Abstract
Backgrounds
CXC chemokine ligand 16 (CXCL16) is a soluble chemokine with a transmembrane domain, playing an important role in inflammatory regulation. NF-κB has a critical role in tumor progression. Recent studies focused on the effect of CXCL16 on tumor progression. However, few reports showed the influence of CXCL16 on lung cancer, especially in regulating NF-κB activity. Here we investigated CXCL16 expression and its clinical significance in lung cancer, as well as the effect on lung cancer cell biological characteristics by regulating NF-κB.
Material/Methods
CXCL16 expression in lung cancer was detected and its associations with clinical characteristics were analyzed. Proliferation and invasion of A549 and PC-9 cells was measured before and after silencing CXCL16 or inhibiting the NF-κB pathway, separately.
Result
The positive rate of CXCL16 in lung cancer tissue was significantly higher than that in adjacent tissue, and that in patients with lymphatic metastasis was significantly higher than that in patients without (all, P<0.05). The positive rate of CXCL16 was significantly (P<0.05) positively corrected with poor prognosis of lung cancer. Silencing CXCL16 not only suppressed proliferation and invasion of A549 and PC-9 cells, but also significantly (P<0.05) inhibited c-Rel, p105, and Rel-B in the NF-κB pathway. Inhibiting NF-κB also suppressed proliferation and invasion of A549 and PC-9 cells, which was similar to the results after silencing CXCL16.
Conclusions
Enhanced CXCL16 expression in lung cancer tissue promoted the proliferation and invasion of lung cancer cells. CXCL16 might promote proliferation and invasion of lung cancer by regulating the NF-κB pathway.
MeSH Keywords: Cell Proliferation; Chemokines, CXC; Lung Neoplasms; Neoplasm Invasiveness; NF-kappa B
Background
Recently, lung cancer has become the most fatal tumor in the world, and its incidence is increasing gradually [1]. Although the treatment of lung cancer has had great advancements, such as surgery, chemotherapy, radiotherapy, and immunotherapy, the 5-year survival rate of patients with advanced lung cancer is only about 15% p[2]. Lung cancer metastasis is the primary factor causing death, and it greatly influences the therapeutic effect. Therefore, identifying the molecular mechanisms in lung cancer progression and metastasis is of clinical significance, and would be helpful for improving the prognosis of patients with lung cancer.
Previous reports demonstrated that malignancies expressed many chemokines and their receptors, indicating the role of chemokine signaling networks in tumor pathogenesis and progression [3–5]. Among these chemokines, CXCL16 was one of the membranes of the CXC chemokine family, which exist in a transmembrane (t) and a soluble (s) form [6,7]. In normal biological processes of the human body, the CXCL16/CXCR6 signaling axis participates in inflammatory regulation [8]. Additionally, more and more studies focused on the effect of CXCL16/CXCR6 signaling axis on different kinds of human cancer progression, including breast cancer [9], prostate cancer [10], nasopharyngeal cancer [11], pancreatic cancer [12], renal cell cancer [13], colorectal cancer [14], bladder cancer [15], and others [16–18]. The CXCL16/CXCR6 signaling axis influences tumor growth, metastasis, angiogenesis, survival, and multiple signaling pathways in malignant cells, suggesting that CXCL16 has a critical role in tumor pathogenesis and progression [16–18]. According to Hu et al. [19], CXCL16 expression is enhanced in human primary lung cancer tissues, and promotes the invasion of A549, 95D, and H292 lung cancer cells in vitro. Those results both suggest the important role of CXCL16 in tumor progression.
NF-κB is a protein complex containing 5 subunits: p105, p100, Rel-A, Rel-B, and c-Rel [20]. NF-κB controls DNA transcription, cytokine production, and cell survival, as well as regulating immune response to infection. The incorrect regulation of NF-κB can result in cancer [21], inflammation [22], and autoimmune disease [23]. Leo et al. [24] suggested that Trim44 promotes non-small cell lung cancer (NSCLC) development by the activation of the NF-κB pathway via up-regulating CXCL16 and MMP9. Chen et al. [25] showed that CXCL16, as an inducer of inflammatory responses, might be involved in Henoch-Schönlein purpura (HSP) pathogenesis, which is regulated by NF-κB pathways. These results indicate that the interaction between CXCL16 and NF-κB pathways might participate in inflammatory reactions during disease or tumor development.
