Abstract
Background
Quercetin, nature’s most common flavonoid, possesses anticarcinogenic properties against various forms of cancer. The aim of this study was to investigate the effect of quercetin on breast cancer stem cells in the MDA-MB-231 cell line, and to elucidate the possible mechanisms for those effects.
Material/Methods
We evaluated breast cancer stem cell proliferation, clone generation, and mammosphere formation to determine the effect of quercetin treatment on breast cancer stem cells.
Results
In our study, quercetin suppressed breast cancer stem cell proliferation, self-renewal, and invasiveness. It also lowered the expression levels of proteins related to tumorigenesis and cancer progression, such as aldehyde dehydrogenase 1A1, C-X-C chemokine receptor type 4, mucin 1, and epithelial cell adhesion molecules.
Conclusions
These results indicate that quercetin targets and destroys breast cancer stem cells, making it a potential novel drug in the fight against cancer.
MeSH Keywords: Neoplastic Stem Cells, Quercetin, Triple Negative Breast Neoplasms
Background
The worldwide incidence of cancer has increased in recent years [1]. Although the mortality rate has declined with early detection [1], recurrence and metastases remain the leading causes of death in patients with cancer [2]. Recent studies have shown that a small subset of cells known as cancer stem cells (CSCs) are capable of self-renewal, differentiation, and infinite propagation, and are resistant to anticancer therapies [3,4]. CSCs have been isolated and identified in solid tumors such as cancers of the colon, liver, head, neck, stomach, and breast.
Breast cancer, the most common type of cancer among women, has a high mortality rate. Since the discovery of breast CSCs in 2003 [5], researchers have reported that a small population of breast CSCs with a CD44+/CD24− phenotype are capable of regenerating malignant cells and generating new tumors [5,6]. Because recurrence, progression, and metastasis of tumors originate from CSCs [7–9], the most promising therapeutic strategy for breast cancer is the targeting of CSCs.
Evidence suggests that naturally occurring compounds have anticancer properties that may be useful for cancer prevention and therapy [10]. Among those compounds, flavonoids reportedly have anticarcinogenic properties [11–13]. Quercetin, the most common flavonoid, has been shown to act against various cancers, including breast cancer [14–18]. Recent studies have reported that quercetin inhibits breast cancer by inhibiting signal transduction, inducing cancer cell apoptosis, and suppressing proliferation, invasion, and metastases of tumor cells [19–21]. However, the effect of quercetin on breast CSCs has not been described.
In the present study, we investigated the effect of quercetin on MDA-MB-231 cells with the CD44+/CD24− phenotype, and we explored its possible mechanisms of action.
Material and Methods
Cell culture and regents
Human MDA-MB-231 breast cancer cells were purchased from American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) that was supplemented with antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) and 10% fetal bovine serum (FBS; Gibco). The cultures were maintained in a humidified incubator at 37°C with 5% CO2. Quercetin was obtained from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China.
Cell proliferation assay
Cell proliferation was measured using CellTiter 96®AQueous One Solution Cell Proliferation Assay (MTS; Promega Biotech Co., Ltd.) according to the product manual. We recorded absorbance at 490 nm using a Vmax® Microplate Reader (Bio-Rad, Benchmark) to calculate the number of cells.
Apoptosis analysis
To detect apoptotic cells, we used an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (MBL International Corporation, Watertown, MA) according to the manufacturer’s instructions, followed by flow cytometry.
Cell cycle assay
We used flow cytometry to elucidate the effect of quercetin on the cell cycle, and more specifically, to analyze apoptosis and determine the apoptotic rate. Briefly, 2×105 cells/mL of MDA-MB-231 cells were seeded in a 6-well plate. After 24 h, we added quercetin. The cells were fixed with 70% ethanol at different time points, stored overnight at 4°C, and then washed and stained with propidium iodide (PI, Sigma-Aldrich, Steinheim, Germany). Cell cycle analysis was performed using a FACSCalibur™ (Becton Dickinson, Franklin Lakes, NJ, USA) flow cytometer.
