Abstract
Purpose: the non-edible fruit parts of Casimiroa edulis Llave et were evaluated for their active constituents and their potential as antioxidants, anti-inflammatory and antitumor activity.
Methods: Fruits peel (FP) and seeds kernel (SK) of Casimiroa edulis Llave et Lex. were extracted successively with hexane and then methanol. Fatty acids were prepared from hexane extracts and identified by GC. Total flavonoid, phenolic acids and tannins contents in methanol extracts were determined by UV spectrophotometer and identified by HPLC. Antioxidant, in-vitro anti-inflammatory activity and antitumor effect against Caco-2 cell line were determined.
Results: GC analysis of hexane extracts showed that oleic acid (47.00%) was the major unsaturated fatty acids in both extracts while lignoceric acid (15.49%) is the most abundant saturated fatty acid in (FP). Total phenolic, flavonoid and tannin contents in (FP) & (SK) methanol extracts were; 37.5±1.5, 10.79±0.66 and 22.28±0.23 for (FP); 53.5±1.5mg/g, 14.44±0.32 mg/g; and 53.73±3.58 mg/g for (SK) respectively. HPLC analysis of methanol extract revealed that; the major phenolic compound was pyrogallol in (FP) and p-hydroxybenzoic acid in (SK), the major flavonoid was luteolin 6-arabinose-8-glucose in (FP) and acacetin in (SK).
Conclusion: This study showed that non-edible parts of C. edulis fruit is a rich source of different phenolic compounds and fatty acids which has great antioxidant, anti-inflammatory and antitumor activities; that could be used as a natural source in pharmaceutical industry.
Keywords: Casimiroa edulis, Fatty acids, Phenolic contents, Antioxidant, Anti-inflammatory, Caco-2
Introduction
Family Rutaceae; is a small family made up of cultivated fruiting trees and medicinal herbs frequently called citrus family, it has a great economic importance because of its several edible Citrus fruits as orange, lemon, etc. Family Rutaceae is dispersed all over the world, particularly in warm climate and tropical areas, mostly found in Africa and Australia.1
Casimiroa edulis Llaveet Lex. is a non-citrus fruit belongs to this family, it is commonly known as Zapote blanco or white sapota and mainly cultivated in Mexico and Central America. C. edulis is widely consumed in different parts of the world for its valuable fruit;2 as it is a rich source of sugar, protein, ascorbic acid, phenols, carotenoids, polyunsaturated fatty acids and minerals like Fe, Cu, Zn, Ca and K.3 It is traditionally used in many countries as a sleep inducer as it has interesting sedative-like effects.2 C. edulis leaves and seeds were found to affect blood pressure, cardiac activity aortic muscular tone,4 and to possess anticonvulsant activity.5 Methanol extract of C. edulis leaves also showed strong antioxidant activity.3
Different classes of compounds were previously separated from different parts of C. edulis; furocoumarins and polymethoxyflavones were isolated from the leaves that exhibited adipogenesis activity.6 Moreover the leaves essential oil had promising antimicrobial activity and mainly contain sesquiterpene hydrocarbons as major constituents.7 Zapotin; a flavanoidal compound which considered as chemo-preventive agent was isolated from the seeds; it was also chemically synthesized because of its great anticancer activity.8 Different compounds were also isolated from the seeds methanolic extract and showed great cardiovascular activity. 9
Several studies reported that; C. edulis can be considered as valuable plant, so the aim of this study is to evaluate the importance of the non-edible parts of C. edulis fruit to evaluate its chemical composition as well as antioxidant, anti-inflammatory and antitumor potential.
Materials and Methods
Plant material
The fruit of C. edulis was collected from a public garden in Helwan, Cairo, Egypt and identified by taxonomist Therese Labib, consultant in the central gardening administration, Orman garden, Giza, Egypt. Fruits were peeled (FP), seeds were separated from the fruit and the kernel was obtained after removing the seed testa (SK). Both were separately dried at room temperature. A voucher specimen (PHG-8) has been deposited in the Pharmacognosy Department, Faculty of Pharmacy, Future University in Egypt (FUE), New Cairo, Egypt.