Therefore, we studied CXCL16 expression and its association with clinical characteristics in patients with lung cancer, as well as the effect of CXCL16 on proliferation and invasion of lung cancer cells, in order to investigate the molecular mechanisms of lung cancer and provide lung cancer treatment based on molecular evidence.
Material and Methods
Patients
Clinical data and samples were collected from 56 patients diagnosed with lung cancer at Inner Mongolia People’s Hospital from May 2009 to April 2016. The 56 patients included 34 males and 22 females, age 38 to 78 years old, with a median age of 58 years old. None of the patients had received any other treatments except for surgical treatment. Informed consent was obtained from each patient. This study was approved by the Medical Ethics Committees of Renmin Hospital of Wuhan University and Inner Mongolia People’s Hospital.
Tissue samples collection
We collected 56 tumor tissues and 21 adjacent tissues and fixed them in 4% paraformaldehyde (Solarbio Life Sciences and Technology Co., Ltd), and then sent them to our Pathology Department. Two pathologists were responsible for making tissue slices. With paraffin embedding, tissue samples were made into serial sections of 4 μm.
CXCL16 detection in tissues
Immunohistochemistry (IHC) was used to detect CXCL16 in tumor and adjacent tissues. Tissue slices were dried at 60°C for 20 min, and then dewaxed with dimethylbenzene (Solarbio) and hydrated with gradient alcohol (Solarbio). Endogenous peroxidase activity was blocked with 3% H2O2 (Solarbio) in methanol at 37°C for 10 min. Antigen retrieval was carried with 0.01 mM sodium citrate buffer (Boster Biological Technology Co., Ltd. Wuhan, China) at 95°C for 20 min. After cooling, normal goat serum (Beyotime Biotechnology. Shanghai, China) was used to block the non-specific antibody binding sites. Slices were incubated with rabbit anti-CXCL16 antibody (1: 500, Abcam plc. Shanghai, China) at 4°C overnight, and then incubated with goat anti-rabbit H&L antibody (1: 500, ZSGB-BIO CO., Ltd. Beijing, China) at 37°C for 30 min. 3,3N-Diaminobenzidine Tetrahydrochloride (DAB) (Thermo Fisher Scientific, Inc. Shanghai, China) was used for staining at 25°C for 3~30 min and hematoxylin (Beyotime) was used for counterstaining. After being dehydrated with gradient alcohol and vitrificated with dimethylbenzene, slices were sealed with glycerol (Thermo) and observed under a microscope (Olympus Corporation, Japan).
Hematoxylin and eosin (HE) staining of tissue slices was performed according to the specifications of the HE staining kit (Beyotime).
Cells culture
A549 and PC-9 cell lines were obtained from American Type Culture Collection (ATCC), Baltimore, USA. A549 and PC-9 cells were cultured in RPMI-1640 medium (Thermo) containing 10% fetal bovine serum (FBS) (Thermo), penicillin (100U/mL) (Sigma-Aldrich Co. LLC. Shanghai, China), and streptomycin (0.1mg/mL) (Sigma), maintaining in the conditions of 37°C, 5% CO2, and saturated humidity.
Silencing CXCL16
RNA interfering (RNAi) was used to silence CXCL16. CXCL16 siRNA (sc-105252, Santa Cruz Biotechnology, Inc. Shanghai, China) was transfected into A549 and PC-9 cells with lipofectamine 2000 (Thermo) according to the specifications. Empty vector transfection was used as the negative control (NC). Transfection was carried on for 48 h, and the cells were used for the following experiments.
Inhibiting the NF-κB pathway
Pyrrolidine dithiocarbamate (PDTC, Beyotime) was dissolved in RPMI-1640 and then sterilized by filtering (Becton, Dickinson and Company. Shanghai, China). A549 and PC-9 cells were cultured in 6-well plates (Corning, New York, USA) with RPMI-1640 without FBS. We used 100 μM PDTC to inhibit NF-κB activity for 24 h and then the cells were used for the following experiments.