Fluorescence-activated cell sorting (FACS)
FACS was utilized to sort breast CSCs according to cluster of differentiation 44 (CD44) and cluster of differentiation 24 (CD24) cell surface markers. Briefly, MDA-MB-231 cells were stained with FITC-labeled mouse anti-human CD44 antibodies and phycoerythrin (PE)-labeled mouse anti-human CD24 (BD Pharmingen™) antibodies. Unbound antibodies were removed by washing with phosphate-buffered saline (PBS) prior to analysis. Then, CD44+/CD24− cells and non-CD44+/CD24− cells were collected.
Soft agar colony formation assay
Sorted CD44+/CD24− CSCs with or without quercetin were seeded in soft agar as previously described [22]. The plates were incubated at 37°C and 5% CO2 for approximately 21 days, and then stained with 0.01% crystal violet. The number of colonies was counted under a microscope.
Mammosphere culture
Sorted CD44+/CD24− CSCs were grown in serum-free DMEM/F12 medium supplemented with B-27 Supplement, 20 ng/mL epidermal growth factor (EGF, Invitrogen), 0.4% bovine serum albumin (BSA), and 4 μg/mL insulin (R&D Systems) as previously reported [23]. Mammospheres were cultured for 6 days, and then quercetin was added. The number of mammospheres was counted 48 h after the addition of quercetin.
Scratch assay
Sorted CD44+/CD24− CSCs were grown in 60-mm tissue culture dishes. After 24 h, the cells were starved in FBS-free DMEM medium for 16 h. We scratched the central region of the confluent culture, removed detached cells, and added fresh culture medium with or without quercetin. Phase-contrast images of the scratched region were recorded at different time points using Image J (National Institutes of Health).
Western blot analysis
Sorted CD44+/CD24− CSCs with or without quercetin treatment were lysed immediately in RIPA buffer, incubated on ice for 30 min, and then centrifuged at 12 000 g for 15 min at 4°C. Equal amounts of total protein (approximately 30 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked with 5% fat-free dry milk/BSA for 2 h, then probed with different primary antibodies: anti-aldehyde dehydrogenase 1A1 (anti-ALDH1A1; 1: 1000; Abcam, #ab9883), anti-epithelial cell adhesion molecule (anti-EpCAM; 1: 1000; Abcam, ab71916), anti-C-X-C chemokine receptor type 4 (anti-CXCR4; 1: 1000; Abcam, #ab124824), anti–mucin 1 (anti-MUC1; 1: 500; CST, #4538), and anti-β-actin (1: 1000; CST, #4970). Quantification of the Western blots was performed using luminol chemiluminescence (ChemiDoc XRS; Bio-Rad).
Statistical analysis
All experiments were conducted more than 3 times. We used one-way ANOVA (analysis of variance) or unpaired, two-tailed t tests for statistical analyses. All results are expressed as means ± standard deviations. Statistically significant differences were declared at p<0.05.
Results
High doses of quercetin inhibit proliferation of MDA-MB-231 cells and CD44+/CD24− CSCs
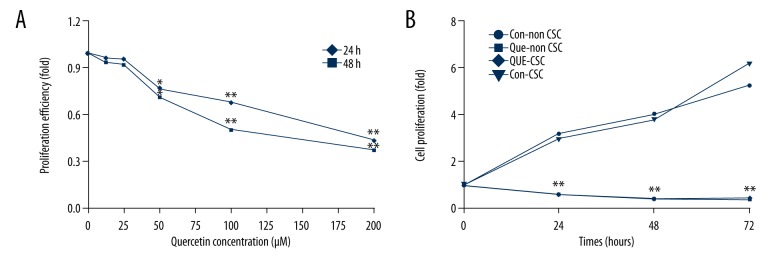
To investigate whether quercetin inhibits proliferation of MDA-MB-231 cells, we performed an MTS assay on MDA-MB-231 cells that had been treated with various concentrations of quercetin (0, 12.5, 25, 50, 100, and 200 μM). No difference was found between untreated cells and those treated with less than 50 μM quercetin, but concentrations of 50 μM and greater significantly inhibited cell proliferation (Figure 1A). We chose 100 μM quercetin for subsequent experiments. We also used FACS to sort CD44+/CD24− CSCs, and used the MTS assay to investigate the effects of quercetin on the proliferation of CD44+/CD24− CSCs. We found that the proliferation of both CD44+/CD24− and non-CD44+/CD24− subpopulations was significantly lower at all time points in the quercetin-treated groups than in the control groups. This suggests that quercetin inhibits both non-CD44+/CD24− and CD44+/CD24− CSCs (Figure 1B).
