodium thiosulphate 5% w/v and Tween-20 and remained for 10 min, to neutralize the subsequent activity of agents and then serial dilutions were prepared.
Statistical analysis
Each experiment was replicated in triplicate and carried out on three separate times and data were expressed as means ± S.E. Data were analyzed by analysis of variance (ANOVA, P<0.05) using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA (www.graphpad.com).
Results
ZEO chemical analysis
As shown in Table 1, 26 different components were identified in ZEO, representing 97.23%, of total EO. General chemical profile of ZEO was characterized by an abundance concentration of thymol (44%). In addition to thymol, carvacrol (14.04%) and p-cymene (11.15%), as main constituents, and traces of linalool, ϒ-terpinene, and α-Pinene were also found in ZEO.
Table 1. Chemical composition of ZEO.
| Compounds | KI a | Area (%) | Compounds | KI | Area (%) |
| α-Thujene | 931 | 0.15 | linalool | 1106 | 6.26 |
| α-Pinene | 639 | 3.66 | Borneol | 1163 | 0.19 |
| Camphene | 953 | 0.17 | Terpinen-4-ol | 1175 | 4.63 |
| β-Pinene | 980 | 1.55 | Thymol methyl ether | 1233 | 0.17 |
| β-Mycrene | 991 | 1.35 | Bornyl acetate | 1284 | 0.17 |
| α-Phellandrene | 1002 | 0.15 | Thymol | 1301 | 44 |
| d-3-Carene | 1009 | 1 | Carvacrol | 1318 | 14.4 |
| α-Terpinene | 1016 | 1.74 | Acetylthymol | 1284 | 0.3 |
| Cis-para-menth-2-en-1-o | 1123 | 0.37 | d-Elemene | 1340 | 0.24 |
| p-Cymene | 1028 | 11.15 | Eugenol | 1360 | 0.82 |
| 1,8-Cineole | 1032 | 0.86 | Trans-Caryophyllene | 1423 | 2.78 |
| γ-Terpinene | 1060 | 0.08 | Caryophyllene oxide | 1583 | 0.93 |
| α-Terpinolene | 1087 | 0.08 | |||
| Total | 97.23% |
aKovats indices calculated against n-alkanes on HP-5 column.
Antibacterial properties of ZEO and SNP
The inhibitory effects of ZEO and SNP alone and in combination against Staph. aureus and Salm. Typhimurium were investigated using microtiter plate assay. For Staph. aureus, the MIC values of ZEO and SNP were 1250 and 25 μg/mL and for Salm. Typhimurium the values were 2500 and 25 μg/mL, respectively. In all cases, MBC values were similar to MICs. The ZEO was found to be more effective on gram-positive than gram-negative bacteria whereas SNP displayed similar antibacterial activity on both bacteria. The MICs for SNP - ZEO combination were 0.78 and 12.5 μg/ mL against Staph. aureus and Salm. Typhimurium, respectively. ZEO-SNP combination inhibited S. aureus and Salm. Typhimurium at 625 μg/ mL. Based on the FICI scale (Table 2), the combination displayed a synergistic action on Staph. aureus (FICI=0.81) and Salm. Typhimurium (FICI= 0.75).
Table 2. Survival population (log CFU/ mL) of Staph. aureus and Salm. Typhimurium treated with ZEO and SNP alone and in combination during 15 min contact time at room temperature.
| Microorganism* | Alone at MIC concentration | In combination | |||
| ZEO | SNP | OIC | 1/2OIC | 1/4OIC | |
| Staph. aureus | 0 | 3.11 × 105± 0.08a | 0 | 3.11 × 105± 0.67a | 4.74 × 105± 0.41a |
| Salm. Typhimurium | 0 | 4.69 × 105± 0.19b | 0 | 3.90 × 105± 0.21b | 5.30 × 105± 0.33b |
*Initial bacterial counts: 106 CFU mL-1. Different letters for each column of indicate a statistically significant difference (P < 0.05).