Proteins detection
Proteins in cells were detected by Western blotting (WB). A lysis kit (Thermo) was used for preparing cell lysates. Proteins were quantified by BCA protein assay kit (Beyotime). We analyzed 35 μg protein by 10% separating gel and 5% stocking gel, and then transferred them to polyvinylidene fluoride (PVDF) (Merck Millipore Corporation, Darmstadt, Germany). We blocked the non-specific antibody binding sites of the membrane with 5% non-fat milk in phosphate buffer solution (PBS) (Boster) containing 0.05% Tween-20 (Sigma). Rabbit anti-c-Rel (1: 1000, Abcam), rabbit anti-Rel-B (1: 5000, Abcam), rabbit anti-NF-κB p105/p50 (1: 10000, Abcam), and mouse anti-GAPDH (1: 10000, Abcam) antibodies were incubated with the membranes at 4°C overnight, separately. Goat anti-rabbit and goat anti-mouse secondary antibodies (1: 10 000, Abcam) were incubated with the membranes at 25°C for 1 h. Electrochemiluminescence (ECL) (Millipore) was used for detecting proteins expressions.
Cell proliferation detection
The Cell counting kit-8 (CCK-8) (Thermo) was used to detect cell proliferation. After transfection, cells were adjusted as the concentration of 0.4×103/well and seeded in 96-well plates (Corning). We added 10 μL of CCK-8 liquid in each well and then incubated them at 37°C for 20 min. Five straight days of optical density (OD) value were measured at 570 nm with a microplate reader (BioTek Instruments, Inc. Beijing, China).
Cell invasion detection
Transwell assay was used for detecting cell invasive ability. Matrigel gel (BD) was diluted in RPMI-1640 (1: 5). We added 80 μL of the mixture to the upper chamber and then incubated at in at 37°C for 4 h. We adjusted the cells as 1×105 cells/mL and staved them for 6 h. Cells were seeded in the upper chamber with RPMI-1640 without FBS, while RPMI-1640 with 10% FBS was added in the lower chamber, and then we continued to culture them for 12 h. We washed the cells with PBS and then fixed them with methanol (Solarbio) for 30 min. Cells were stained with 0.4% crystal violet (Macklin Inc. Shanghai, China) in 20% methanol for 20 min and then we cleaned the cells on the lower surface of the chamber. Under microscopy, we selected 5 fields (20×) randomly and counted the cells.
Statistical analysis
All data were analyzed by SPSS 19.0 software. The t-test or one-way ANOVA was for analyzing comparisons between groups. Chi-square test was used for analyzing the proportion rate. Kaplan-Meier analysis was used for estimating the survival curve. P<0.05 was considered as significance.
Results
CXCL16 expression was enhanced in tumor tissue
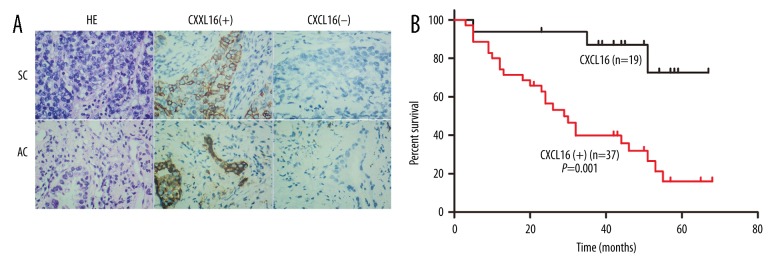
CXCL16 expression in tumor and adjacent tissues are shown in Figure 1A and Table 1. The positive rate of CXCL16 expression in tumor tissues was significantly higher than in adjacent tissues (14.29% vs. 66.07%, P<0.05). The positive staining was accumulated on the membrane and in the cytoplasm, while the nucleus was stained negative.
Figure 1.
CXCL16 expression in lung tumor tissues and survival curve of patients with lung cancer. (A) Immunohistochemistry of lung cancer tissue; (B) Survival curve of patients with lung cancer according to CXCL16 expression in tumor tissue; HE – hematoxylin and eosin; SC – squamous cell carcinoma; AC – adenocarcinoma; ‘+’ – positive expression; ‘−’ – negative expression.
Table 1.