Figure 1.
Proliferation of MDA-MB-231 cells and cancer stem cells (CSCs) inhibited by quercetin. (A) Cell proliferation was significantly inhibited in a concentration-dependent manner at quercetin concentrations ≥50 μM. (B) Proliferation of quercetin-treated CD44+/CD24− and non-CD44+/CD24− subpopulations decreased at all time points. Data represent the means ± standard deviations (n=3). * P<0.05; ** P<0.01 vs. control group.
Quercetin induces apoptosis of MDA-MB-231 cells
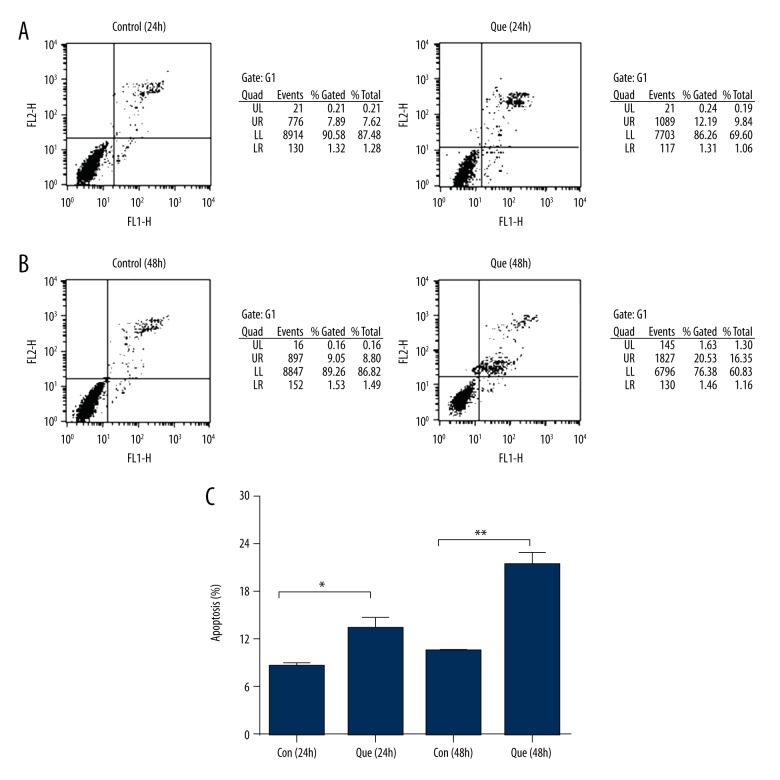
To examine whether quercetin inhibits cell proliferation associated with induction of cell apoptosis, we assessed apoptosis of MDA-MB-231 cells using flow cytometry. The number of apoptotic cells was significantly higher in the quercetin group than in the control group (Figure 2). These results show that quercetin has apoptotic effects against human MDA-MB-231 cells and inhibits cell proliferation.
Figure 2.
Induction of apoptosis by quercetin. (A) Flow cytometry analysis comparing apoptosis levels of 24-h quercetin-treated cells to that of the control group. (B) Flow cytometry analysis comparing apoptosis levels of 48-h quercetin-treated cells to that of the control group. (C) Levels of apoptosis in MDA-MB-231 cells. These results represent 3 independent experiments. Con – control; Que – quercetin. Data represent the means ± standard deviations (n=3). * P<0.05; ** P<0.01 vs. control group.