Loss of 260 nm absorbing material
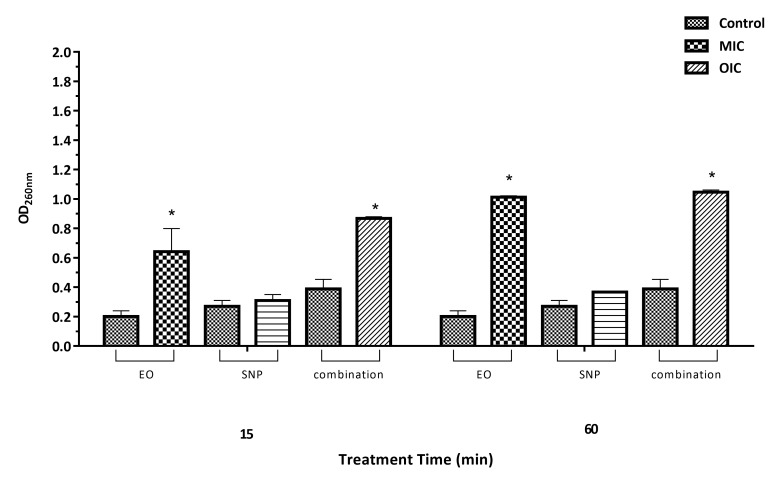
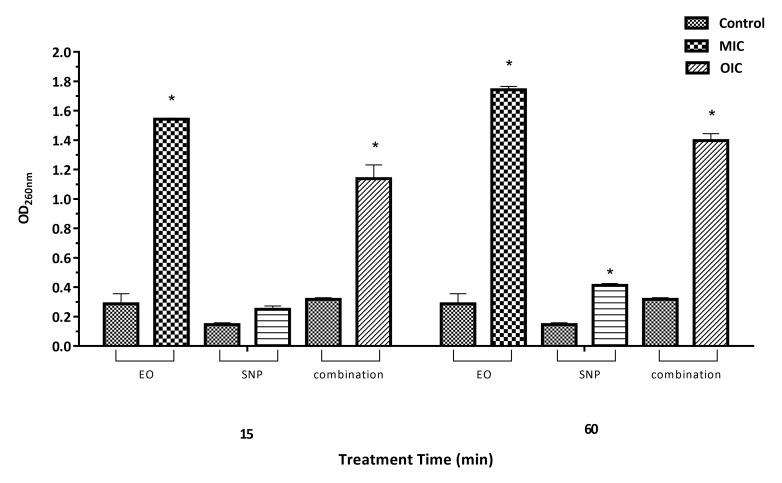
Exposure of Staph. aureus and Salm. Typhimurium to ZEO at MIC concentrations over 15 min resulted in a significant increase in loss of 260 nm absorbing material from the bacterial cell compared with the control (P<0.05), indicating a stronger disruption of the cell membrane by ZEO (Figure 1, 2). Results also showed that the loss began before 15 min and continued up to 60 min. In general, loss of cytoplasmic materials by ZEO from both bacteria was faster than SNP. The leakage pattern of 260-nm absorbing material was directly linked to the sanitizing activity of both antimicrobial compounds. As shown in Figures 1 and 2, no significant release of 260 nm absorbing material with SNP- treated at MIC concentration over 15 min for both bacteria was found. Higher exposure time (60 min) significantly increased the cell leakage of Salm. Typhimurium, but not in case of Staph. aureus. Longer exposures (60 min) with OIC concentration of ZEO and SNP (Figures 1 and 2), caused 3 and 4-fold more gross membrane damage, in Staph. aureus and Salm. Typhimurium culture compared to the control sample, respectively.
Figure 1.
260-nm absorbing material released from Staph. aureus cells after treatment with MIC concentration of silver nanoparticle (SNP), Zataria multiflora essential oil (EO) and their combination. Asterisks indicate significantly different values (P<0.05) when comparing optical density (OD) of control and each treatment at the same exposure time.
Figure 2.