CXCL16 expression in tumor tissue and its association with clinical characteristics of patients with lung cancer.
| Group | Subgroup | CXCL16 | χ2 | P value | |
|---|---|---|---|---|---|
| + | − | ||||
| Age | <65 | 23 | 7 | 3.23 | 0.07* |
| ≥65 | 14 | 12 | |||
| Gender | Male | 20 | 14 | 2.03 | 0.15 |
| Female | 17 | 5 | |||
| Pathological type | SC | 28 | 14 | 0.01 | 0.92 |
| AC | 9 | 5 | |||
| Tumor size | <3 cm | 16 | 10 | 0.45 | 0.51 |
| ≥3 cm | 21 | 9 | |||
| Lymphatic metastasis | Yes | 26 | 6 | 7.64 | 0.01* |
| No | 11 | 13 | |||
SC – squamous cell carcinoma; AC – adenocarcinoma; ‘+’ – positive expression; ‘−’ – negative expression;
P<0.05.
Comparing CXCL16 expressions in different pathological types, there was no significant difference between that in squamous cell carcinoma (SC) and that in adenocarcinoma (AC) (66.66% vs. 64.29%, P>0.05).
Analyzing the association of CXCL16 expression with clinical characteristics, there was no significant association of CXCL16 expression with age, gender, or tumor size. The positive rate of the patients with lymphatic metastasis was significantly higher than those without (81.25% vs. 45.83%, P<0.05).
High CXCL16 expression shortens the survival time of patients with lung cancer
The association of CXCL16 expression with survival time of patients with lung cancer is shown in Figure 1B. Survival time of the patients with positive (+) CXCL16 in tumor tissue was significantly (P=0.001) lower than in those with negative (−) CXCL16, which indicates that low CXCL16 expression in tumor tissue prolongs the survival time of patients with lung cancer.
Silencing CXCL16 decreased NF-κB activity
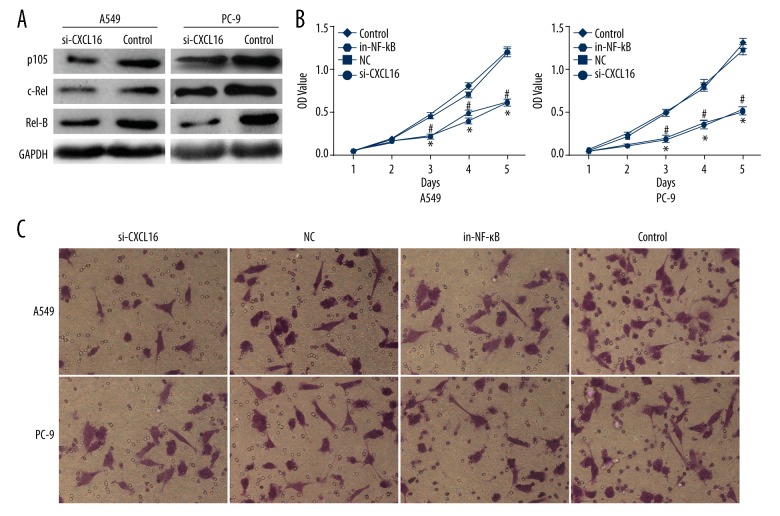
Relative proteins expression of the NF-κB pathway before and after silencing CXCL16 are shown in Figure 2A. p105, c-Rel, and Rel-B expressions after silencing CXCL16 were all obviously decreased in A549 and PC-9 cells compared to the controls, which indicates that silencing CXCL16 decreases NF-κB activity in lung cancer cells.
Figure 2.
Silencing CXCL16 suppressing proliferation and invasion of lung cancer cells. (A) NF-κB activity in lung cancer cells; (B) Cell proliferation; (C) Cell invasion; SC – squamous cell carcinoma; AC – adenocarcinoma; si-CXCL16 – silencing CXCL16; in-NF-κB – inhibiting NF-κB; NC – negative control; Con. – control.
Silencing CXCL16 or inhibiting NF-κB pathway suppresses proliferation of lung cancer cells
Proliferation of A549 (Figure 2B-a) and PC-9 (Figure 2B-b) cells is shown in Figure 2B. From the 3rd day, there were fewer A549 and PC-9 cells in the NC and Control groups than in the si-CXCL16 and NF-κB groups, separately and significantly (P<0.05), implying that silencing CXCL16 or inhibiting NF-κB both suppressed the proliferation of A549 and PC-9 cells.