Quercetin changes the cell cycle of MDA-MB-231 cells
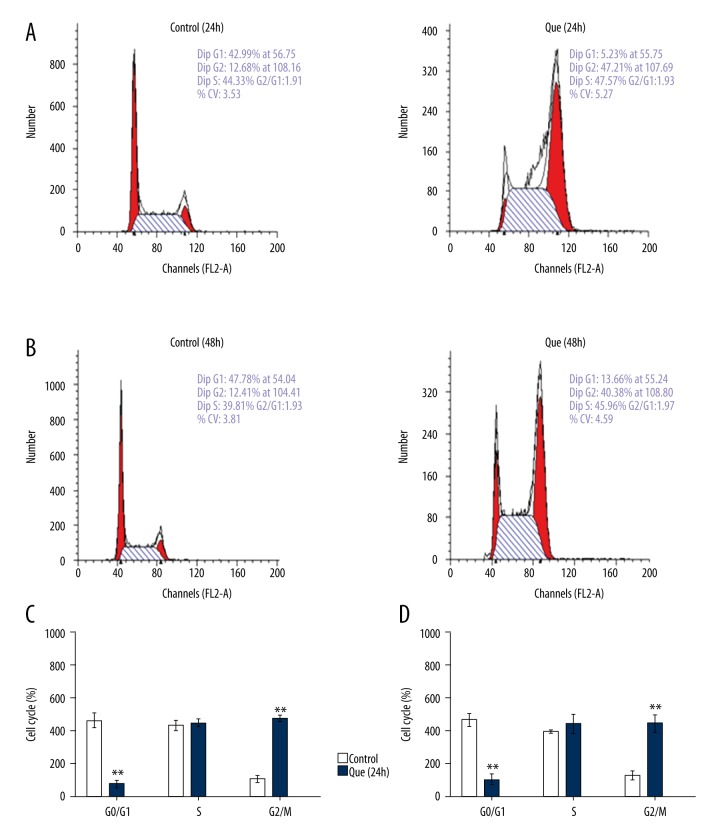
We used flow cytometry to elucidate changes in the MDA-MB-231 cell cycle to further investigate the possible mechanisms through which quercetin inhibits cell proliferation. The proportion of cells in G1 phase was significantly lower in the quercetin group than in the control group (Figure 3). The proportion of cells in G2/M phase was significantly higher in the quercetin group than in the control group (Figure 3). The proportion of cells in S phase was similar between the quercetin and control groups (Figure 3). These results indicate that quercetin alters the MDA-MB-231 cell cycle.
Figure 3.
MDA-MB-231 cell cycles change with quercetin treatment. (A) Cell cycles of 24-h quercetin-treated cells and those of the control group. (B) Cell cycles of 48-h quercetin-treated cells and those of the control group. (C) Changes in the proportion of cells in each phase of the cell cycle at 24 h, as determined by flow cytometry. (D) Changes in the proportion of cells in each phase of the cell cycle at 48 h, as determined by flow cytometry. Que – quercetin. Bars represent the mean numbers of cells ± standard deviations (n=3). ** P<0.01 vs. control group.