260-nm absorbing material released from Salm. Typhimurium cells after treatment with MIC concentration of silver nanoparticle (SNP), Zataria multiflora essential oil (EO) and their combination. Asterisks indicate significantly different values (P<0.05) when comparing optical density (OD) of control and each treatment at the same exposure time.
Biofilm removal
As compared with Salm. Typhimurium (Figure 3b), higher removal of Staph. aureus biofilms were achieved at 15 min exposure time for SNP-ZEO (Figure 3a). Results demonstrated that biofilm of Staph. aureus is more sensitive to SNP - ZEO than Salm. Typhimurium. SNP at MIC, 2MIC, and 4MIC concentrations resulted in 93, 87 and 71.33% and 82, 79 and 58% removal of Staph. aureus and Salm. Typhimurium cells, respectively. For Staph. aureus and Salm. Typhimurium, 90.6%, and 89% bacteria were eliminated at a concentration of MIC for ZEO. Concerning the hydrophobicity property of ZEO, it can be observed in Figure 3, a reduction in the biofilm removal with increasing ZEO concentration supporting the use of surfactants could help in the disruption of such shortcoming.
Figure 3.
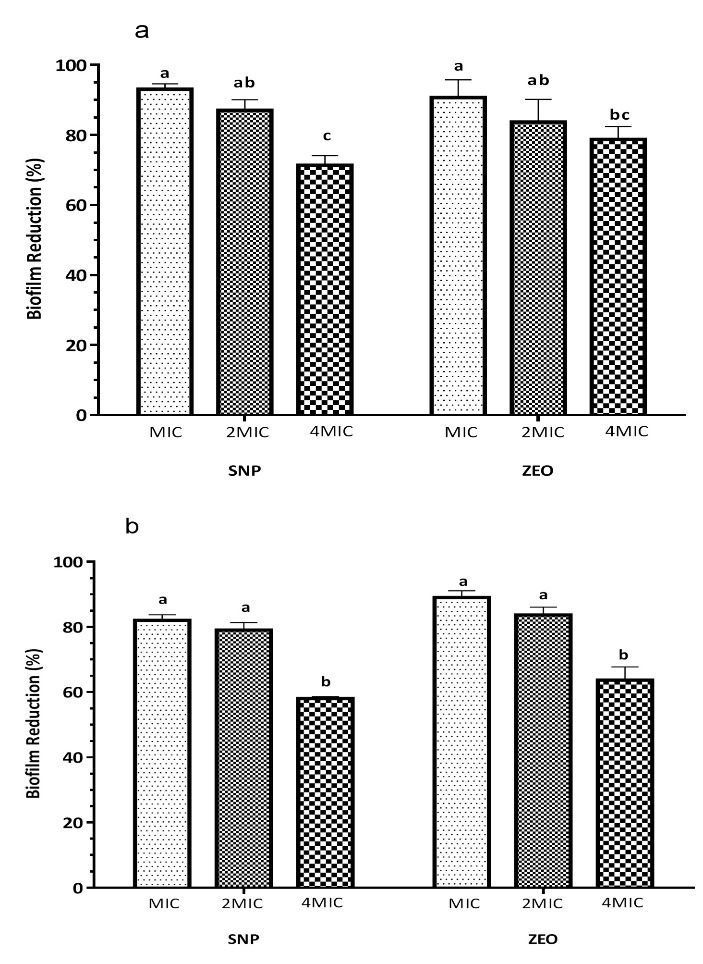
The effect of silver nanoparticle (SNP) and Zataria multiflora essential oil (ZEO) on removal one-day old biofilm of Staph. aureus (a) and Salm. Typhimurium (b) developed on polystyrene surface with 15 min contact time. Different letters for each concentration of antimicrobial indicate a statistically significant difference (P < 0.05).