Preparation of plant extract
100 gm fruit peel (FP) and 100 gm seed kernel (SK) of C. edulis were separately coarsely powdered and extracted with n-hexane then by methanol for 72 h using a Soxhlet extractor at 60°C. All the extracts were dried separately under reduced pressure.
Chemical composition
GC analysis of the Fatty Acids composition of hexane extract
Hexane extracts of (FP)He and (SK)He were subjected separately to direct methylation in 1.5% sulfuric acid –methanol at 95°C for 2 h.10
Total Flavonoid, Phenolic acids &Tannins content in methanol extract of C. edulis
This was determined for the methanol extracts of (FP)Me and (SK)Me according to methods described previously.11,12
HPLC Analysis of the methanol extracts
The phenolic and flavonoid compounds of (FP)Me and (SK)Me of C. edulis were extracted according to the method described by Mattila et al.13
Biological activity for methanol and hexane extracts
Antioxidant activity of C. edulis extracts using ABTS, DPPH and Total antioxidant activity
It was carried out according to Arnao et al.,14 Ye et al. method.15,16
In vitro Antitumor activity
The activity was tested on Caco-2 cell line using sulforhodamin B assay.17
In vitro Anti-inflammatory activity using bovine albumin serum
This was tested using the method of Rahman et al.18
Statistical analysis
All result is expressed as mean value of three replicate. Data were statistically analyzed through analysis of variance (ANOVA) and Duncans test at P>0.01 using CoStat Statistics Software.
Results and Discussion
Chemical composition
Fatty acids composition of C. edulis hexane extracts
"Table 1" showed that Both (FP)He and (SK)He extracts revealed high percentage of total unsaturated fatty acids 71.15% and 94.20% respectively. The monounsaturated fatty acids oleic acid (omega-9) is the most abundant in both extracts; (36%) in (FP)He and (47%) in (SK)He; Also palmitoleic acid was found in (FP)He (20%) and (SK)He (21%). Furthermore, the hexane extracts showed the presence of different long chain mono and poly unsaturated fatty acids. The unsaturated fatty acids have a great role in decreasing the risk of certain cancers, as colon cancers, breast and prostate.19
Table 1. GC analysis of unsaturated fatty acid% in hexane extracts .
| Unsaturated fatty acid | Fatty acid % | ||
| (FP)He extract | (SK)He extract | ||
| C14:1 | Myristoleic | 1.64 | 0.5 |
| C15:1 | Pentadecanoic acid | 1.29 | 0.32 |
| C16:1 | Palmitoleic acid | 20.00 | 21.00 |
| C17:1 | Heptadecanoic acid | 0.35 | 0.63 |