Quercetin inhibits mammosphere formation, and migration of CD44+/CD24− CSCs
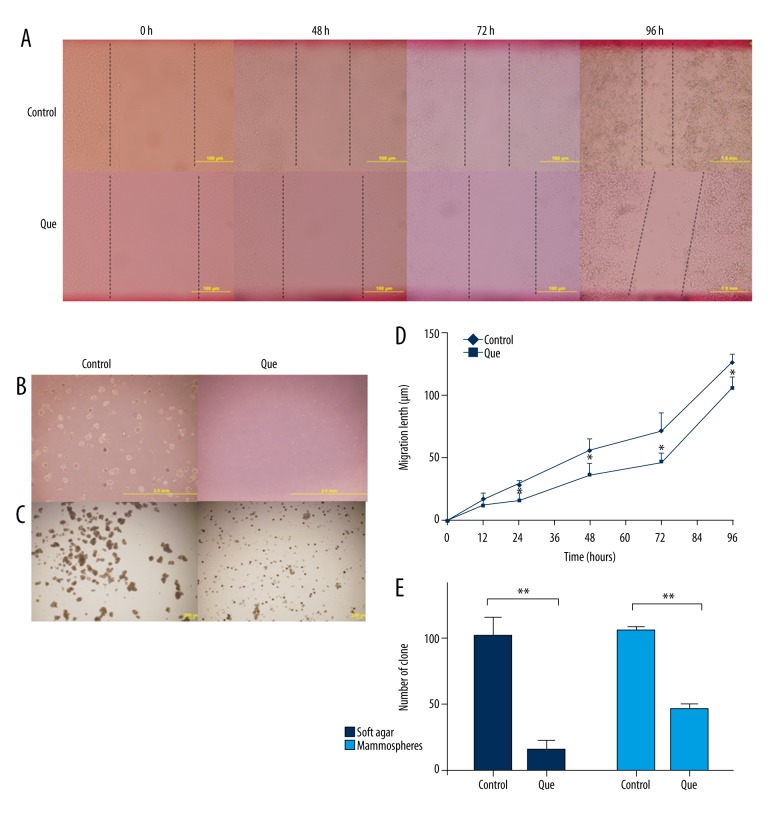
We examined the effect quercetin on colony formation and mammosphere generation in CD44+/CD24− CSCs to determine if inhibition of stem cell properties by quercetin reflects the degree to which it inhibits malignancy. The number of foci was significantly lower in the quercetin-treated group than in the control group (Figure 4B, 4E). Using phase-contrast microscopy, we observed that the size of the colonies and mammospheres in the quercetin group was significantly smaller than that in the control group (Figure 4C). Indeed, the number of mammospheres dramatically declined within 48 h of quercetin treatment due to cell death. Because mammospheres are composed primarily of CSCs, our results suggest that quercetin kills CSCs (Figure 4C, 4E).
Figure 4.
Analysis of invasion, clonal expansion, and mammosphere formation in CD44+/CD24− CSCs. (A, D) Scratch assay analysis of migration in control and quercetin groups (B) Clone formation in control and quercetin-treated CSCs. (C) Mammosphere generation in control and quercetin-treated CSCs. (E) Analysis of the numbers of clone formations and mammospheres in control and quercetin-treated CSCs. CD – cluster of differentiation; CSC – cancer stem cell. Data represent the means ± standard deviations (n=3). * P<0.05; ** P<0.01 vs. control group.
To characterize the effect of quercetin on migration of CD44+/CD24− CSCs, a scratch assay was performed. We found that after 24 h, the migration of cells in the quercetin-treated group significantly declined compared to that in the control group (Figure 4A, 4D). Our results suggest that quercetin treatment inhibits proliferation, clonal expansion, mammosphere formation, and migration of CD44+/CD24− CSCs.
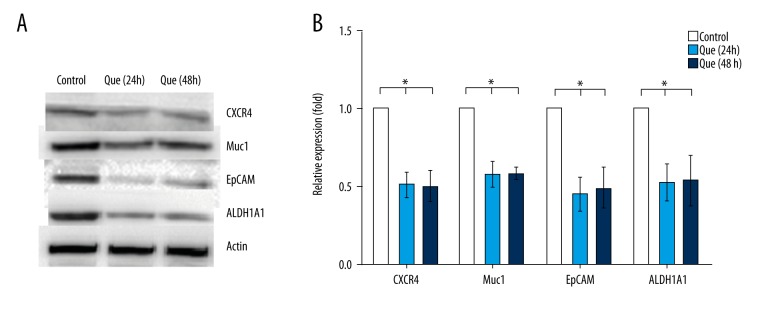
Quercetin-induced CSC inactivation is associated with suppression of ALDH1A1, CXCR4, EpCAM, and MUC1 levels
To further investigate the mechanisms by which quercetin inhibits proliferation, clonal expansion, mammosphere formation, and migration of CD44+/CD24− CSCs, we examined the expression of proteins involved in cancer tumorigenesis and progression. We found that the levels of ALDH1A1, CXCR4, EpCAM, and MUC1 proteins were significantly lower in the quercetin group than in the control group (Figure 5), which was concordant with our experiments on mammosphere formation and migration. These results suggest that ALDH1A1, CXCR4, EpCAM, and MUC1 play crucial roles in quercetin inhibition of CD44+/CD24− CSCs.