Synergistic sanitizing activity
The purpose of this experiment was to achieve a best effective concentration of SNP and ZEO as a sanitizing solution. To assess the disinfectants efficiencies, MIC and OIC concentrations were determined. The reductions in the Staph. aureus and Salm. Typhimurium counts after 15 min exposure to MIC and various OIC concentrations were shown in Table 2. A reduction of 100% was achieved for both bacteria after 15 min exposure to ZEO. A similar reduction was also found for OIC concentration. Whereas, less than 0.4 log10 CFU/ mL reduction was observed for the samples treated with SNP in alone.
As shown by 260 nm absorbing material measurements, SNP displayed an antimicrobial activity after a long time of exposure (at least after 1 h) compared with ZEO. In comparison with Staph. aureus, Salm. Typhimurium had higher resistance to SNP. Additionally, no significant reduction (P < 0.05) was observed in the microbial counts of 1/2OIC and 1/4OIC with SNP treated samples.
Discussion
The components identified in ZEO (Table 1) in this study was similar to previous works. According to Saedi Dezaki et al. (2007),19 the major components of ZEO were thymol (41.81%), carvacrol (28.85%), and p-cymene (5.63-13.16%). In the study of Saei-Dehkordi, et al. (2010),7 a variation of the major components, thymol (27.5-64.87%), carvacrol (2.7-22.39), p-cymene (8.36%) and linalool (0-7.92%) of ZEO were observed, due to the collection of plant material from five main phytogeographic grown towns in Iran. Factors such as growth phase and geographic origin of plant, method of plant drying and processing affect EO content and chemical compositions.7
In our study, the antibacterial properties of ZEO was not different to the results reported from other studies, apart from some detected variable bacterial susceptibilities that may cause the differences in the chemical composition of ZEOs and the bacterial strains used by others. The hydrophobic property of most EOs could increase their permeability and accumulation in the bacterial cell membranes.6,7
Silver ions and silver-based compounds are widely known as highly toxic to 16 major species of bacteria due to their multiple mechanisms of action. Zarei et al. (2014) reported the MIC values of SNP against Listeria monocytogenes, S. Typhimurium, Escherichia coli O157:H7 and Vibrio parahaemolyticus in the range of 3.12- 6.25 µg mL-1 according to microdilution method in Tryptic soy broth.20 The small size and high ratio of surface to volume in SNPs, allow them to more effectively contact with microorganisms and induce their antimicrobial activities.21 It has been shown that antibacterial efficacy of SNP varies between different prepared SNPs. Given the fact that factors such as the type of bacteria, the media of inoculation, type and the size of nanoparticle and the method of preparation must be considered when comparing the antibacterial activity of different nanoparticles.1
The additive or partial synergy effect is a type of interaction in which the combined effect is equal to the sum of the effects of the individual agents. As shown previously,22 SNP interaction with EO is a bacteria-dependent phenomenon. The synergistic properties of SNP with ZEO on Staph. epidermidis and Staph. aureus (FICI= 0.5-1) has been demonstrated, but no synergistic effects were found against Methicillin-resistant Staph. aureus and Ps. aeruginosa (FICI value of 3 and 1.25, respectively).
The MBC: MIC ratio ≥ 8 is considered as an indicator of bacteriostatic activity.23 In the current study, MIC/MBC of both SNP and ZEO were 1:1, suggesting a bactericidal effect on Staph. aureus and Salm. Typhimurium. It was demonstrated that oregano EO reveals a synergistic activity with common antibiotics such as gentamicin against some potential pathogens,24 additive activity in combination with another antibiotic such as amoxicillin and polymyxin on Extended-Spectrum Beta-Lactamase (ESBL)-producing E.coli,25 and synergistic with other EO obtained from Thymus vulgare and Rosmarinus officinalis.26
Measuring the loss of 260-nm absorbing material from the bacteria is an indicator of cell leakage which could be used to understand the mechanism of action of antimicrobial compounds. The presence of materials with an absorbance at 260 nm in the supernatant of bacterial solution indicates a loss of nucleic acids from the cell. Based on the results of this study (Figures 1 and 2), the higher values determined by the measurements at 260 nm are an indication for the leakage of bacterial contents which subsequently confirms the physical damages of bacterial cell walls by ZEO.4 However, the reductions in the efficacy of SNP may be best explained by bacterial blocking caused by higher concentrations of SNP, which could reduce the contact surface of nanoparticles with bacteria and its antibacterial efficacy.