| C18:1 | Oleic acid | 36.00 | 47.00 |
| C18:1 | Vaccenic acid | 0.3 | ND |
| C18:2 | Linoleic acid | 2.18 | 9.00 |
| C18:3 | α-Linolenic acid | 2.56 | 1.09 |
| C18:3 | γ-Linolenic acid | ND | 9.01 |
| C20:2 | Eicosadienoic acid | 1.09 | 2.20 |
| C20:3 | Eicosatrienoic acid | 1.28 | 1.9 |
| C22:1 | Erucic acid | 1.89 | ND |
| C24:1 | Nervonic acid | 2.57 | 1.55 |
| Saturated fatty acid | (FP)He extract | (SK)He extract | |
| C6:0 | Caproic acid | 0.77 | ND |
| C8:0 | Caprylic acid | 0.60 | ND |
| C10:0 | Capric acid | 0.13 | ND |
| C11:0 | Undecylic acid | 0.2 | ND |
| C12:0 | Lauric acid | 0.67 | 0.15 |
| C13:0 | Tridecylic acid | 2.47 | ND |
| C14:0 | Myristic acid | 3.4 | 0.2 |
| C15:0 | Pentadecylic acid | 2.06 | 0.63 |
| C16:0 | Palmitic acid | 1.23 | 3.01 |
| C17:0 | Heptadecanoic acid | ND | 1.30 |
| C21:0 | Heneicosylic acid | 0.45 | ND |
| C22:0 | Behenic acid | 1.28 | 0.3 |
| C23:0 | Tricosylic acid | 0.1 | 0.21 |
| C24:0 | Lignoceric acid | 15.49 | ND |
| Total mono-unsaturated fatty acid% | 64.04 | 71 | |
| Total poly-unsaturated fatty acid % | 7.11 | 23.2 | |
| Total saturated fatty acid % | 28.85 | 5.8 | |
ND: not detectable (FP)He: fruit peel hexane extract (SK)He: seed kernel hexane extract
(FP)He has higher percent of total saturated fatty acid 28.85% than that in (SK)He 5.8%; lignoceric acid 15.49% was the major in (FP)He while palmitic acid 3.01% was the highest in (SK)He; these fatty acids play important role in increasing LDL cholesterol level.20
Lipid profile presented in "Table 1" showed that both (FP)He and (SK)He extracts have great percentage of unsaturated fatty acids more than the saturated one; this indicate that the non-edible parts of C. edulis can be considered as a valuable natural source that offer a way of increasing the availability of unsaturated fatty acids especially oleic, palmiotleic, linoleic and γ-linolenic acid. Previous studies proved that those acids have a role in inflammation suppression.20
Total Flavonoid, Phenolic acids & Tannins contents in C. edulis methanol extract
The results of qualitative analysis of both extracts (FP)Me & (SK)Me revealed the presence of considerable amount of secondary metabolites which could be an indication for their pharmaceutical potential. The results in "Table 2" showed that they are more abundant in (SK)Me than that in (FP)Me.
Table 2. Total phenolic acid, flavonoid and tannin contents in C. edulis methanol extracts .
| Methanol extract | Phenolic (mg/g) DW | Flavonoids (mg/g) DW | Tannins (mg/g) DW |