Figure 5.
Changes in the expression of proteins (ALDH1A1, CXCR4, EpCAM, and MUC1) associated with tumorigenesis and progression of CSC. (A, B) The expressions of ALDH1A1, CXCR4, EpCAM, and MUC1 in MDA-MB-231 cells were detected by western blot and analyzed using Quantity One software. The data represent the means ± standard deviations (n=3). ALDH1A1 – aldehyde dehydrogenase 1A1; CXCR4 – C-X-C chemokine receptor type 4; EpCAM – epithelial cell adhesion molecule; MUC1 – mucin 1; Que – quercetin. * P<0.05 vs. control group.
Discussion
Although the number of deaths in patients with breast cancer has decreased in Western countries, that decline is partially associated with early detection. Many patients continue to die of recurrence or metastases [1]. Researchers have proposed that a small subset of cells (the so-called CSCs) is resistant to chemotherapy and radiotherapy, and these cells are capable of self-renewal, differentiation, and clonal expansion. Hence, targeting CSCs is considered the most promising treatment strategy against cancer [24].
Phytochemicals are potential anticancer agents because they reportedly target breast CSCs and their signaling pathways [24]. Quercetin is a phytochemical that can inhibit breast cancer [19–21], but its effect on breast CSCs has not been reported until now. In the present study, we focused on the effect of quercetin on breast CSCs and the possible mechanisms by which it may exert those effects.
In our study, quercetin inhibited proliferation of MDA-MB-231 cells and CSC populations (Figure 1). When we analyzed the effect of quercetin on apoptosis, we found that quercetin inhibited cell proliferation by increasing the rate of apoptosis (Figure 2).
We also discovered that MDA-MB-231 cells treated with quercetin had a lower proportion of G1 phase cells and a higher proportion of G2/M phase cells compared to those not treated with quercetin (Figure 3). These results are similar to those previously reported [25]. Quercetin-mediated cell cycle arrest in the G2/M phase is possibly associated with upregulation of cyclin D1, which has been reported in other cell lines [26,27].
Until now, the role of quercetin on CD44+/CD24− CSCs was unclear, so we investigated the effect of quercetin on these cells. An MTS assay of sorted CD44+/CD24− CSCs showed that those treated with quercetin had significantly lower proliferative ability than those not treated with quercetin (Figure 1B). We also investigated the effect of quercetin on mammosphere formation and found that quercetin significantly reduced mammosphere formation, which suggests that quercetin inhibits proliferation and self-renewal of CD44+/CD24− CSCs (Figure 4).
In addition to self-renewal and proliferation of CD44+/CD24− CSCs, another key malignant characteristic is invasion. In this study, we used a scratch assay to estimate the ability of CD44+/CD24− CSCs to invade and repair. We found that quercetin-treated CD44+/CD24− CSCs were less invasive than were CD44+/CD24− CSCs in the control group (Figure 4A, 4D). Specifically, quercetin reduced the malignancy of CD44+/CD24− CSCs.
In accordance with the above results, we therefore assessed several candidate molecules related to tumorigenesis and progression of CSCs: ALDH1A1, CXCR4, EpCAM, and MUC1. It is known that ALDH1A1 can be used as a marker for breast CSCs, which have high tumor-initiating and self-renewing capabilities [28]. Cancer cell lines with high expression levels of ALDH1 exhibit high clonogenicity, tumorigenicity, and invasiveness [28,29]. Not surprisingly, studies have associated high expression levels of ALDH1 in CSCs with the development of drug resistance in cancers. In breast cancer, ALDH expression has been positively correlated with grade, stage, and poor prognosis [30].