SNP can cause agglomeration after adding to the nutritious media such as LB and as shown previously,2 treatment of Staph. aureus with a combination of EO and SNP, induced a reduction in cell density, exopolysaccharide, morphology changes, and cell destruction. However, membrane permeability created by EO might allow the small molecules of SNP to enter the cell.
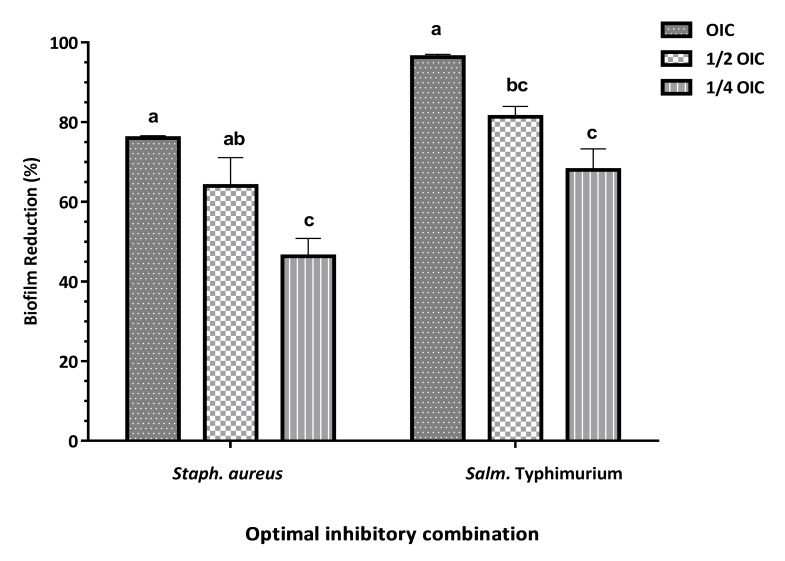
Researchers are aware of the importance of biofilms in causing diseases and drug resistance, therefore finding a safe and effective method for biofilm removal is of great importance. Natural agents are considered as a safe way to remove biofilms. Although the research on SNP interaction with biofilm is still in its early phases, we currently know that removal of biofilm by SNP could occur in three steps: transportation to the vicinity of the biofilm, attachment, and penetration within the biofilm.27 It has been shown that eradication of biofilms by SNP is not a concentration-dependent process, rather it occurs in a time-dependent manner.28 The main compounds of ZEO are thymol, a monoterpene phenolic derivative, and its phenol isomer, carvacrol. Both of them contribute to antimicrobial and antioxidant properties of Lamiaceae family.4 Additionally, hydrophobic characteristics of these ingredients allow the penetration of ZEO into outer exopolysaccharide and inner layers of biofilms.29 In our study, by decreasing the OIC concentration from 1/2OIC to 1/4OIC (Figure 4), the biofilm removal properties were significantly decreased (P<0.05) from 64 % to 43.33 % for Staph. aureus (Figure 4) whereas OIC concentration, removed 76% of biofilm mass. For Salm. Typhimurium (Figure 4) the values were 96.46%, 81.50% and 68 % for OIC, 1/2OIC and 1/4OIC concentrations, respectively. It means that combined use of SNP and ZEO boosted biofilm removal potential of both antibacterial compounds against different pathogens. According to Gurunathan et al. (2014), the combined use of NPs and antibiotic such as ampicillin exhibit antibiofilm activity on Gram-positive and Gram-negative bacteria by 55 and 70%, whereas combining those NPs with vancomycin revealed a 75 and 55% reduction of biofilm of Gram-positive and Gram-negative bacteria.1 It is worth mentioning that, differences in the method used to evaluate biofilm removal activity and differences in the sensitivity of different bacteria, the age of biofilm and type of surface which biofilm developed could cause different results in the biofilm removal percentage in previous studies.