Silencing CXCL16 or inhibiting the NF-κB pathway suppresses invasion of lung cancer cells
Invasion of A549 and PC-9 cells is shown in Figure 2C. Compared to NC and control groups, the number of invasive cells in A549 and PC-9 cells with silencing CXCL16 or inhibiting NF-κB both decreased obviously, which indicates that the effect of silencing CXCL16 on invasion of lung cancer cells was similar with inhibiting NF-κB.
Discussion
In our study, we firstly found that CXCL16 expression was enhanced in lung tumor tissue, which shortened the survival time of patients with lung cancer. Secondly, with cellular experiments, we found that silencing CXCL16 or inhibiting NF-κB activity not only decreased proteins expression related to NF-κB pathway, but also suppressed proliferation and invasion of lung cancer cells.
In the process of tumor pathogenesis and progression, chemokines and their receptors provide proliferation, adhesion, and metastasis under certain conditions [26]. CXCL16, with a scavenger receptor that binds phosphatidylsedne and oxidized lipoprotein (SR-PSOX), belongs to the CXC family [27]. CXCL16 has with many biological functions. Huang et al. [28] suggested that CXCL16 is highly expressed in various tumors, such as breast cancer, pancreatic cancer, and prostatic cancer. Ke et al. [29] showed that CXCL16 is highly expressed in patients with NSCLC, and also influences the survival time of patients with NSCLC. In our study, we also found that CXCL16 expression was enhanced in lung tumor tissues compared to the adjacent tissue, accumulating on the membrane and cytoplasm. By analyzing the survival curve, the results suggest that lower CXCL16 expression in tumor tissue prolongs the survival time of patients with lung cancer, indicating that enhancing CXCL16 expression in tumor tissue results in poor prognosis. Additionally, the positive rate of the patients with lymphatic metastasis was higher than in those without, indicating that increasing CXCL16 expression in tumor tissue promotes lymphatic metastasis. Our results are in accord with previous investigations.
In different kinds of carcinoma, CXCL16 might have different effects on the prognosis of bladder cancer [15] and ovarian cancer [30]. CXCL16 promotes tumor metastasis, as well as participating in the malignant transformation and differentiation of lymphoma [31]. However, CXCL16 was reported to suppress the invasion and metastasis of gastric [32] and renal [33] carcinomas, improving the prognosis of those patients. There have been few studies on the relationship between CXCL16 and lung cancer. Hu et al. [19] found that the CXCL16/CXCR6 signal axis is highly expressed in lung tumor tissue, and the exogenous CXCL16 clearly promoted tumor proliferation and invasion, while inhibiting CXCL16 blocked the stimulation of CXCL16, indicating that CXCL16/CXCR6 might play a critical role in lung cancer metastasis. Actually, for CXCL16 with transmembrane and soluble forms, there might be a different effect of CXCL16 expression on metastasis of different carcinomas. sCXCL16 was reported to stimulate the proliferation and metastasis of tumor cells, while tCXCL16 was shown to suppress metastasis of some kinds of carcinomas, such as breast cancer [34].
Therefore, we silenced CXCL16 in lung cancer cells and analyzed the biological characteristics. We found that silencing CXCL16 not only suppressed proliferation of A549 and PC-9 cells, but also decreased their invasive ability, suggesting that promotion of CXCL16 affects proliferation and invasion of lung cancer cells. However, IHC results indicated the CXCL16 appear as tCXCL16, which was different from a previous report [34]. We consider that this difference was mainly caused by the use of different kinds of tumors between our study and other studies.
In order to discover the molecular mechanisms of CXCL16 expression acting on promoting proliferation and invasion of lung cancer cells, we detected the activity of NF-κB. NF-κB has an important role in tumor progression, which was reported to be related to lung tumor development [35,36]. In NSCLC, the activation of NF-κB up-regulated cyclin A, cyclin B, and cyclin D1, as well as down-regulating P21cip/Waf1 and Rb expressions, which promoted cell cycle transiting from G1 to S and induced proliferation of lung cancer cells [37,38]. In our study, after silencing CXCL16, we found that c-Rel, Rel-B, and p105 expressions were all decreased, showing inhibition of the NF-κB pathway. Next, we compared the proliferation and invasion of A549 and PC-9 cells between silencing CXCL16 and inhibiting the NF-κB pathway, and the results showed that both proliferation and invasion of A549 and PC-9 cells were suppressed, which implied that silencing CXCL16 might suppress the proliferation and invasion of lung cancer cells through inhibiting NF-κB activity. Our results are in agreement with similar outcomes in previous studies.