MUC1 is a transmembrane glycoprotein that can bind to epidermal growth factor receptor (EGFR) family members and β-catenin to activate signal transduction and growth-related pathways [31,32]. Overexpression of MUC1 is associated with increased cell proliferation and promotion of invasion and metastases [32]. Indeed, high expression levels of MUC1 have been correlated with nodal metastases and a worse prognosis in a variety of epithelial cancers, including breast cancer [31].
EpCAMs play an important role in cancer by possibly upregulating c-myc and cyclin A, activating the Wnt signaling pathway, and inducing proliferation [33,34]. Several in vivo and in vitro studies have shown that EpCAMs may act as CSC markers for many tumors, including breast, colon, liver, and pancreatic cancers [35,36]. In addition, high EpCAM expression levels are often correlated with poor prognoses in cancers such as breast and gallbladder cancers [37,38].
CXCR4 is an alpha-chemokine receptor specific for C-X-C motif ligands (CXCLs) expressed in various cancer cells, such as breast cancer [39]. A considerable number of recent studies have revealed that CXCR4s play a key role in cell proliferation, migration, and metastasis in breast cancer [40].
Researchers have reported that ALDH1A1, CXCR4, EpCAM, and MUC1 may all be potential targets for breast CSC treatment. In our research, MDA-MB-231 cells showed significantly lower ALDH1A1, CXCR4, EpCAM, and MUC1 levels compared to those of the control group (Figure 5). We surmise that lower expression levels of ALDH1A1, CXCR4, EpCAM, and MUC1 may be important factors mediating quercetin inhibition of CSC activation.
Conclusions
Our study demonstrated that quercetin inhibits cell proliferation and induces apoptosis in breast cancer cells. From our results, we conclude that it also reduces proliferation, mammosphere formation, and invasiveness of sorted CD44+/CD24− CSCs, perhaps by inhibiting ALDH1A1, CXCR4, EpCAM, and MUC1. Our findings indicate that quercetin is a potential novel drug for targeting CSCs.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by grants from the Natural Science Fund of the Inner Mongolia Autonomous Region (grant no. 2013MS1115), the Chifeng University Key Laboratory and Higher School Science Research Project of the Inner Mongolia Autonomous Region (Grant Number NJZC13316), and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630052016008)
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–88. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czemplik M, Mierziak J, Szopa J, Kulma A. Flavonoid C-glucosides derived from flax straw extracts reduce human breast cancer cell growth in vitro and induce apoptosis. Front Pharmacol. 2016;7:282. doi: 10.3389/fphar.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40–41:192–208. doi: 10.1016/j.semcancer.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng J, Wang L, Chen H, et al. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013;32:535–51. doi: 10.1007/s10555-013-9423-y. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Heredia JM, Verdugo Sivianes EM, Lucena-Cacace A, et al. Numb-like (NumbL) downregulation increases tumorigenicity, cancer stem cell-like properties and resistance to chemotherapy. Oncotarget. 2016;7:63611–28. doi: 10.18632/oncotarget.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastl G, Spizzo G, Obrist P, et al. Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet. 2000;356:1981–82. doi: 10.1016/S0140-6736(00)03312-2. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson RW, Finger EC, Olcina MM, et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016;18:1078–89. doi: 10.1038/ncb3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan F, Niaz K, Maqbool F, et al. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients. 2016;8:E529. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606–13. doi: 10.1007/s00534-012-0542-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee TJ, Kim OH, Kim YH, et al. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006;240:234–42. doi: 10.1016/j.canlet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Lv L, Liu C, Chen C, et al. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget. 2016;7:32184–99. doi: 10.18632/oncotarget.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–71. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 14.Marsden CG, Wright MJ, Pochampally R, Rowan BG. Breast tumor-initiating cells isolated from patient core biopsies for study of hormone action. Methods Mol Biol. 2009;590:363–75. doi: 10.1007/978-1-60327-378-7_23. [DOI] [PubMed] [Google Scholar]