| (FP)Me | 37.5±1.5b | 10.79±0.66b | 22.28±0.23b |
| (SK)Me | 53.5±1.5a | 14.44±0.32a | 53.73±3. 58a |
| LSD | 1.9 | 5.6 | 9.5 |
DW: dry weight, (FP)Me: fruit peel methanol extract, (SK)Me: seed kernel hexane extract
HPLC Analysis of phenolic compounds and flavonoid contents in C. edulis methanol extracts
"Table 3" recorded that the (FP)Me and (SK)Me extracts contained different phenolic and flavonoid compounds.
Table 3. HPLC analysis of the phenolic and flavonoids compounds in C. edulis methanol extracts .
| Phenolic compounds | (FP)Me (mg/100g) DW | (SK)Me (mg/100g) DW |
| 3,4,5-methoxycinnamic acid | 3.43 | 37.34 |
| 4-amino benzoic acid | 79.86 | 4.86 |
| Benzoic acid | 252.60 | 251.11 |
| Caffeic acid | 15.38 | 48.76 |
| Catechein | 169.77 | 240.81 |
| Catechol | 230.60 | 190.87 |
| Chlorogenic acid | 175.36 | 410.98 |
| Cinnamic acid | 6.36 | 24.44 |
| Ellagic acid | 52.37 | 133.42 |
| Epicatechein | 176.30 | 60.97 |
| e-vanillic acid | 457.57 | 344.81 |
| Ferulic acid | 53.20 | 58.32 |
| Gallic acid | 21.56 | 28.94 |
| Iso-ferulic acid | 100.11 | 22.34 |
| p-coumaric acid | 52.04 | 55.63 |
| P-hydroxy benzoic acid | 185.72 | 1571.13 |
| Protocatechuic acid | 79.86 | 89.72 |
| Pyrogallol | 1846.16 | 695.98 |
| Reversetrol | 7.00 | 14.45 |
| Rosmarinic acid | 30.37 | 11.27 |
| Salycilic acid | 18.39 | 60.80 |
| Vanillic acid | 53.48 | 49.70 |
| α- coumaric acid | 7.75 | 36.95 |
| Flavonoids compounds | (FP)Me (mg/100g) DW | (SK)Me (mg/100g) DW |
| Luteolin-6-arabinose-8-glucose | 1907.92 | 1242.72 |
| Luteolin-6-glucose-8-arabinose | 537.94 | 561.91 |
| Apigenin-6-arabinose-8-galactose | 97.63 | 41.01 |
| Apigenin-6-rhamnose-8-glucose | 322.24 | 592.74 |
| Apigenin-6-glucose-8-rhamnose | 823.66 | 129.61 |
| Apigenin-7-O-neohespiroside | - | 17.94 |
| Apigenin-7-O-glucose | - | 54.66 |
| Luteolin-7-O-glucose | - | 26.09 |
| Kampferol-3,7-dirhamoside | - | 47.02 |
| Luteolin | 1103.24 | 150.63 |
| Acacetin | 103.93 | 2560.78 |
| Naringin | 3.48 | 291.92 |
| Rutin | 238.18 | 181.26 |
| Hespirdin | 196.86 | - |
| Quercetrin | 25.10 | 37.75 |
| Quercetin | 35.92 | 298.65 |
| Kampferol | 6.66 | 14.70 |
| Hespirtin | 10.45 | 26.26 |
| Apigenin | 0.48 | 87.31 |
| Rhamnetin | 2.58 | 66.26 |
| Total identified compounds | 16 | 19 |