Figure 4.
The effect of silver nanoparticle (SNP) and Zataria multiflora essential oil (ZEO) combination on removal one-day old biofilm of Staph. aureus and Salm. Typhimurium developed on polystyrene surface with 15 min contact time. Different letters for each concentration of antimicrobial indicate a statistically significant difference (P < 0.05).
Conclusion
The results of our study demonstrated that SNP and ZEO have synergistic antibacterial activities against Staph. aureus, and Salm. Typhimurium. It was also shown that the antimicrobial and biofilm removal properties of SNP and ZEO were affected by the type of microorganisms and concentrations of both compounds. ZEO displayed a fast antimicrobial activity. Both antimicrobials represented considerable biofilm removal activity on both bacteria. The combination of SNP and ZEO was additive, which means significant antibacterial and antibiofilm activity could achieve by use of agents at concentrations without compromising their antibacterial effects. The best concentrations for SNP- ZEO sanitizing solution were 12.5 μg/ mL for SNP and 625 μg/ mL for ZEO. Our results highlighted the powerful combination activity of SNP and ZEO which accelerated antibacterial activity, alleviated undesirable sensorial property of ZEO and reduced the concentration of both compounds.
Acknowledgments
This study was funded by a grant from Faculty of Veterinary Medicine and Institute of Biotechnology, Urmia University. The authors would like to thank Dr. Mahmoudian for his assistance.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interest related to this work.
References
- 1.Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against gram-negative and gram-positive bacteria. Nanoscale Res Lett. 2014;9(1):373. doi: 10.1186/1556-276X-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scandorieiro S, de Camargo LC, Lancheros CA, Yamada-Ogatta SF, Nakamura CV, de Oliveira AG. et al. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front Microbiol. 2016;7:760. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora boiss. (shirazi thyme)--an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145(3):686–98. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Shokri H, Sharifzadeh A. Zataria multiflora boiss.: A review study on chemical composition, anti-fungal and anti-mycotoxin activities, and ultrastructural changes. J HerbMed Pharmacol. 2017;6(1):1–9. [Google Scholar]
- 6.Moradi M, Tajik H, Razavi Rohani SM, Mahmoudian A. Antioxidant and antimicrobial effects of zein edible film impregnated with Zataria multiflora Boiss. LWT - Food Sci Technol. 2016;72:37–43. doi: 10.1016/j.lwt.2016.04.026. [DOI] [Google Scholar]
- 7.Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in zataria multiflora boiss. From different parts of iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48(6):1562–7. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18(7):800–5. doi: 10.1016/j.foodcont.2006.04.002. [DOI] [Google Scholar]
- 9.Desbois AP, Lang S, Gemmell CG, Coote PJ. Surface disinfection properties of the combination of an antimicrobial peptide, ranalexin, with an endopeptidase, lysostaphin, against methicillin-resistant Staphylococcus aureus (MRSA) J Appl Microbiol. 2010;108(2):723–30. doi: 10.1111/j.1365-2672.2009.04472.x. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Righi E. New antibiotics and antimicrobial combination therapy for the treatment of gram-negative bacterial infections. Curr Opin Crit Care. 2015;21(5):402–11. doi: 10.1097/mcc.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez J, Barry-Ryan C, Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol. 2008;124(1):91–7. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Taghizadeh M, Solgi M. The application of essential oils and silver nanoparticles for sterilization of bermudagrass explants in in vitro culture. Int J Hort Sci Technol. 2014;1(2):131–40. [Google Scholar]
- 13. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. Document M100-S22. Wayne, Pa, USA: CLSI; 2012.
- 14. Moody JA. Synergism testing: Broth microdilution checkerboard and broth microdilution methods. In: Isenberg HD, editor. Clinical microbiology procedures handbook. Washington, DC: American Society for Microbiology; 2003. PP. 1-28.