- 15.Moreb JS, Ucar D, Han S, et al. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195:52–60. doi: 10.1016/j.cbi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–29. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 17.Ediriweera MK, Tennekoon KH, Adhikari A, et al. New halogenated constituents from Mangifera zeylanica Hook.f. and their potential anti-cancer effects in breast and ovarian cancer cells. J Ethnopharmacol. 2016;189:165–74. doi: 10.1016/j.jep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 18.Ediriweera MK, Tennekoon KH, Samarakoon SR, et al. Protective effects of six selected dietary compounds against leptin-induced proliferation of oestrogen receptor positive (MCF-7) breast cancer cells. Medicines (Basel) 2017;4(3) doi: 10.3390/medicines4030056. pii: E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath S, Mukherjee P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–42. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolaou KC, Talianidis I. Hepatic cancer stem cells may arise from adult ductal progenitors. Mol Cell Oncol. 2016;3:e1021946. doi: 10.1080/23723556.2015.1021946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noolu B, Gogulothu R, Bhat M, et al. In vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Anticancer Agents Med Chem. 2016;16:1605–14. doi: 10.2174/1871520616666160520112210. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan S, Halagowder D, Sivasithambaram ND. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One. 2015;10:e0141370. doi: 10.1371/journal.pone.0141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redig AJ, McAllister SS. Breast cancer as a systemic disease: A view of metastasis. J Intern Med. 2013;274:113–26. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 25.Rivera Rivera A, Castillo-Pichardo L, Gerena Y, Dharmawardhane S. Anti-breast cancer potential of quercetin via the Akt/AMPK/Mammalian target of rapamycin (mTOR) signaling cascade. PLoS One. 2016;11:e0157251. doi: 10.1371/journal.pone.0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X, Liu D, Zhang J, et al. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complement Altern Med. 2016;16:245. doi: 10.1186/s12906-016-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava S, Somasagara RR, Hegde M, et al. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Q, Peng M, Zhang Y, et al. Quercetin induces bladder cancer cells apoptosis by activation of AMPK signaling pathway. Am J Cancer Res. 2016;6:498–508. [PMC free article] [PubMed] [Google Scholar]
- 30.Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer stem cells: A novel paradigm for cancer prevention and treatment. Mini Rev Med Chem. 2010;10:359–71. doi: 10.2174/138955710791330954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–41. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 32.Varga M, Obrist P, Schneeberger S, et al. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res. 2004;10:3131–36. doi: 10.1158/1078-0432.ccr-03-0528. [DOI] [PubMed] [Google Scholar]
- 33.Wicha MS, Liu S, Dontu G. Cancer stem cells: An old idea – a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Zhao H, Chen H, Yao Q. CXCR4 in breast cancer: Oncogenic role and therapeutic targeting. Drug Des Devel Ther. 2015;9:4953–64. doi: 10.2147/DDDT.S84932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan H, Lu X, Ma Q, et al. Flavonoids from Artemisia sacrorum Ledeb. and their cytotoxic activities against human cancer cell lines. Exp Ther Med. 2016;12:1873–78. doi: 10.3892/etm.2016.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–53. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Guo L, Zhao H, et al. CXCR4 over-expression and survival in cancer: A system review and meta-analysis. Oncotarget. 2015;6:5022–40. doi: 10.18632/oncotarget.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Y, Shen S, Zhou Y, et al. ALDH1 is a better clinical indicator for relapse of invasive ductal breast cancer than the CD44+/CD24– phenotype. Med Oncol. 2014;31:864. doi: 10.1007/s12032-014-0864-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhou N, Wang R, Zhang Y, et al. Staurosporine induced apoptosis may activate cancer stem-like cells (CD44(+)/CD24(−)) in MCF-7 by upregulating Mucin1 and EpCAM. J Cancer. 2015;6:1049–57. doi: 10.7150/jca.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]