Twenty three phenolic compounds were identified in both (FP)Me and (SK)Me by comparison with authentic reference compounds. In (FP)Me pyrogallol is the most abundant phenolic compound 1846.16 mg/100g followed by e-vanillic acid 457.57 mg/100g, benzoic acid 252.6 mg/100g and catechol tannins 230.6 mg/100g. The major phenolic compound in (SK)Me was P-hydroxy benzoic acid 1571.13 mg/100g followed by pyrogallol 695.98 mg/100g then cholinergic acid 410.98 mg/100g and e-vanillic acid 344.81 mg/100g.
The total flavonoid compounds identified in (FP)Me extract was 16 compounds the major compound was luteolin 6-arabinose-8-glucose 1907.92 mg/100g.
Biological activity
Antioxidant activity of C. edulis extracts
The antioxidant activity of the methanol and hexane extracts of both (FP) and (SK) was evaluated using the ABTS and DPPH free radical-scavenging assay; “Figure 1a and 1b” showed that the (SK) extracts has higher antioxidant activity than the (FP) extracts, this may be attributed to the higher unsaturated fatty acid, phenolic & flavonoid contents.
Figure 1.
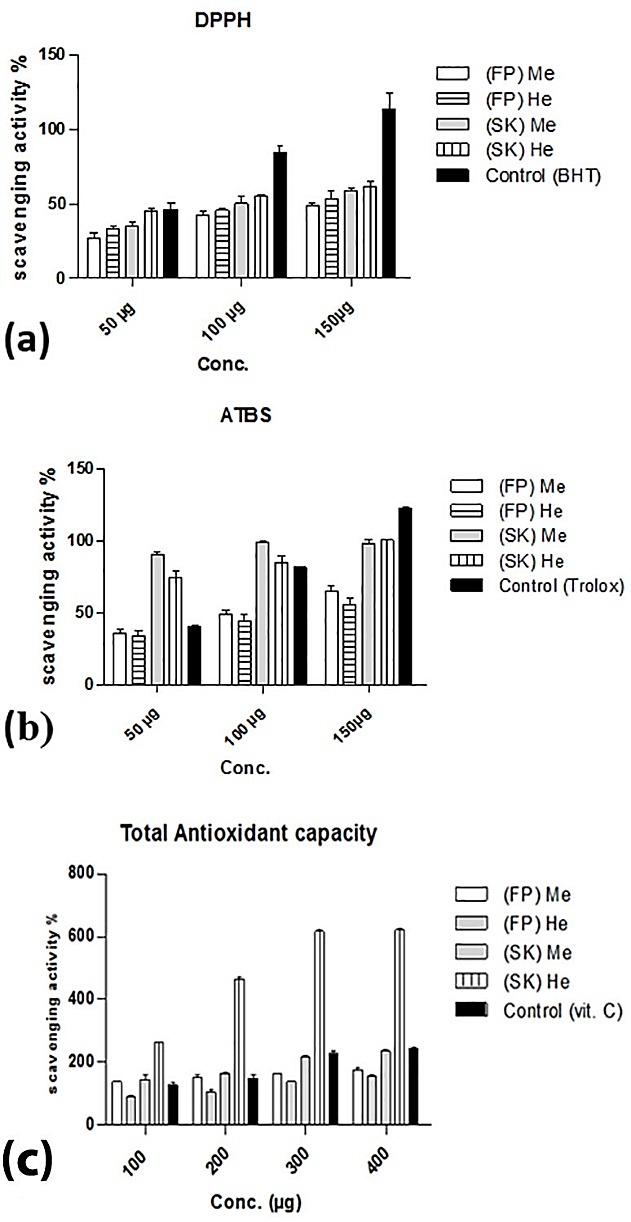
Antioxidant activity of C. edulis extracts(FP) Me: fruit peel methanol extract, (SK) Me: seed kernel hexane extract, (FP) He: fruit peel hexane extract, (SK) He: seed kernel hexane extract
On the other hand the results showed that most powerful antioxidant activity is presented in the (SK)He extract "Figure 1c"; this could be due to the high percentage of the unsaturated fatty acids 94.2% "Table 1" especially oleic acid which has great role in protection of cell membranes from free radicals.21
The antioxidant activity was also previously reported in the edible parts and leaves methanol extract of C. edulis.3
In vitro Anti-inflammatory activity
Results in "Figure 2" showed that the (SK)He extract at different doses (50, 100 and 150µg/ml) has the most potent anti-inflammatory activity compared with (Diclofenac) as control drug. This effect may be due to the high percentage of the unsaturated fatty acids in (SK)He extract;22 the potential anti-inflammatory activity of the methanol extracts can be also attributed to the presence of higher percentage of phenolic contents.
Figure 2.
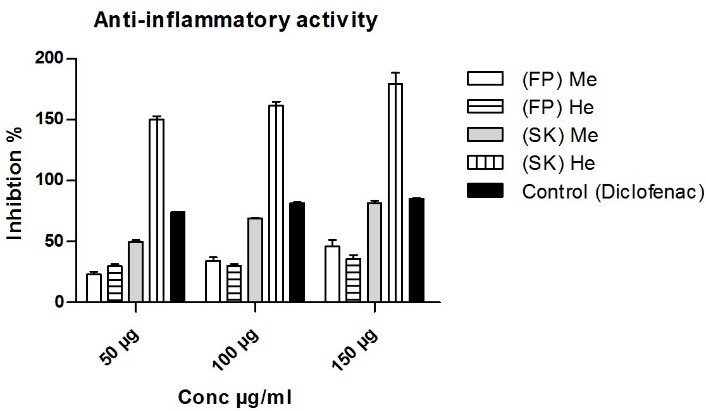
Anti-inflammatory activity of C. edulis extracts
(FP) Me: fruit peel methanol extract, (SK) Me: seed kernel hexane extract, (FP) He: fruit peel hexane extract ,(SK) He: seed kernel hexane extract
Antitumor activity of C. edulis extracts
"Figure 3" reveled that (FP) and (SK) extracts have certain inhibition effect against the Caco-2 cell line but the most active extract is the (FP)He extract when compared with reference drug doxorubicin, where the IC50 is 45 µg/ml.
Figure 3.