Wang et al. [39] blocked the CXCR6/AKT/mTOR pathway and found the suppression of tumor metastasis, which indicated that the CXCL16/CXCR6 signal axis promotes metastasis of prostatic carcinoma by regulating AKT/mTOR. Another science team found that CXCL16 could up-regulate MMP2 and MMP9 expressions by AKT/PI3K and NF-κB pathways and then promoted the metastasis and invasion of lung cancer tumors [40].
There are some limitations in our study. Firstly, the number of the patients with lung cancer was small, leading to the weak representativeness of the analytical results. Secondly, we silenced CXCL16 and detected the activity of NF-κB, but we did not detect CXCL16 expression after inhibiting the NF-κB pathway. The co-interaction between CXCL16 expression and the NF-κB pathway is still unclear. Thirdly, we only measured CXCL16 expression but did not focus on CXCR6, so the total activity of the CXCL16/CXCR6 signal axis is unknown. Last but not least, we did not perform analysis of apoptosis and cell cycle. Further studies with larger sample sizes and in vivo experiments are needed to determine the effects of CXCL16 expression in lung cancer on tumor metastasis, patient survival time, and biological characteristics of lung cancer cells.
Conclusions
Our results show that tCXCL16 is highly expressed in lung tumor tissue, promoting the proliferation and invasion of lung tumors. CXCL16 is a potential target in treating lung cancer and may be useful as a biological marker for diagnosing the progression of lung cancer.
Acknowledgement
Prof. Ke Hu and Renmin Hospital of Wuhan University supported this study.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558–66. doi: 10.1016/j.ctrv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Gerber PA, Hippe A, Buhren BA, et al. Chemokines in tumor-associated angiogenesis. Biol Chem. 2009;390:1213–23. doi: 10.1515/BC.2009.144. [DOI] [PubMed] [Google Scholar]
- 4.Sarvaiya PJ, Guo D, Ulasov I, et al. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matloubian M, David A, Engel S, et al. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 7.Shimaoka T, Kume N, Minami M, et al. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. 2000;275:40663–66. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- 8.Wilbanks A, Zondlo SC, Murphy K, et al. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–45. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2011;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W, Zhen X, Xiong B, et al. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci. 2008;99:1362–69. doi: 10.1111/j.1349-7006.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou DL, Chen CL, Lin SB, et al. Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J Pathol. 2006;210:363–73. doi: 10.1002/path.2053. [DOI] [PubMed] [Google Scholar]
- 12.Gaida MM, Wente MN, Mayer C, et al. [The role of the inflammatory mediator CXCL16 in pancreatic cancer – increase of invasiveness and potential marker?]. Chirurgisches Forum fur Experimentelle und Klinische Forschung. 2007:109–10. [in German] [Google Scholar]
- 13.Gutwein P, Schramme A, Sinke N, et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer. 2009;45:478–89. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Kee JY, Ito A, Hojo S, et al. CXCL16 suppresses liver metastasis of colorectal cancer by promoting TNF-α-induced apoptosis by tumor-associated macrophages. BMC Cancer. 2014;14:949. doi: 10.1186/1471-2407-14-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JT, Sang DL, Lee JZ, et al. Expression analysis and clinical significance of CXCL16/CXCR6 in patients with bladder cancer. Oncol Lett. 2013;5:229–35. doi: 10.3892/ol.2012.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng L, Chen N, Li Y, et al. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim Biophys Acta. 2010;1806:42–49. doi: 10.1016/j.bbcan.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Darash-Yahana M, Gillespie JW, Hewitt SM, et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One. 2009;4:e6695. doi: 10.1371/journal.pone.0006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijer J, Ogink J, Kreike B, et al. The chemokine receptor CXCR6 and its ligand CXCL16 are expressed in carcinomas and inhibit proliferation. Cancer Res. 2008;68:4701–8. doi: 10.1158/0008-5472.CAN-08-0482. [DOI] [PubMed] [Google Scholar]