- 15.Dong X, Chen F, Zhang Y, Liu H, Liu Y, Ma L. In vitro activities of rifampin, colistin, sulbactam and tigecycline tested alone and in combination against extensively drug-resistant acinetobacter baumannii. J Antibiot (Tokyo) 2014;67(9):677–80. doi: 10.1038/ja.2014.99. [DOI] [PubMed] [Google Scholar]
- 16.Rhayour K, Bouchikhi T, Tantaoui-Elaraki A, Sendide K, Remmal A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on escherichia coli and bacillus subtilis. J Essent Oil Res. 2003;15(5):356–62. doi: 10.1080/10412905.2003.9698611. [DOI] [Google Scholar]
- 17.Mahdavi M, Jalali M, Kasra Kermanshahi R. The effect of nisin on biofilm forming foodborne bacteria using microtiter plate method. Res Pharm Sci. 2007;2(2):113–8. [Google Scholar]
- 18.Phongphakdee K, Nitisinprasert S. Combination inhibition activity of nisin and ethanol on the growth inhibition of pathogenic gram negative bacteria and their application as disinfectant solution. J Food Sci. 2015;80(10):M2241–6. doi: 10.1111/1750-3841.13015. [DOI] [PubMed] [Google Scholar]
- 19.Saedi Dezaki E, Mahmoudvand H, Sharififar F, Fallahi S, Monzote L, Ezatkhah F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm Biol. 2016;54(5):752–8. doi: 10.3109/13880209.2015.1079223. [DOI] [PubMed] [Google Scholar]
- 20.Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J Microbiol. 2014;7(1):e8720. doi: 10.5812/jjm.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Diaz M, Alvarado-Gomez E, Magana-Aquino M, Sanchez-Sanchez R, Velasquillo C, Gonzalez C. et al. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater Sci Eng C Mater Biol Appl. 2016;60:317–23. doi: 10.1016/j.msec.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 22.Sheikholeslami S, Mousavi SE, Ahmadi Ashtiani HR, Hosseini Doust SR, Mahdi Rezayat S. Antibacterial activity of silver nanoparticles and their combination with zataria multiflora essential oil and methanol extract. Jundishapur J Microbiol. 2016;9(10):e36070. doi: 10.5812/jjm.36070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parhi AK, Zhang Y, Saionz KW, Pradhan P, Kaul M, Trivedi K. et al. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg Med Chem Lett. 2013;23(17):4968–74. doi: 10.1016/j.bmcl.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honorio VG, Bezerra J, Souza GT, Carvalho RJ, Gomes-Neto NJ, Figueiredo RC. et al. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front Microbiol. 2015;6:1223. doi: 10.3389/fmicb.2015.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosato A, Piarulli M, Corbo F, Muraglia M, Carone A, Vitali ME. et al. In vitro synergistic antibacterial action of certain combinations of gentamicin and essential oils. Curr Med Chem. 2010;17(28):3289–95. doi: 10.2174/092986710792231996. [DOI] [PubMed] [Google Scholar]
- 26.Si H, Hu J, Liu Z, Zeng ZL. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum β-lactamase-producing Escherichia coli. FEMS Immunol Med Microbiol. 2008;53(2):190–4. doi: 10.1111/j.1574-695X.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikuma K, Decho AW, Lau BL. When nanoparticles meet biofilms-interactions guiding the environmental fate and accumulation of nanoparticles. Front Microbiol. 2015;6:591. doi: 10.3389/fmicb.2015.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khameneh B, Zarei H, Fazly Bazzaz BS. The effect of silver nanoparticles on staphylococcus epidermidis biofilm biomass and cell viability. Nanomed J. 2014;1(5):302–7. doi: 10.7508/nmj.2015.05.003. [DOI] [Google Scholar]
- 29.Vazquez-Sanchez D, Cabo ML, Rodriguez-Herrera JJ. Antimicrobial activity of essential oils against staphylococcus aureus biofilms. Food Sci Technol Int. 2015;21(8):559–70. doi: 10.1177/1082013214553996. [DOI] [PubMed] [Google Scholar]