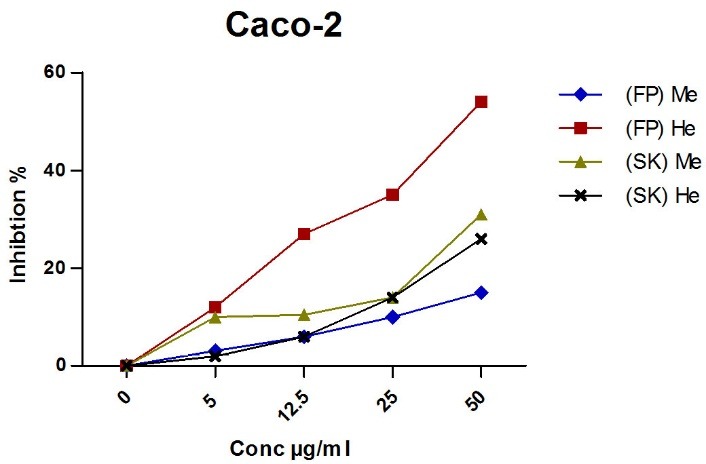
Antitumor activity of C. edulis extracts
(FP) Me: fruit peel methanol extract, (SK) Me: seed kernel hexane extract, (FP) He: fruit peel hexane extract, (SK) He: seed kernel hexane extract
Conclusion
C. edulis non edible fruit parts could be considered as a valuable source for different useful metabolites as unsaturated fatty acid in the hexane extract and poly-phenolic, flavonoids and tannins in methanol extract; both extracts revealed great importance as antioxidant, anticancer and anti-inflammatory activities. Thus the non-edible part of fruit which is considered as waste product may be phyto-therapeutically used. However; further in vivo studies are required to authenticate such biological activities in order to formulate safe effective pharmaceutical herbal product.
Ethical Issues
Methods are done after approval of the research ethics committee; the approval form has serial no. REC-FPSPI-5/34.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Pollio A, De Natale A, Appetiti E, Aliotta G, Touwaide A. Continuity and change in the mediterranean medical tradition: Ruta spp. (rutaceae) in hippocratic medicine and present practices. J Ethnopharmacol. 2008;116(3):469–82. doi: 10.1016/j.jep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Romero ML, Escobar LI, Lozoya X, Eniquez RG. High-performance liquid chromatographic study of casimiroa edulis: I. Determination of imidazole derivatives and rutin in aqueous and organic extracts. J Chromatogr A. 1983;281:245–51. doi: 10.1016/S0021-9673(01)87882-1. [DOI] [Google Scholar]
- 3.Moo-Huchin VM, Estrada-Mota I, Estrada-Leon R, Cuevas-Glory L, Ortiz-Vazquez E, Vargas y Vargas Mde L . et al. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from yucatan, mexico. Food Chem. 2014;152:508–15. doi: 10.1016/j.foodchem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Magos GA, Vidrio H. Pharmacology of casimiroa edulis; part i. Blood pressure and heart rate effects in the anesthetized rat. Planta Med. 1991;57(1):20–4. doi: 10.1055/s-2006-960008. [DOI] [PubMed] [Google Scholar]
- 5.Garzon-De la Mora P, Garcia-Lopez PM, Garcia-Estrada J, Navarro-Ruiz A, Villanueva-Michel T, Villarreal-de Puga LM. et al. Casimiroa edulis seed extracts show anticonvulsive properties in rats. J Ethnopharmacol. 1999;68(1-3):275–82. doi: 10.1016/s0378-8741(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 6.Nagai H, Tanaka T, Goto T, Kusudo T, Takahashi N, Kawada T. Phenolic compounds from leaves of casimiroa edulis showed adipogenesis activity. Biosci Biotechnol Biochem. 2014;78(2):296–300. doi: 10.1080/09168451.2014.877821. [DOI] [PubMed] [Google Scholar]
- 7.Awaad AS, Al-Jaber NA, Soliman GA, Al-Outhman MR, Zain ME, Moses JE. et al. New biological activities of casimiroa edulis leaf extract and isolated compounds. Phytother Res. 2012;26(3):452–7. doi: 10.1002/ptr.3690. [DOI] [PubMed] [Google Scholar]
- 8.Toton E, Romaniuk A, Budzianowski J, Hofmann J, Rybczynska M. Zapotin (5,6,2',6'-tetramethoxyflavone) modulates the crosstalk between autophagy and apoptosis pathways in cancer cells with overexpressed constitutively active pkc. Nutr Cancer. 2016;68(2):290–304. doi: 10.1080/01635581.2016.1134595. [DOI] [PubMed] [Google Scholar]