- 19.Hu W, Liu Y, Zhou W, et al. CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and mediate the invasion of lung cancer cell lines in vitro. PLoS One. 2014;9:e99056. doi: 10.1371/journal.pone.0099056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabel GJ, Verma IM. Proposed NF-kappa B/I kappa B family nomenclature. Genes Dev. 1993;7:2063. doi: 10.1101/gad.7.11.2063. [DOI] [PubMed] [Google Scholar]
- 21.Yan L. Expression and Relationship of TGF-β RII, NF-κB, tumor-associated macrophages in lung cancers. Acta Medicinae Universitatis Scientiae Et Technologiae Huazhong. 2010 [in Chinese] [Google Scholar]
- 22.Hong HH, Yang JC, Cai WR. Effects of Qidong Huoxue decoction on caveolin-1/NF-κB inflammation signal pathway in acute lung injury rats. China Journal of Traditional Chinese Medicine & Pharmacy. 2016 [in Chinese] [Google Scholar]
- 23.Kim S, Millet I, Kim HS, et al. NF-κB prevents β cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA. 2007;104:1913–18. doi: 10.1073/pnas.0610690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Q, Lin H, Ye X, et al. Trim44 facilitates the migration and invasion of human lung cancer cells via the NF-κB signaling pathway. Int J Clin Oncol. 2015;20:508–17. doi: 10.1007/s10147-014-0752-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Guo ZP, Jiao XY, et al. CCL5, CXCL16, and CX3CL1 are associated with Henoch-Schönlein purpura. Arch Dermatol Res. 2011;303:715–25. doi: 10.1007/s00403-011-1150-z. [DOI] [PubMed] [Google Scholar]
- 26.Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2:1125–31. doi: 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kume N. [New oxidized LDL receptors and their functions in atherogenesis]. Nihon Ronen Igakkai Zasshi. 2002;39:264–67. doi: 10.3143/geriatrics.39.264. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Zhang J, Cui ZM, et al. Expression of the CXCL12/CXCR4 and CXCL16/CXCR6 axes incervical in traepithelial neoplasia and cervical cancer. Chin J Cancer. 2013;32(5):289–96. doi: 10.5732/cjc.012.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ke C, Ren Y, Lv L, et al. Association between CXCL16/CXCR6 expression and the clinicopathological features of patients with non-small cell lung cancer. Oncol Lett. 2017;13:4661–68. doi: 10.3892/ol.2017.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Cui ZM, Zhang J, Huang Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin J Cancer. 2011;30(5):336–43. doi: 10.5732/cjc.010.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hattermann K, Heldfeindt J, Ludwig A, Mentlein R. The CXCL16-CXCR6 chemokine axis in glial tumors. J Neuroimmunol. 2013;260:47–54. doi: 10.1016/j.jneuroim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Xing Y, Xu X, Nie X, et al. Role and clinicopathologic significance of CXC chemokine ligand 16 and chemokine (C-X-C motif) receptor 6 expression in gastric carcinomas. Hum Pathol. 2012;43:2299–307. doi: 10.1016/j.humpath.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Gutwein P, Schramme A, Sinke N, et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer. 2009;45:478–89. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Fang YY, Lei QQ, Li Y, Chen NY. [Expression of non-secretory CXCL16 and its impact on biological characteristics in breast cancer cell lines]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2013;44(4):522–25. 530. [PubMed] [Google Scholar]
- 35.Li D, Wei Y, Wang D, et al. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-κB/MMP-9/VEGF pathways. Biochem Biophys Res Commun. 2016;472:465–70. doi: 10.1016/j.bbrc.2016.01.163. [DOI] [PubMed] [Google Scholar]
- 36.Liao CG, Yao L, Xie W, et al. Basigin-2 upregulated by receptor activator of NF-κB ligand enhances lung cancer-induced osteolytic lesions. Cancer Cell Int. 2016;16:28. doi: 10.1186/s12935-016-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Zhou Y, Li Y, et al. Mutations of p53 and KRAS activate NF-κB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett. 2015;357:520–26. doi: 10.1016/j.canlet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Hyunkyoung L, Jong-Shu K, Euikyung K. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One. 2012;7(11):e50624. doi: 10.1371/journal.pone.0050624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lu Y, Wang J, et al. CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Res. 2008;68:10367–76. doi: 10.1158/0008-5472.CAN-08-2780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Wang YH, Dong YY, Wang WM, et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Vasc Intervent Radiol. 2013;32:51. doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]