- 9.Magos GA, Vidrio H, Reynolds WF, Enriquez RG. Pharmacology of casimiroa edulis iv. Hypotensive effects of compounds isolated from methanolic extracts in rats and guinea pigs. J Ethnopharmacol. 1999;64(1):35–44. doi: 10.1016/s0378-8741(98)00101-9. [DOI] [PubMed] [Google Scholar]
- 10.Barnsteiner A, Lubinus T, di Gianvito A, Schmid W, Engel KH. Gc-based analysis of plant stanyl fatty acid esters in enriched foods. J Agric Food Chem. 2011;59(10):5204–14. doi: 10.1021/jf104930z. [DOI] [PubMed] [Google Scholar]
- 11.Saenkod C, Liu Z, Huang JYG. Antioxidative biochemical properties of extracts from some chinese and thai rice varieties. Afr J Food Sci. 2013;9(7):300–5. doi: 10.5897/AJFS2013.1010. [DOI] [Google Scholar]
- 12.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidant by means of folin-ciocalteu reagent. Method Enzymol. 1999;299:152–78. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 13.Mattila P, Astola J, Kumpulainen J. Determination of flavonoids in plant material by hplc with diode-array and electro-array detections. J Agric Food Chem. 2000;48(12):5834–41. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- 14.Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73(2):239–44. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- 15.Ye H, Zhou C, Sun Y, Zhang X, Liu J, Hu Q. et al. Antioxidant activities of ethanol extracts from brown seaweed sargassum pallidum. Eur Food Res Technol. 2009;230:101–9. doi: 10.1007/s00217-009-1147-4. [DOI] [Google Scholar]
- 16.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin e. Anal Biochem. 1999;269(2):337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 17.Tsai AC, Pai HC, Wang CY, Liou JP, Teng CM, Wang JC. et al. In vitro and in vivo anti-tumour effects of mpt0b014, a novel derivative aroylquinoline, and in combination with erlotinib in human non-small-cell lung cancer cells. Br J Pharmacol. 2014;171(1):122–33. doi: 10.1111/bph.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman H, Eswaraiah CM, Dutta AM. In-vitro anti-inflammatory and anti-arthritic activity of oryza sativa var. Joha rice (an aromatic indigenous rice of assam) American Eurasian J Agric Environ Sci. 2015;15(1):115–21. doi: 10.5829/idosi.aejaes.2015.115.121. [DOI] [Google Scholar]
- 19.Lunn J, Theobald HE. The health effects of dietary unsaturated fatty acids. Brit Nutr Bull. 2006;31(3):178–224. doi: 10.1111/j.1467-3010.2006.00571.x. [DOI] [Google Scholar]
- 20.Bernstein AM, Roizen MF, Martinez L. Purified palmitoleic acid for the reduction of high-sensitivity c-reactive protein and serum lipids: A double-blinded, randomized, placebo controlled study. J Clin Lipidol. 2014;8(6):612–7. doi: 10.1016/j.jacl.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Haug A, Hostmark AT, Harstad OM. Bovine milk in human nutrition--a review. Lipids Health Dis. 2007;6:25. doi: 10.1186/1476-511x-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugur G, Chris S. N-3 omega fatty acids: A review of current knowledge. Int J Food Sci Tech. 2010;45:417–36. doi: 10.1111/j.1365-2621.2009.02151.x. [DOI] [Google Scholar]


